Back to Journals » Journal of Pain Research » Volume 11
Traumatic osteoarthritis-induced persistent mechanical hyperalgesia in a rat model of anterior cruciate ligament transection plus a medial meniscectomy
Authors Tsai HC , Chen TL, Chen YP, Chen RM
Received 13 October 2017
Accepted for publication 16 November 2017
Published 22 December 2017 Volume 2018:11 Pages 41—50
DOI https://doi.org/10.2147/JPR.S154038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Hsiao-Chien Tsai,1–3 Ta-Liang Chen,2–4 Yu-Pin Chen,5 Ruei-Ming Chen1,3,6
1Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan; 2Department of Anesthesiology, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan; 3Department of Anesthesiology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan; 4Anesthesiology and Health Policy Research Center, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan; 5Department of Orthopedic Surgery, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan; 6Comprehensive Cancer Center, Taipei Medical University, Taipei, Taiwan
Background: Osteoarthritis (OA) is a degenerative joint disease characterized by progressive cartilage degeneration, subchondral bone changes, osteophyte formation, and synovitis. A major symptom is pain that is triggered by peripheral and central changes within the pain pathways. Some surgery-induced joint instability rat models of OA were described to mimic traumatic OA. Several behavioral tests were developed to access OA-induced pain. However, follow-up in most studies usually only occurred for about 4 weeks. Since traumatic OA is a chronic disease which gradually develops after trauma, the pattern of pain might differ between early and late stages after the trauma.
Purpose: To observe the time-dependent development of hypersensitivity after traumatic OA and to determine the best timing and methods to investigate traumatic OA-induced pain.
Methods: Anterior cruciate ligament transection plus medial meniscectomy was used to induce traumatic OA in Sprague-Dawley rats. Traumatic OA-induced pain was evaluated using four different behavioral tests for 15 weeks.
Results: A significant difference in mechanical hypersensitivity developed throughout the observational period. It was worst in the first 3 weeks after the operation, then became less significant after 5 weeks but persisted. There were no differences in thermal hyperalgesia or motor coordination.
Conclusion: Traumatic OA induced mechanical hyperalgesia but did not cause thermal hyperalgesia or influence motor coordination. Furthermore, to investigate chronic pain induced by OA, the observational period should be at least 5 weeks after the intervention. These findings may help in further research and improve our understanding of traumatic OA-induced pain mechanisms.
Keywords: traumatic osteoarthritis, acute and chronic pain, mechanical hyperalgesia, thermal hyperalgesia, motor coordination
Introduction
Osteoarthritis (OA) is characterized by the progressive loss of articular cartilage, subchondral bone lesions, and synovitis.1 It causes disability in elderly people and also young adults. In the last decade, the incidence of total knee replacement (TKR) in younger patients has rapidly increased. This might be associated with previous trauma. Performing TKR in young patients is still controversial. Although TKRs seem a promising procedure in patients aged ≤45 years, long-term septic and aseptic loosening rates are high.2 In cases of traumatic OA, despite complicated variances of clinical phenomena, chronic pain is the most prominent and disabling symptom.3 If OA pain can be eased or eliminated, TKR could be delayed and thus might provide some benefits in lowering rates of revisions due to aseptic loosening or infection over a patient’s lifetime.
OA-induced pain is thought to develop as the joint structure changes after a previous trauma. Therefore, TKR, which reconstructs the joint, could theoretically terminate OA-induced pain. However, there has been increasing evidence demonstrating that spinal and supraspinal neurological adaptations (central sensitization) essentially contribute to the spread and facilitation of pain sensations in OA patients.4,5 This could explain why some patients still suffer from joint pain after TKR. Currently, understanding the mechanisms that drive this chronic pain state remains challenging, which largely hampers the optimistic management of chronic pain in OA patients. Animal models could provide some help in elaborating the mechanisms underlying OA-induced pain and proposing potential therapeutic strategies.6
Many studies used a monoiodoacetate (MIA) model to induce OA pain.7–9 In studies using MIA models, observational period was usually <4 weeks, since significant changes in pain behaviors, histology, and radiologic images are seen in a short time.10,11 Pajak et al further proved that the MIA model showed a biphasic progressive pattern of pain-like behaviors over 4 weeks.8 In their study, an initial enormously increased weight-bearing deficit on day 2 after the MIA injection was followed by an alleviation of symptoms on days 6–12. On days 14–28, a second phase of chronic, progressive pain was observed. This two-stage development of OA pain in 4 weeks corresponded to the expression of selected matrix metalloproteinases. However, using a surgical OA rat model would be more reasonable for observing traumatic OA-induced pain.12–14 Since this kind of model can mimic articular joint destruction after a trauma, we could observe the development of behavioral hypersensitivity in chronic posttraumatic OA (PTOA)-induced pain. PTOA is more likely a chronic disease that gradually progresses after the initial injury. Although pain can develop immediately after a trauma, it is usually alleviated after the acute inflammatory phase and then results in a chronic persistent or progressive pain state thereafter. Taking the MIA model for instance, during the first week, pain is mainly induced by inflammation after the iodoacetate injection, but afterward inflammation plays only a minor role in pain.15 A traumatic OA model might exhibit a similar biphasic pain profile but has a longer time course of pain development than the MIA model. Previous studies used the traumatic model to evaluate OA-induced pain in the acute phase.16,17 Even though some studies conducted the same animal experiments for 4–20 weeks, they also focused on the acute phase of OA-induced pain.12,13,18,19 However, the study period was not long enough, so differences in pain behaviors might be due to acute alterations after trauma rather than chronic OA-induced pain. Therefore, this study attempted to identify pain in OA and observe the time course of pain behaviors following surgery in a rat model of PTOA to determine a suggested observational period.
Furthermore, the time course of pain development and also characteristics of pain profiles might differ between chemical- and surgery-induced OA models. There are numerous tests for evaluating pain behaviors which can represent different mechanisms of pain, such as von Frey tests for mechanical allodynia and plantar tests for thermal hyperalgesia.20,21 Both mechanical and thermal hyperalgesia can be observed in the MIA model.11 However, previous studies using the surgery-induced OA model usually used mechanical allodynia or weight-bearing asymmetry to quantify the pain severity rather than thermal hyperalgesia.22,23 We hypothesized that PTOA, unlike the MIA model, might induce pain by a different mechanism which would not lead to thermal hyperalgesia. In the present study, four different kinds of tests were performed to determine which tests are suitable for pain evaluation in a traumatic OA model.
Materials and methods
Establishment of a traumatic OA-induced pain model
Male Sprague-Dawley (SD) rats (250 ± 20 g) purchased from BioLASCO (Taipei, Taiwan) were housed under standard diurnal light/dark conditions, fed a standard commercial diet, and allowed access to tap water ad libitum. All procedures were performed according to the National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Taipei Medical University - Wan Fang Hospital (Taipei, Taiwan). After acclimation for 1 week, animals were randomly allocated into three groups: a naïve control, a sham operation, and an operation group. All surgical procedures were performed under sterile conditions and general anesthesia, which involved inhalation of 1%–2% isoflurane. The traumatic OA-induced pain model was established as described previously.13 Clinically, anterior cruciate ligament (ACL) injury is usually accompanied with meniscus tear.24,25 Concomitant meniscal tears occurred in 40%–82% of patients with sustained ACL injuries. Therefore, a combination of ACL transection (ACLT) and medial meniscectomy (MMx) was performed in this study to mimic the clinical conditions. In the operation group, the right knee was exposed using an 8–10 mm medial parapatellar approach with the patella laterally dislocated before the ACL was transected, and the medial meniscus (MM) was completely resected (ACLT/MMx) in a manner that did not injure the articular cartilage (Figure 1A and B). The skin was then closed in layers. In the sham-operated group, the right knee was exposed using the same approach, but the ACL and MM were not destroyed. The control group received anesthesia only. All rats were allowed to move, eat, and drink freely after surgery.
Evaluation of mechanical hyperalgesia
During OA development, the pain parameters were assessed weekly from the day before the ACLT/MMx surgery to postoperative week 15. Experiments were performed in a sound-attenuated and air-conditioned (20°C) laboratory. Mechanical nociception and hyperalgesia were assessed by a modified von Frey test.26 The paw withdrawal threshold in response to mechanical stimuli was measured with an electronic von Frey anesthesiometer (IITC Life Science, Woodland Hills, CA, USA) (Figure 1C). One hour before testing, rats were transported to the experimental room to minimize any environmental stimuli (visual or auditory) or stress related to handling. Animals were then placed in a clear chamber with a metal grid floor that gave access to the underside of their paws. After acclimatization to the test apparatus for 30 min, measurements were conducted on both hind paws. The paw withdrawal threshold of the contralateral limb (unaffected) served as an intra-animal control. A clean plastic tip on the force transducer was applied to the midplantar surface of the hind paw (at the center of the footpad). Slight pressure was given and gradually increased until the rat withdrew its hind paw. The maximal strength (threshold) was automatically measured. A minimum of three measurements were taken for each rat. Steps were repeated until three close withdrawal thresholds were observed (<10% difference) and recorded. Stimulation of the same hind paw occurred at least 3–5 min apart. A measurement was excluded if the rat moved during the stimulation. The data are expressed as the withdrawal threshold in grams.
Evaluation of motor coordination
Motor coordination and balance were evaluated by a rotarod performance test.27 The time to fall was measured using an accelerating rotarod performance test (model MK-630B; Muromachi, Tokyo, Japan) (Figure 1D). Each rat received a 300 s training course at a constant speed of 4 rpm. The first trial began at least 30 min after training. A rat was placed on the rod, and the rotarod speed was increased from 4 to 40 rpm over a 300 s period (with a 4 rpm increase every 30 s). Each trial was completed when the animal fell off the rotarod. Animals were then repeatedly tested weekly for their endurance performance on the accelerating rotarod (the longest time spent on the rotarod, with a cutoff time of 300 s). Data are expressed as the time to fall in seconds.
Evaluation of thermal hyperalgesia by a hot plate test
Thermal hyperalgesia was also tested using a modified hot plate test.28 Time to paw licking was measured by the hot plate test using a hot plate analgesia meter T (model I-39; IITC Life Science) (Figure 1E). After acclimatization for 10–15 min prior to testing, a rat was placed on the platform which was heated at 50 °C. The heat source was maintained at a constant intensity. Exposure to heat continued until a nocifensive reaction of either hind paw occurred, and the time necessary for a response to occur to the painful stimulus was manually recorded. The maximum exposure time on the hot surface was 30 s to prevent tissue damage. Animals were tested in only one series of measurements, and typical responses were hind paw shaking and/or lifting. Data are expressed as the time to paw licking in seconds. Hyperalgesia to heat was defined as a decrease in the withdrawal latency.
Evaluation of thermal hyperalgesia with a plantar test
Thermal nociception was evaluated using a plantar test as described previously.29 The paw withdrawal latency in response to radiant heat stimuli was assessed using a plantar stimulator analgesia meter (model 390G; IITC Life Science) (Figure 1F). An animal was placed in a clear apparatus consisting of individual boxes on an elevated, temperature-regulated glass platform maintained at 29 °C. After acclimating to the environment for 10–15 min prior to testing, a radiant heat source was located under the table and focused on the midplantar surface of the hind paw. This focused thermal heat stimulus was delivered from a fixed distance to the plantar surface of the hind paw for up to 30 s to prevent burning injury. The paw withdrawal latency was defined as the time taken by the rat to remove its hind paw from the heat source. A full leg raise specifically at the site at which the heat stimulus was directed was considered a reaction to the thermal stimulus. At each test exposure, the left and right paws were tested 1 min apart in random order. Three determinations of the withdrawal latency for each paw were separated by 5 min. Presumably, this demonstrated secondary hyperalgesia because the testing was done on the hindpaw remote from the joint inflammation site. Data are expressed as the withdrawal latency in seconds.
Statistical analysis
Data are expressed as mean ± standard error of the mean and were analyzed with IBM SPSS Statistics 18.0 software (IBM, Armonk, NY, USA). Statistical analysis for behavioral experiments was carried out on raw data using a one-way analysis of variance and a multiple paired Student’s t-test. Statistical significance was corrected for multiple comparisons using Bonferroni’s post hoc test and the Holm–Sidak method. p < 0.05 was set as the level of statistical significance. Fitted curves of behavioral parameters were analyzed by a fifth-order polynomial nonlinear regression performed using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA, USA).
Results
Induction of traumatic OA did not cause significant alterations in general behaviors
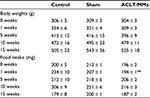
SD rats were subjected to ACLT/MMx and then evaluated at 1, 5, 10, and 15 weeks, and their body weights and food intake were recorded (Table 1). Compared to the control and sham groups, body weights of animals subjected to ACLT/MMx were not found to be influenced at 1, 5, 10, and 15 weeks (Table 1). Although food intake was significantly lower in the ACLT/MMx group compared to the control group 1 week after surgery, there was no significant difference afterward (Table 1). The general health of the animals was good, with no signs of spontaneous nociceptive behavior, impaired locomotion, or distress thereafter.
Traumatic OA-induced persistent mechanical hyperalgesia
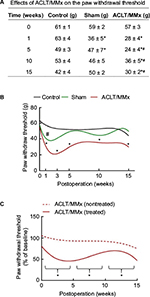
After ACLT/MMx, mechanical hyperalgesia was determined weekly by measuring the paw withdrawal threshold (Figures 2 and 3). The values at 0, 1, 3, 5, 10, and 15 weeks were listed (Figure 2A). In the sham group, values of the paw withdrawal threshold were reduced by 43% compared to control animals at 1 week after surgery (Figure 2A). However, the reduction in the paw withdrawal threshold between the control and sham group recovered at 5, 10, and 15 weeks after surgery. In contrast, 1 week after ACLT/MMx, a significant 56% decrease in the paw withdrawal threshold was seen compared to the control group (Figure 2A). Three, 5, 10, and 15 weeks later, paw withdrawal threshold values significantly decreased by 56%, 51%, 32%, and 28% in the ACLT/MMx group compared to control animals. Compared to the sham group, ACLT/MMx group showed 47%, 49%, 22%, and 40% reduction in paw withdrawal threshold values at 3, 5, 10, and 15 weeks, respectively (Figure 2A).
The fitting curve showing the time-dependent effects of the sham operation and ACLT/MMx on paw withdrawal thresholds was further analyzed (Figure 2B). Our results revealed that mechanical hyperalgesia in the ACLT/MMx group reached a maximum at week 3, with a mean paw withdrawal threshold value of 23 ± 2 g, and this was maintained for the duration of the study. In addition, a significant difference was observed between the bilateral paws during the entire observational period following ACLT/MMx (Figure 2C). During the entire observational period, the contralateral (nonoperated side) paws of the controls, and sham-operated and operated animals did not statistically differ from each other. No mechanical hyperalgesia was observed in the controls or the sham-operated group (data not shown).
In the ACLT/MMx group, only mechanical hyperalgesia developed on the treated side after the operation (Figure 3A and B). Although slight improvement was noted after 5 weeks, mechanical hyperalgesia remained significantly different from the control group for the entire observational period. However, no mechanical hyperalgesia was observed in the control or sham group after 1 week (Figure 3A and B).
Traumatic OA did not trigger thermal hyperalgesia
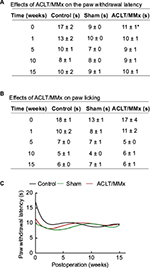
After the ACLT/MMx surgery, thermal hyperalgesia was determined by measuring the paw withdrawal latency and the time to paw licking (Figure 4). A comparison of thermal hyperalgesia measured by the plantar test and hot plate test revealed no significant difference among the three groups at any time points following ACLT/MMx (Figure 4A). Although the baseline paw withdrawal latency was significantly higher in the control group, there was no difference among the groups postoperatively. The times to paw licking in the hot plate test were similar among the groups during the study period (Figure 4B). However, the times to paw licking declined from 13–18 to 6–7 s as time went by in all groups. Figure 4C shows the fitting cure analyzed by the fifth-order polynomial nonlinear regression, which revealed similar patterns among the groups.
Traumatic OA did not change motor coordination or balance
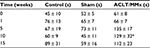
Motor coordination and balance were determined by counting the time to fall using a rotarod test (Table 2). There was no abnormal coordination noted in any of the three groups (Table 2). In contrast, times on rod even increased after weekly training and tests, especially in the ACLT/MMx group. The time on rod increased from 45 to 89 s in the control group, while it increased from 61 to 112 s in the ACLT/MMx group. Although the time on the rod greatly increased in the ACLT/MMx group, a significant difference was only found in postoperative week 10 compared to the sham group.
Discussion
PTOA-induced pain in the early and late phases
The hallmark symptom of knee OA is pain, which is a very complex phenomenon. Two different patterns of pain were described according to the disease stage.3 In the early phase, pain is linked to activity. In the late stage, it becomes unpredictable “background pain”. It is critical to know the reasons explaining why this occurs with precise timing in the progression of joint disease.30,31 Anderson et al demonstrated that acute joint trauma causes three overlapping phases of cartilage injury: early, intermediate, and late phases. At 1–2 weeks after the trauma, the early phase is characterized by apoptosis and inflammation, including augmentations in levels of proinflammatory cytokines, caspases, reactive oxygen species, basic fibroblast growth factor, metalloproteinases, and aggrecanases. An intermediate phase then follows, in which catabolic responses become subsided, and anabolic responses are initiated. Finally, a late phase with limited repair/remodeling/matrix formation occurs.32 In the MIA model, Pajak et al found a biphasic progression pattern of pain-like behavior which was compatible with the stages after trauma.8 This biphasic pattern of pain progression was also observed in our PTOA model. Mechanical hyperalgesia worsened after traumatic insults, and then slightly improved but persisted thereafter. The pain in the first 5 weeks might be correlated with early-phase inflammation due to trauma rather than chronic OA-induced pain. If one wants to study chronic pain in OA, one should focus on the late phase. According to our results, mechanical hyperalgesia became steady after 5 weeks and was more likely the chronic pain that developed in traumatic OA, while hyperalgesia in the early phase might only represent the pain due to the acute trauma.
Mechanical rather than thermal hyperalgesia developed in PTOA
There are several experimental models for OA-induced pain, including the MIA model and the ACLT/MMx model. An intra-articular injection of MIA causes inflammation followed by joint destruction,9,33,34 while ACLT/MMx leads to direct joint destruction and subsequent inflammation. Both models can mimic clinical OA. However, they might induce OA and pain through different mechanisms. Meller et al demonstrated that thermal and mechanical hyperalgesia depend on activation of two different cascades in the spinal cord.35 Thermal hyperalgesia relies mainly on activation of spinal N-methyl-D-aspartate receptors, translocation of protein kinase C, and the production of nitric oxide and cyclic guanosine monophosphate. Mechanical hyperalgesia depends on activation of spinal α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors, metabotropic glutamate receptors, and phospholipase A2, and production of cyclooxygenase products.35 Since the mechanism involved in OA-induced pain might differ between the MIA and ACLT/MMx models, behavioral changes might also differ. Therefore, different behavioral tests might be suitable for different conditioned models. Although both mechanical hyperalgesia evaluated by the von Frey test and thermal hyperalgesia evaluated by the plantar test or hot plate test can be used to quantify pain in the MIA model, no difference in thermal hyperalgesia was observed with the surgery-induced OA model in our study. This might have been due to different pathways for conducting thermal and mechanical stimuli. While Kelly et al reported spontaneous firing of C-fibers and increased mechanical sensitivity in A-fibers of knee joint mechanoreceptive neurons in the MIA model,36 Wu et al demonstrated that Aβ-fiber low-threshold mechanoreceptors but not C- or Aδ-fiber nociceptors underwent changes in electrophysiological properties in a surgery-induced OA model.37 This supports the primary sensory neurons involved in the pathogenesis of OA pain possibly differing in different OA models. The surgery-induced OA model causes changes in Aβ-fibers and thus leads to mechanical hypersensitivity. Since the surgery-induced OA model causes no changes in C fibers, which are known to be associated with thermal nociception, this could explain why we observed no thermal hyperalgesia in our ACLT/MMx model. In contrast, the MIA model, as a chemical-induced OA model, might lead to changes in both Aβ- and C-fibers, and thus could cause both mechanical and thermal hyperalgesia. In this study in which the ACLT/MMx model was used to mimic traumatic OA, the von Frey test used for evaluating paw withdrawal threshold was the best and the only test that could be used to observe PTOA-induced pain.
Young male rat model for evaluating PTOA
Experimental models tend to use genetically homogeneous groups of young rodents in restricted and unvarying environments, and might not closely mimic natural conditions.38,39 Thus, animal models might not adequately reflect clinical pain phenomena pertinent to human patients. For example, experimental models usually use young adult rats, which may not reflect OA in older humans who have long lived in a complex environment. However, PTOA develops secondary to joint injury, and the injury can occur at a young age, and a surgery-induced OA model in young adult rats can still be used to observe PTOA-induced pain.40 With a similar pathophysiology, the ACLT/MMx rat model can still present generalizable findings and improve the translation potential for humans.
Improved motor coordination and shortened hot plate latency after repeated tests
As shown in Table 2, the time to licking in the hot plate test homogeneously became shorter in all groups. Compared to results of the plantar test, another test for thermal hyperalgesia, the shortened time to a response was unlikely due to increasing pain. Contrarily, it may have been due to constrained learning after the weekly tests. The decrease in the hot plate latency after repeated testing was reported before.41–43 This decrease occurs when animals are tested once a day or once a week, regardless of whether rats are exposed to a hot or a room-temperature plate. Therefore, it is unlikely caused by learning but probably due to habituation to stimuli associated with the test apparatus.44 Otherwise, motor coordination seemed to gradually improve among all groups. This cannot be explained as decreased pain intensity, but indicates that all rats had learned and become skillful in running on the rod after the weekly tests. Buitrago et al demonstrated that rats developed a motor strategy by modifying their gait patterns during training.45 Improvement on the rotarod was not a result of reduced pain or enhanced general locomotor ability, but due to changes in the motor strategy to master the task after repeated training on the rod. Indeed, intolerable pain may lead to a shorter time on the rod;38 however, a longer time on rod does not always mean less pain. Motor coordination involves the balance, grip strength, and motor planning. Pain might impair the motor performance; however, it might also be altered or compensated for by other mechanisms. Our results indicated that OA-induced pain did not worsen the motor coordination in the ACLT/MMx model. Contrarily, motor coordination improved after repeated training. The rotarod performance test might not be suitable for measuring chronic OA-induced pain.
Conclusion
In a PTOA rat model, mechanical hyperalgesia is more suitable for detecting the development of hypersensitivity than thermal hyperalgesia or motor coordination. Since OA is a chronic disease and induces pain as the disease progresses, the observational period for investigating traumatic OA-induced pain should be longer than 5 weeks. According to the trend of changes in mechanical hypersensitivity, it worsened in the first 3 weeks after the operation, and then became less significant after 5 weeks but persisted. The pain intensity remained in a relatively steady state thereafter, which was more like chronic pain rather than acute or subacute pain caused by traumatic damage. There were no differences in thermal hyperalgesia or motor coordination. These findings may help in further research and improve our understanding of mechanisms of traumatic OA-induced pain.
Acknowledgments
We thank Wei-Hua Chang and Mei-Hsiu Liao for assistance with model establishment and behavioral test designs, and Wei-Hua Chang for comments that greatly improved the manuscript. This study was supported by grants from Welfare Surcharge of Tobacco Products (MOHW106-TDU-B-212-144001) and Chi-Mei Medical Center (106CM-TMU-16-1), Taiwan.
Author contributions
Hsiao-Chien Tsai, Ruei-Ming Chen, and Yu-Pin Chen substantially contributed to the conception and design of the study, and acquisition, analysis, and interpretation of data. Hsiao-Chien Tsai drafted the article. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther. 2009;11(3):227. | ||
Castagnini F, Sudanese A, Bordini B, Tassinari E, Stea S, Toni A. Total knee replacement in young patients: survival and causes of revision in a registry population. J Arthroplasty. 2017;32(11):3368–3372. | ||
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21(9):1145–1153. | ||
Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. 2014;18(10):1367–1375. | ||
Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(7):1043–1056. | ||
Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthr Cartil. 2013;21(9):1308–1315. | ||
Kelly S, Dobson KL, Harris J. Spinal nociceptive reflexes are sensitized in the monosodium iodoacetate model of osteoarthritis pain in the rat. Osteoarthr Cartil. 2013;21(9):1327–1335. | ||
Pajak A, Kostrzewa M, Malek N, Korostynski M, Starowicz K. Expression of matrix metalloproteinases and components of the endocannabinoid system in the knee joint are associated with biphasic pain progression in a rat model of osteoarthritis. J Pain Res. 2017;10:1973–1989. | ||
Pomonis JD, Boulet JM, Gottshall SL, et al. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain. 2005;114(3):339–346. | ||
Otis C, Guillot M, Moreau M, et al. Spinal neuropeptide modulation, functional assessment and cartilage lesions in a monosodium iodoacetate rat model of osteoarthritis. Neuropeptides. 2017;65:56–62. | ||
Ogbonna AC, Clark AK, Gentry C, Hobbs C, Malcangio M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur J Pain. 2013;17(4):514–526. | ||
Iijima H, Ito A, Nagai M, et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthr Cartil. 2017;25(6):964–975. | ||
Iijima H, Aoyama T, Tajino J, et al. Subchondral plate porosity colocalizes with the point of mechanical load during ambulation in a rat knee model of post-traumatic osteoarthritis. Osteoarthr Cartil. 2016;24(2):354–363. | ||
Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine. 2015;11(4):939–946. | ||
Beyreuther B, Callizot N, Stohr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9(1):R14. | ||
Nwosu LN, Mapp PI, Chapman V, Walsh DA. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheum Dis. 2016;75(6):1246–1254. | ||
Kc R, Li X, Kroin JS, et al. PKCδ null mutations in a mouse model of osteoarthritis alter osteoarthritic pain independently of joint pathology by augmenting NGF/TrkA-induced axonal outgrowth. Ann Rheum Dis. 2016;75(12):2133–2141. | ||
Chia WT, Pan RY, Tseng FJ, et al. Experimental osteoarthritis induced by surgical realignment of the patella in BALB/c mice. J Bone Joint Surg Br. 2010;92(12):1710–1716. | ||
Ruan MZ, Patel RM, Dawson BC, Jiang MM, Lee BH. Pain, motor and gait assessment of murine osteoarthritis in a cruciate ligament transection model. Osteoarthr Cartil. 2013;21(9):1355–1364. | ||
Detloff MR, Clark LM, Hutchinson KJ, Kloos AD, Fisher LC, Basso DM. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp Neurol. 2010;225(2):366–376. | ||
Harvey VL, Dickenson AH. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain. 2009;5:18. | ||
Fernihough J, Gentry C, Malcangio M, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112(1–2):83–93. | ||
Ogawa S, Awaga Y, Takashima M, Hama A, Matsuda A, Takamatsu H. Knee osteoarthritis pain following medial meniscectomy in the nonhuman primate. Osteoarthr Cartil. 2016;24(7):1190–1199. | ||
Bellabarba C, Bush-Joseph CA, Bach BR Jr. Patterns of meniscal injury in the anterior cruciate-deficient knee: a review of the literature. Am J Orthop (Belle Mead NJ). 1997;26(1):18–23. | ||
Kilcoyne KG, Dickens JF, Haniuk E, Cameron KL, Owens BD. Epidemiology of meniscal injury associated with ACL tears in young athletes. Orthopedics. 2012;35(3):208–212. | ||
Campana G, Rimondini R. Mechanical nociception measurement in mice and rats with automated Von Frey equipment. Methods Mol Biol. 2015;1230:229–231. | ||
Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158(2):219–223. | ||
Bannon AW. Models of pain: hot-plate and formalin test in rodents. Curr Protoc Pharmacol. 2001;Chapter 5:Unit 5.7. | ||
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. | ||
Favero M, Ramonda R, Goldring MB, Goldring SR, Punzi L. Early knee osteoarthritis. RMD Open. 2015;1(Suppl 1):e000062. | ||
Im HJ, Kim JS, Li X, et al. Alteration of sensory neurons and spinal response to an experimental osteoarthritis pain model. Arthritis Rheum. 2010;62(10):2995–3005. | ||
Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. | ||
Combe R, Bramwell S, Field MJ. The monosodium iodoacetate model of osteoarthritis: a model of chronic nociceptive pain in rats? Neurosci Lett. 2004;370(2–3):236–240. | ||
Chandran P, Pai M, Blomme EA, Hsieh GC, Decker MW, Honore P. Pharmacological modulation of movement-evoked pain in a rat model of osteoarthritis. Eur J Pharmacol. 2009;613(1–3):39–45. | ||
Meller ST, Gebhart GF. Spinal mediators of hyperalgesia. Drugs. 1994;47(Suppl 5):10–20; discussion 46–47. | ||
Kelly S, Dunham JP, Murray F, Read S, Donaldson LF, Lawson SN. Spontaneous firing in C-fibers and increased mechanical sensitivity in A-fibers of knee joint-associated mechanoreceptive primary afferent neurones during MIA-induced osteoarthritis in the rat. Osteoarthr Cartil. 2012;20(4):305–313. | ||
Wu Q, Henry JL. Changes in Abeta non-nociceptive primary sensory neurons in a rat model of osteoarthritis pain. Mol Pain. 2010;6:37. | ||
Percie du Sert N, Rice AS. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014;171(12):2951–2963. | ||
Klinck MP, Mogil JS, Moreau M, et al. Translational pain assessment: could natural animal models be the missing link? Pain. 2017;158(9):1633–1646. | ||
Schultz M, Molligan J, Schon L, Zhang Z. Pathology of the calcified zone of articular cartilage in post-traumatic osteoarthritis in rat knees. PLoS One. 2015;10(3):e0120949. | ||
Lai YY, Chan SH. Shortened pain response time following repeated algesiometric tests in rats. Physiol Behav. 1982;28(6):1111–1113. | ||
Plone MA, Emerich DF, Lindner MD. Individual differences in the hotplate test and effects of habituation on sensitivity to morphine. Pain. 1996;66(2–3):265–270. | ||
Gunn A, Bobeck EN, Weber C, Morgan MM. The influence of non-nociceptive factors on hot-plate latency in rats. J Pain. 2011;12(2):222–227. | ||
Hunskaar S, Berge OG, Hole K. A modified hot-plate test sensitive to mild analgesics. Behav Brain Res. 1986;21(2):101–108. | ||
Buitrago MM, Schulz JB, Dichgans J, Luft AR. Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem. 2004;81(3):211–216. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.