Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Treatment burden, clinical outcomes, and comorbidities in COPD: an examination of the utility of medication regimen complexity index in COPD
Authors Negewo NA, Gibson PG, Wark PAB , Simpson JL , McDonald VM
Received 8 March 2017
Accepted for publication 29 August 2017
Published 6 October 2017 Volume 2017:12 Pages 2929—2942
DOI https://doi.org/10.2147/COPD.S136256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Netsanet A Negewo,1,2 Peter G Gibson,1–3 Peter AB Wark,1–3 Jodie L Simpson,1,2 Vanessa M McDonald1–4
1Priority Research Centre for Healthy Lungs, 2Hunter Medical Research Institute, Faculty of Health and Medicine, The University of Newcastle, Callaghan, 3Department of Respiratory and Sleep Medicine, John Hunter Hospital, Newcastle, 4School of Nursing and Midwifery, Faculty of Health and Medicine, The University of Newcastle, Callaghan, NSW, Australia
Background: COPD patients are often prescribed multiple medications for their respiratory disease and comorbidities. This can lead to complex medication regimens resulting in poor adherence, medication errors, and drug–drug interactions. The relationship between clinical outcomes and medication burden beyond medication count in COPD is largely unknown.
Objectives: The aim of this study was to explore the relationships of medication burden in COPD with clinical outcomes, comorbidities, and multidimensional indices.
Methods: In a cross-sectional study, COPD patients (n=222) were assessed for demographic information, comorbidities, medication use, and clinical outcomes. Complexity of medication regimens was quantified using the validated medication regimen complexity index (MRCI).
Results: Participants (58.6% males) had a mean (SD) age of 69.1 (8.3) years with a postbronchodilator forced expiratory volume in 1 second % predicted of 56.5 (20.4) and a median of five comorbidities. The median (q1, q3) total MRCI score was 24 (18.5, 31). COPD-specific medication regimens were more complex than those of non-COPD medications (median MRCI: 14.5 versus 9, respectively; P<0.0001). Complex dosage formulations contributed the most to higher MRCI scores of COPD-specific medications while dosing frequency primarily drove the complexity associated with non-COPD medications. Participants in Global Initiative for Chronic Obstructive Lung Disease quadrant D had the highest median MRCI score for COPD medications (15.5) compared to those in quadrants A (13.5; P=0.0001) and B (12.5; P<0.0001). Increased complexity of COPD-specific treatments showed significant but weak correlations with lower lung function and 6-minute walk distance, higher St George’s Respiratory Questionnaire and COPD assessment test scores, and higher number of prior year COPD exacerbations and hospitalizations. Comorbid cardiovascular, gastrointestinal, or metabolic diseases individually contributed to higher total MRCI scores and/or medication counts for all medications. Charlson Comorbidity Index and COPD-specific comorbidity test showed the highest degree of correlation with total MRCI score (ρ=0.289 and ρ=0.326; P<0.0001, respectively).
Conclusion: In COPD patients, complex medication regimens are associated with disease severity and specific class of comorbidities.
Keywords: medication burden, medication counts, complex pharmacotherapy, clinical scores
Introduction
COPD is a major public health problem and is expected to remain a challenge for health care systems well into the 21st century.1 Despite falling death rates, COPD is still among the leading causes of death worldwide.1 The disease burden associated with COPD is further exacerbated by the presence of comorbidities, which influence disease expression and survival.2,3 Pharmacotherapy is one of the principal approaches used in the management of COPD. Treatments for COPD can improve symptoms, health status, and exercise capacity, in addition to reducing the frequency and severity of exacerbations.4 People with COPD are also highly likely to be prescribed multiple other therapies, to effectively combat their nonrespiratory comorbid conditions.2 As a result, individuals with COPD may have complex medication regimens.5,6
Complex pharmacotherapy is a key contributor to nonadherence,7,8 increased risk of drug–drug interactions and adverse drug events,9 poor disease control,10 and substantial cost of illness.11 As Winner12 pointed out, “the first step in minimizing complex medication regimens is to identify and quantify them.” The medication regimen complexity index (MRCI) is a tool that has been developed and validated for this purpose.5 MRCI measures the multiple aspects of medication regimens including type of dosage forms, dosing frequencies, and additional instructions that guide dosage administration.5 The relationships between MRCI and clinical outcomes including quality of life,13,14 unplanned hospitalization,15 all-cause mortality,16 rehospitalization for adverse drug events,9 and risk of hospital readmissions and/or emergency visits17–19 have been investigated. Although MRCI was originally developed and validated in people with COPD,5 so far only a few studies have applied this tool in studies involving COPD population.17–19 Nevertheless, these studies as such did not specifically investigate the relationships of MRCI with COPD-related clinical outcomes and other dimensions of the disease.
The present study aimed to explore the associations of medication burden in COPD patients, as measured by medication count and MRCI, with COPD outcomes (including disease severity, disease-specific health-related quality of life, exercise capacity, and prior year COPD exacerbation and hospitalization history) and multidimensional prognostic indices. Furthermore, we sought to determine the contributions of comorbid conditions to complex medication regimens in COPD. The two hypotheses we tested were that, 1) MRCI would be associated with COPD disease severity and comorbidities, and 2) MRCI may potentially serve as an alternative tool to the existing comorbidity-specific indices and other multidimensional COPD indices.
Methods
Study design and participants
This study was a cross-sectional study involving 222 COPD patients (postbronchodilator forced expiratory volume in 1 second [FEV1] to forced vital capacity [FVC] ratio <0.70).4 The data for 72 participants were obtained from our previously published studies.20,21 The remaining 150 participants were recruited from the respiratory ambulatory care clinics at John Hunter Hospital (Newcastle, Australia), the clinical research databases of the Priority Research Centre for Healthy Lungs at the University of Newcastle and the Hunter Medical Research Institute (Newcastle, Australia), and through community advertisement. All participants provided written informed consent, and ethics approval was obtained from the Human Research Ethics Committee of the Hunter New England Local Health District (12/12/12/3.06) and the University of Newcastle (H-2013-0010).
Clinical assessment
Participants attended up to two visits and demographic information, lung function, smoking history, medical history, medication use, dyspnea (modified Medical Research Council [mMRC] scale),22 self-reported prior year exacerbation and hospitalization history, and health-related quality of life (St George’s Respiratory Questionnaire [SGRQ]23 and COPD assessment test [CAT]24) were recorded. Airflow limitation was assessed using spirometry (Medgraphics, CPFS/D USB™ Spirometer; BreezeSuite v7.1, Saint Paul, MN, USA) to measure pre- and postbronchodilator FEV1, FVC, and FEV1/FVC ratio. Severity of COPD was graded according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification system of airflow limitation based on postbronchodilator FEV1% predicted (ie, GOLD grade 1, FEV1 ≥80%; GOLD grade 2, 50%≤ FEV1 <80%; GOLD grade 3, 30%≤ FEV1 <50%; GOLD grade 4, FEV1 <30%).4 Participants were evaluated using GOLD quadrants according to the 2011 GOLD “ABCD” assessment tool (using mMRC for symptom assessment).4 Participants also performed a 6-minute walk test.25
Comorbidities
Comorbidities were recorded by interviewing participants using a medical history tool that examines 14 different body or organ systems. Participants were asked if they have any medical conditions relating to these organ systems. Self-reported comorbidity diagnosis was confirmed by reviewing patient’s medical records or current medications list.
Exacerbation capture
Exacerbation history was recorded by asking participants how many times in the last 12 months they experienced a COPD-related episode that led to hospitalization, emergency visit, or the need for oral corticosteroids and/or antibiotic for at least 3 days.
Comorbidity-specific and other multidimensional COPD indices
Participants were evaluated using the following three comorbidity-specific indices, namely, Charlson Comorbidity Index (CCI),26 comorbidity test (COTE),27 and comorbidity, airway obstruction, dyspnea, and previous exacerbation (CODEx)28 and four multidimensional indices, namely, body mass index, airflow obstruction, dyspnea and exercise capacity (BODE),29 body mass index, airflow obstruction, dyspnea and severe exacerbations (BODEx),30 age, dyspnea and airflow obstruction (ADO),31 and dyspnea, airflow obstruction, smoking status and exacerbation (DOSE).32 All indices were calculated as previously prescribed.26–32
Medication assessment and MRCI scoring
Complexity of medication regimens was quantified using the MRCI developed and validated by George et al.5 MRCI is the summation of individual component scores for dosage form (section A), dosing frequency (section B), and medication administration instructions (section C). Higher MRCI scores indicate greater regimen complexity.5
Participants’ current prescription and nonprescription COPD and non-COPD medication lists were reviewed, and MRCI scores were calculated using the University of Colorado’s electronic MRCI Data Capture Tool33 and its accompanying MRCI additional instructions document.34 Akin to the suggestions of Abou-Karam et al,18 if recommendations in the MRCI additional instructions were different from those described in the original MRCI article,5 scoring was conducted in accordance with the latter. For situations where no guidance for scoring was provided in either MRCI additional instructions or the original MRCI article, working guidelines were developed by the authors (MRCI weighting score of 3 for Respimat© and continuous positive airway pressure).
Statistical analysis
Data were analyzed using Stata 13 (StataCorp LP, College Station, TX, USA) and were reported as mean (SD) or median (interquartile range [q1, q3]) depending on the distribution. Wilcoxon rank-sum test was used to compare medication counts, total MRCI scores, and MRCI sub-scores between COPD and non-COPD medications. Comparison of categorical data was performed using Fisher’s exact test. Spearman’s rank correlation coefficient was used to examine the associations of medication count and regimen complexity with clinical outcomes and multidimensional indices. The differences in total medication counts and total MRCI scores for all medications in patients with and without a given class of comorbidity were assessed using either Student’s t-test (for normally distributed data) or Wilcoxon rank-sum test (for skewed data). Using the class of comorbidities that resulted in statistically significant differences in medication counts or MRCI scores in the abovementioned comparison analyses, we performed multiple regression analyses, adjusting for age and sex, to identify which classes of comorbidities individually contributed to higher medication counts and complex medication regimens. Prior to fitting the regression models, multicollinearity of variables was assessed using the variance inflation factor (VIF), which measures how much the variance of an estimated regression coefficient increases if predictors are correlated. A VIF score of between 5 and 10 is often taken as an indication that multicollinearity may be overly influencing the least squares estimates.35 Kruskall–Wallis test was performed to compare medication counts and MRCI scores in patients grouped in the different GOLD grades and quadrants. All results were reported as significant when P<0.05.
Results
Cohort characteristics
Participants (58.6% males) had a mean (SD) age of 69.1 (8.3) years and mild-to-severe airflow limitation (Table 1). Over one-third (38%) of participants were GOLD grade 3 or higher, and over half (52.7%) were in GOLD quadrant D. Participants had significantly impaired health-related quality of life, with mean (SD) CAT and SGRQ scores of 20.6 (6.9) and 54.1 (16), respectively.
Comorbidities
A total of 118 comorbidities were recorded. The number of comorbidities per participant ranged from 0 to 11 with a median (q1, q3) of 5 (3, 7). Figure 1 shows the prevalence of comorbidities by disease categories. The prevalence of individual comorbidities is provided in Table S1.
  | Figure 1 Prevalence of comorbidities by disease categories. |
Medication count and MRCI
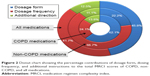
Table 2 presents medication counts and MRCI scores. The percentage contributions of dosage form (section A), dosing frequency (section B), and dosage instructions (section C) to the total MRCI scores of COPD, non-COPD, and all medications are shown in Figure 2. The median (q1, q3) total MRCI score for all medications was 24 (18.5, 31). Nearly half of the participants (47.8%) were taking nine or more medications. Even though participants were taking a higher number of non-COPD medications compared to COPD-specific medications (median of 5 versus 3, respectively; P<0.0001), the latter had greater complexity (median total MRCI score: 14.5 versus 9; P<0.0001) (Table 2). MRCI section A sub-score (dosage form) was the greatest contributor to total MRCI score of COPD medications accounting for 62.1% (Figure 2) and was significantly greater than that for non-COPD medications (9 [7, 10] versus 2 [1, 3], respectively; P<0.0001) (Table 2). MRCI section A quantifies complexity associated with using different dosage forms and may serve as a measure of inhaler device polypharmacy for the COPD medications we considered. In this study, 68 (30.6%) participants had three or more different inhaler devices prescribed concurrently. In the case of non-COPD medications, MRCI section B sub-score (dosing frequency) primarily drove the total MRCI score accounting for 66.7% (Figure 2).
Comparisons of medication counts and regimen complexity based on GOLD quadrants and GOLD grades
When considering all medications, participants in GOLD quadrant D had significantly higher total MRCI score compared to those in quadrant A (P=0.0004) (Figure 3). With regard to COPD-specific medications, participants in GOLD quadrant D had significantly higher total MRCI scores compared to those in GOLD quadrants A (P=0.0001) and B (P<0.0001) (Figure 3). Furthermore, both sections A (dosage form) and B (dosing frequency) sub-scores of the total MRCI score of COPD medications were significantly higher for participants in GOLD quadrant D (10 [7, 10] and 4.5 [3.5, 6.5], respectively) compared to those in GOLD quadrants A (7 [6, 10], P=0.003 and 3.5 [2, 4.5], P=0.0003, respectively) and B (7 [7, 10], P=0.001 and 3.5 [2.5, 4], P<0.0001, respectively). Higher usage of nebulizer or oxygen (Table S2) and the combined higher occurrences of “three times daily”, “four times daily”, “every 6 hours”, and “oxygen use over 15 hours” in participants in GOLD quadrant D may partly be responsible for the aforementioned complexity of COPD-specific regimens in this group. Only GOLD quadrants D and B had significantly different MRCI section C sub-scores (dosage instructions) (3 [1, 2] and 2 [1, 2], respectively; P=0.002). Both non-COPD medication regimen complexity and medication counts (COPD, non-COPD, and all medications) were similar among the different GOLD quadrants (Table S3).
Our analysis also showed that participants in GOLD grade 4 were taking significantly higher number of COPD medications (median 4 [3, 4]) compared to those in GOLD grades 1 (median 3 [2, 3]; P<0.0001) and 2 (median 3 [2, 3]; P=0.0002). In addition, participants in GOLD grades 4 (median MRCI 18 [13.5, 21]) and 3 (median MRCI 15.5 [12.5, 19.5]) were found to have more complex COPD medication regimens than their counterparts in GOLD grades 1 (median MRCI 12.5 [9.5, 15.5]; P<0.0001) and 2 (median MRCI 13.5 [11, 15.5]; P=0.0002).
Associations between medication burden and COPD outcomes
There was a weak but significant positive association between total MRCI score for all medications and CAT score, SGRQ, and prior year exacerbation history (Table 3). We found a significant negative association between total MRCI score for all medications and 6-minute walk distance (6MWD). There were weak but significant positive relationships between total medication count for all medications and SGRQ and exacerbation history in the previous 12 months. Total medication counts negatively correlated with 6MWD. In contrast, we found no significant relationship between total medication counts and CAT score (Table 3). Both total MRCI score and total medication counts for all medications (ie, COPD and non-COPD medications together) did not show any correlations with postbronchodilator FEV1% predicted and prior year hospitalization history.
With regard to COPD-specific medications, significant but weak positive correlations were found between total MRCI score for COPD drugs and CAT score, SGRQ, and preceding year exacerbation and hospitalization history. Increased complexity of COPD-specific treatments showed weak correlations with lower lung function and 6MWD (Table 3). Similarly, SGRQ, CAT score, and number of exacerbations in the previous 12 months correlated positively with the number of COPD medications. In contrast, correlation between number of COPD medications and number of hospitalizations in the previous 12 months was absent. Higher number of COPD medications correlated with lower lung function and 6MWD (Table 3).
Medication counts and regimen complexity in relation to class of comorbidities
In our study, total medication count for all medications was significantly higher for participants with comorbid cardiovascular diseases, gastrointestinal diseases, musculoskeletal conditions, metabolic disorders, respiratory diseases, psychiatric disorders, skin disorders, or neurological conditions compared to those without these conditions. Comorbid cancer did not contribute to higher total medication counts (Table 4).
With regard to medication regimen complexity, our preliminary analysis has shown that participants with comorbid cardiovascular diseases, gastrointestinal diseases, metabolic disorders, other respiratory conditions, psychiatric disorders, or neurological conditions tended to have significantly higher total MRCI scores for all medications compared to those without these conditions (Table 4). Further examination revealed that non-COPD medications primarily drove medication complexity in the cases of comorbid cardiovascular diseases, gastrointestinal diseases, other respiratory conditions, or neurological conditions (Figure 4A). In contrast, both COPD and non-COPD medications significantly contributed to complex medication regimens in the cases of comorbid metabolic or psychiatric disorders (Figure 4B). Comorbid musculoskeletal diseases, skin disorders, and cancer did not contribute to complex medication regimen.
In order to identify which categories of comorbid conditions individually contributed to higher medication counts or greater complexity of regimen, multiple regression analyses were performed with either total medication counts or total MRCI score for all medications as dependent variables, after adjusting for age and sex. Accordingly, cardiovascular diseases (β=2.381; 95% confidence interval [CI]: 1.360–3.403; P<0.0001), gastrointestinal diseases (β=0.927; 95% CI: 0.014–1.841; P=0.047), and metabolic disorders (β=1.003; 95% CI: 0.068–1.939; P=0.036) were found to be the three classes of comorbidities that significantly contributed to higher total medication counts. The proportion of variance explained by the regression model of total medication count (R2) was 0.29 (P<0.0001) (Table 5). Cardiovascular diseases (β=3.225; 95% CI: 0.565–5.885; P=0.018) were the only class of comorbidities that individually contributed to the regression model of medication complexity (overall model fit [R2]=0.18; P<0.0001) (Table 6).
  | Table 5 Results of multiple regression analysis for comorbidities that contributed to higher medication count |
Associations between medication burden and clinical scores in COPD
The correlations between total MRCI score for all medications and each of the following multidimensional indices CCI, COTE, CODEx, BODE, BODEx, DOSE, and ADO, as well as total medication count and the before mentioned indices are summarized in Table 7. The comorbidity-specific indices CCI and COTE showed the highest degree of correlation with both total MRCI score and medication counts. Total MRCI score was significantly associated with each of the remaining five indices considered, whereas medication count was only weakly associated with CODEx.
Discussion
The present study set out to explore the associations of medication burden, as measured by medication count and MRCI, with clinical outcomes, comorbidities, and clinical scores in COPD. While similar previous studies in COPD mainly investigated the relationships between medication count and clinical outcomes only,36,37 to the best of our knowledge, the present study is the first to describe the relationships of COPD outcomes, comorbidities, and multidimensional indices with medication burden beyond medication count.
Our findings have shown that complex medication regimens are associated with COPD disease severity and specific class of comorbidities. According to our data, COPD medication counts and regimen complexity significantly related to both GOLD grades and GOLD quadrants. For instance, participants in GOLD grades 4 (median MRCI 18) and 3 (median MRCI 15.5) were found to have significantly higher MRCI scores compared to those in GOLD grades 1 (median MRCI 12.5; P<0.0001) and 2 (median MRCI 13.5; P=0.0002). Moreover, participants in GOLD grade 4 were prescribed higher number of COPD medications compared to their counterparts in GOLD grades 1 and 2. This finding is consistent with previous reports indicating similar associations between increase in number of respiratory medications with severity of airflow limitation based on FEV1% predicted36 and GOLD grades.37 Our study has also revealed that participants in GOLD quadrant D had the most complex COPD-specific pharmacotherapy in all the three aspects of MRCI, especially when compared with those in quadrants A and B, even though they were taking a similar number of COPD medications as participants in all the other GOLD quadrants. This suggests that complexity of pharmacotherapy goes beyond the number of prescribed medications and that MRCI is a tool that can differentiate the true nature of treatment complexity.
Higher usage of nebulizer (MRCI weightings: 5) or oxygen (MRCI weightings: 3) in participants in GOLD quadrant D (Table S2) may have partly contributed to the aforementioned complexity of COPD-specific regimens in this group, as they are among the least convenient dosage forms due to the relative degree of difficulty in their administrations.5 In contrast, while no single dosing frequency was able to individually account for the higher MRCI section B score in patients in GOLD quadrant D, the combined higher occurrences of three times daily, four times daily, every 6 hours, and oxygen use over 15 hours may partly be responsible. Of note, the use of pressurized metered-dose inhaler with a spacer for bronchodilator therapy has been shown to be as effective as nebulizer therapy, at least in acute asthma.38 In relation to this, the findings of the present study demonstrate how nebulizers increase complexity of medication regimens and add support to the conversion of therapy to inhaler devices. It is worth mentioning here that long-term noninvasive ventilation (NIV) is increasingly being employed in COPD patients, particularly in those with pronounced hypercapnia.39 Unfortunately, however, data on the use of home NIV were not collected in our study. The absence of this data may potentially underestimate the overall burden of treatment, especially in the case of the severe COPD patients.
Our data also demonstrate significant but weak correlations of poor lung function, poor 6MWD, impaired health-related quality of life, and higher number of prior year exacerbations with both higher number and increased complexity of COPD-specific treatments. Similar associations between several clinical outcomes, including 6MWD, SGRQ score, and number of exacerbations in the previous 12 months with the number of respiratory medications in COPD patients, have been previously reported.37
Consistent with previous reports of Divo et al27 and Vanfleteren et al,40 participants in our study had a median of 5 (3, 7) comorbidities. As might be expected, the presence or absence of comorbid conditions can considerably influence the number of medications that patients take as well as the complexity of regimens.12 Most but not all of the comorbidities we studied were found to relate to overall medication burden in COPD. Our results illustrated that having comorbid musculoskeletal diseases, skin disorders, or cancer may not lead to a more complex pharmacotherapy in COPD patients. One possible explanation for this finding could be that these comorbidities may have been managed surgically and/or by using other non-pharmacological approaches (eg, radiation therapy and physiotherapy), the treatment burden of which cannot be captured by MRCI. In contrast, total MRCI score for all medications was significantly higher for patients with comorbid cardiovascular diseases, gastrointestinal diseases, metabolic disorders, other respiratory conditions, psychiatric disorders, or neurological conditions compared to those without these conditions. Multiple regression analysis, with age and sex as confounding variables, revealed that the presence of a comorbid cardiovascular disease can contribute approximately two additional medications to total medication counts and lead to an increase in total MRCI score of 3.23. Conversely, all the remaining comorbidities failed to individually contribute to the total MRCI score and medication count with the exception of gastrointestinal diseases and metabolic disorders, which individually contributed one medication each to total medication counts. It should be noted here that only 18% of the variance in total MRCI score was explained by the regression model of medication complexity (Table 6), suggesting that other factors probably also contribute, for instance, the effects of two or more concurrently existing comorbidities. It would be worthwhile to further explore how the co-occurrence of different types of comorbidities could potentially impact the complexity of pharmacotherapy in COPD. Moreover, investigating the contributions of specific comorbid diseases to complex medication regimens in COPD may provide clinically important insights.
Further analysis has shown that the complexity of medication regimens associated with having comorbid cardiovascular diseases, gastrointestinal diseases, other respiratory conditions, or neurological conditions was largely due to multiple dosing frequencies of non-COPD medications (Figures 2 and 4A and B). Interestingly, in the case of comorbid metabolic and psychiatric disorders, however, the complexity of the medication regimen involved both COPD and non-COPD medications (Figure 4B). While this finding may be indicative of more prevalent usage of respiratory medications in COPD patients with coexistent metabolic or psychiatric illnesses, further investigation is warranted.
In this study, we identified a weak relationship between medication regimen complexity and some of the currently available multidimensional indices that are widely used for the assessment or prognostication of COPD and its comorbidities (Table 7). Multidimensional indices are important in the management of COPD due to their ability to stratify and prognosticate.41 Despite this, however, they have not been widely used outside the research setting. With this in mind, one of the objectives of this study was to determine whether MRCI (and/or medication count) could serve as alternative tool(s) that can easily be applied in clinical settings, given that clinicians routinely review patient’s medications in their day-to-day practice. While MRCI related to multidimensional indices in our study, the strength of the relationships we observed would not justify the use of MRCI or medication count in place of the existing multidimensional indices. Nevertheless, as in the case of related previous studies,12,33 the present study has illustrated that MRCI is a valuable clinical tool that can provide an insight into several aspects of disease and medication burden, which can form the basis for informed decision making and comprehensive medication review.
It is interesting to note that participants in our study were taking a higher number of medications compared to both slightly younger (median 62.7 [61.7, 63.8] years)42 and older (mean [SD] 73.7 [8.9] years)36 COPD patients in other studies. In our cohort, the median total MRCI score for all medications was 24 (18.5, 31). This relatively high MRCI score is similar to what previous studies reported in people with COPD (mean [SD] 28.7 [5])19 and other chronic diseases including geriatric depression (mean [SD] 25.4 [11.7]),33 diabetes mellitus (mean [SD] 23 [11.6]),33 chronic kidney disease (mean [SD] 22.4 [10.2]),43 and HIV (median 21 [12, 29])44 but lower than that reported in hospitalized COPD patients (mean [SD] 35.3 [14.4])18 and elderly inpatients (≥65 years, mean [SD] 30.3 [14]).45
We found that COPD-specific medication regimens were significantly more burdensome than those of non-COPD medications despite the fact that patients were taking a fewer number of COPD medications compared to non-COPD ones. COPD medication complexity was largely driven by complex dosage formulations (Figure 2). The concurrent use of multiple different types of inhaler devices was also prevalent in our study population with one out of three patients having inhaler device polypharmacy (IDP) (defined as the use of three or more different inhaler devices).21,46 In people with respiratory diseases, complex medication regimens and IDP could lead to unintentional nonadherence to treatment, suboptimal device technique, and poor disease control.47,48 IDP is a recognizable attribute of airways disease that can be rectified via medication review and device rationalization.21,46 Our findings suggest that MRCI is a tool that can potentially assist clinicians in this regard by serving as a risk assessment tool for identifying COPD patients who may benefit from simplification of a complex dosage regimen as originally intended by George et al.5
Conclusion
This study illustrated that patients with COPD face dual disadvantages with medication regimen complexity as a result of, first, the complex dosage formulations of their COPD medications and, second, the multiple dosing frequencies of non-COPD medications they may take for their comorbid diseases. Clinicians need to carefully consider all aspects of the disease burden of COPD patients together with the multiple aspects of their pharmacotherapy to identify ways of reducing medication regimen complexity in order to improve treatment adherence, achieve better treatment outcomes, and reduce potential adverse drug events. Based on our findings, minimizing multiple dosing frequencies where possible, minimizing the use of nebulizers, reevaluating the use of inhaler devices, and/or opting for combination therapies can be suggested as ways of reducing the considerable burden of treatment in COPD patients. Future studies in this area could benefit from investigating the effects of modifying medication regimen complexity on clinically relevant outcomes such as adherence, patient satisfaction, health status, unplanned hospitalization, and adverse drug events. One of the important findings of the present study was that comorbidities play a significant role in driving medication complexity in COPD. Given that COPD patients commonly suffer from multiple comorbidities, the relative significance of individual comorbidities and their co-occurrences on medication regimen complexity in COPD deserves further investigation.
Acknowledgments
The authors would like to acknowledge Kelly Steel, Amber Smith, Gabrielle LeBrocq, Hayley Scott, Penny Baines, Clare Powell, Brooke Emmett, and Kate Morgan for their role in data collection and Kellie Fakes, Bridgette Donati, and Michelle Gleeson for their role in sample processing. This study was supported by National Health and Medical Research Council (NHMRC), Australia, Grant ID: 1045230 (PGG, VMM, JLS, and PABW), Ramaciotti Foundation (VMM), and Lung Foundation of Australia (VMM). NAN was supported by the Priority Research Centre for Asthma and Respiratory Diseases PhD Scholarship and Emlyn and Jennie Thomas Postgraduate Medical Research Scholarship through the Hunter Medical Research Institute.
Disclosure
NAN reports grants from Emlyn and Jennie Thomas Postgraduate Medical Research Scholarship through the Hunter Medical Research Institute, during the conduct of the study. PGG reports grants and personal fees from AstraZeneca, GlaxoSmithKline, outside the submitted work. PABW and JLS have nothing to disclose. VMM reports grants from NHMRC, Lung Foundation of Australia, and Ramaciotti Foundation, during the conduct of the study and personal fees from GSK, Menarini, and Astra Zeneca, outside the submitted work. The authors report no other conflicts of interest in this work.
References
López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. | ||
Vanfleteren LEGW, Spruit MA, Wouters EFM, Franssen FME. Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med. 2016;4(11):911–924. | ||
Decramer M, Janssens W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013;1(1):73–83. | ||
Global Initiative for Chronic Obstructive Lung Disease [webpage on the Internet]. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2016. Available from: http://goldcopd.org/. Accessed 11 Oct, 2016. | ||
George J, Phun YT, Bailey MJ, Kong DCM, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369–1376. | ||
Dolce JJ, Crisp C, Manzella B, Richards JM, Hardin JM, Bailey WC. Medication adherence patterns in chronic obstructive pulmonary disease. Chest. 1991;99(4):837–841. | ||
Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. | ||
Trotta MP, Ammassari A, Melzi S, et al; AdICoNA Study Group. Treatment-related factors and highly active antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2002;31(suppl 3):S128–S131. | ||
Willson MN, Greer CL, Weeks DL. Medication regimen complexity and hospital readmission for an adverse drug event. Ann Pharmacother. 2014;48(1):26–32. | ||
Yeh A, Shah-Manek B, Lor KB. Medication regimen complexity and A1C goal attainment in underserved adults with type 2 diabetes. Ann Pharmacother. 2017;51(2):111–117. | ||
Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155(18):1949–1956. | ||
Winner L. Medication regimen complexity in patients with comorbid conditions. Proceedings of the National Conference on Undergraduate Research (NCUR). Lexington, KY: University of Kentucky; 2014. | ||
Lalic S, Jamsen KM, Wimmer BC, et al. Polypharmacy and medication regimen complexity as factors associated with staff informant rated quality of life in residents of aged care facilities: a cross-sectional study. Eur J Clin Pharmacol. 2016;72(9):1117–1124. | ||
Frohlich SE, Zaccolo AV, da Silva SL, Mengue SS. Association between drug prescribing and quality of life in primary care. Pharm World Sci. 2010;32(6):744–751. | ||
Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K. Medication regimen complexity and number of medications as factors associated with unplanned hospitalizations in older people: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2016;71(6):831–837. | ||
Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K. Medication regimen complexity and polypharmacy as factors associated with all-cause mortality in older people: a population-based cohort study. Ann Pharmacother. 2016;50(2):89–95. | ||
Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713–721. | ||
Abou-Karam N, Bradford C, Lor KB, Barnett M, Ha M, Rizos A. Medication regimen complexity and readmissions after hospitalization for heart failure, acute myocardial infarction, pneumonia, and chronic obstructive pulmonary disease. SAGE Open Med. 2016;4:1–9. | ||
Schoonover H, Corbett CF, Weeks DL, Willson MN, Setter SM. Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J Patient Saf. 2014;10(4):186–191. | ||
McDonald VM, Gibson PG, Scott HA, et al. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology. 2016;21(5):875–882. | ||
McDonald VM, Simpson JL, Higgins I, Gibson PG. Multidimensional assessment of older people with asthma and COPD: clinical management and health status. Age Ageing. 2011;40(1):42–49. | ||
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. | ||
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. | ||
Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. | ||
Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428–1446. | ||
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. | ||
Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–161. | ||
Almagro P, Soriano JB, Cabrera FJ, et al; Working Group on COPD, SpanishSociety of Internal Medicine. Short- and medium-termprognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145(5):972–980. | ||
Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. | ||
Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez LS, Tordera MP, Sanchez PR. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2009;103(5):692–699. | ||
Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374(9691):704–711. | ||
Jones RC, Donaldson GC, Chavannes NH, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(12):1189–1195. | ||
Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385.e1–398.e1. | ||
UCDENVER. MRCI Additional Instructions. 2016. Available from: http://www.ucdenver.edu/academics/colleges/pharmacy/Research/researchareas/Documents/POR/MRCI_Additional_Instructions_Walkthru.pdf. Accessed 5 November, 2016. | ||
Kutner MH, Nachtsheim CJ, Neter J. Applied Linear Regression Models. 4th ed. Irwin: McGraw-Hill; 2004. | ||
Diez-Manglano J, Barquero-Romero J, Mena PA, et al; ECCO Study Researchers. Polypharmacy in patients hospitalised for acute exacerbation of COPD. Eur Respir J. 2014;44(3):791–794. | ||
Franssen FM, Spruit MA, Wouters EF. Determinants of polypharmacy and compliance with GOLD guidelines in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:493–501. | ||
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052. | ||
Janssens JP, Derivaz S, Breitenstein E, et al. Changing patterns in long-term noninvasive ventilation: a 7-year prospective study in the Geneva Lake area. Chest. 2003;123(1):67–79. | ||
Vanfleteren LEGW, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. | ||
Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015;20(8):1160–1171. | ||
Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med. 2012;12:26. | ||
Cardone KE, Manley HJ, Grabe DW, Meola S, Hoy CD, Bailie GR. Quantifying home medication regimen changes and quality of life in patients receiving nocturnal home hemodialysis. Hemodial Int. 2011;15(2):234–242. | ||
Metz KR, Fish DN, Hosokawa PW, Hirsch JD, Libby AM. Patient-level medication regimen complexity in patients with HIV. Ann Pharmacother. 2014;48(9):1129–1137. | ||
Mansur N, Weiss A, Beloosesky Y. Looking beyond polypharmacy: quantification of medication regimen complexity in the elderly. Am J Geriatr Pharmacother. 2012;10(4):223–229. | ||
Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. | ||
Bryant J, McDonald VM, Boyes A, Sanson-Fisher R, Paul C, Melville J. Improving medication adherence in chronic obstructive pulmonary disease: a systematic review. Respir Res. 2013;14:109. | ||
McDonald VM, Gibson PG. Inhalation-device polypharmacy in asthma. Med J Aust. 2005;182(5):250–251. |
Supplementary materials
  | Table S1 Prevalence of self-reported diagnosed comorbidities |
  | Table S2 Use of nebulizer or oxygen in participants in different GOLD quadrants |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.