Back to Journals » Local and Regional Anesthesia » Volume 17
Transversus Abdominis Plane Block versus Epidural Anesthesia for Pain Management Post-Caesarean Delivery: A Pilot Study
Authors Salazar-Flórez JE , Arenas-Cardona LT, Marhx N, López-Guerrero E, Echeverri-Rendón ÁP , Giraldo-Cardona LS
Received 16 October 2023
Accepted for publication 16 January 2024
Published 15 April 2024 Volume 2024:17 Pages 39—47
DOI https://doi.org/10.2147/LRA.S444947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Jorge Emilio Salazar-Flórez,1 Leidy Tatiana Arenas-Cardona,2 Ninemy Marhx,2 Eduardo López-Guerrero,2 Ángela Patricia Echeverri-Rendón,1 Luz Stella Giraldo-Cardona1
1Department of Medicine, San Martín University Foundation, Sabaneta, Antioquia, Colombia; 2Department of Medicine, Hospital General de Occidente, University Health Sciences Center of University of Guadalajara, Jalisco, Mexico
Correspondence: Jorge Emilio Salazar-Flórez, Faculty of Medicine, San Martín University Foundation, Calle 75 sur # 34-50, Block 2, Sabaneta, Antioquia, Colombia, Tel +604-5906983 – 5025, Email [email protected]
Background: Effective post-operative analgesia profoundly influences patient recovery and outcomes after caesarean delivery. The Transversus Abdominis Plane (TAP) block represents a potential alternative, potentially offering greater effectiveness than epidural analgesia while causing fewer adverse effects.
Objective: To assess if the abdominal transverse block provides superior postoperative pain relief in patients undergoing caesarean delivery compared to epidural analgesia.
Methods: Participants were divided into parallel groups: an experimental group receiving TAP block (n=25) and a control group receiving epidural analgesia (n=24). All patients received a 10 mg dose of hyoscine at the end of the surgery. Experimental Group received a total of 20 mL of 0.2% ropivacaine. In Epidural group received 0.2% ropivacaine at 4 mL/h for 24 hours. All participants were administered combined with neuroaxial block anesthesia. The patients selected for epidural analgesia received the mentioned dose, while the other group block had the epidural catheter removed after the cesarean section. The primary outcome was post-caesarean pain, evaluated using the Visual Analog Scale (VAS) at four intervals (0, 6, 12, and 24 hours). Also, surgical bleeding and residual motor were evaluated. VAS pain scores between the groups were compared using the Friedman test and Generalized Linear Model (GLM) for non-normally distributed data. The effect size was estimated with Eta Square (), considering values ≥ 0.38 as indicative of large effects. A two-tailed p-value < 0.05 was deemed statistically significant.
Results: Statistically significant differences in pain scores were noted at 0 and 6 hours post-surgery (p< 0.01). The TAP block group reported lower pain scores at 0 hours (mean=0.04) and 6 hours (mean=1.16) compared to the epidural group, reflecting a substantial effect size.
Conclusion: The TAP block proves advantageous in mitigating postoperative pain for women post-caesarean delivery, particularly in the initial 6 postpartum hours. This relief promotes early mother-infant bonding and facilitates breastfeeding.
Keywords: analgesics, anesthetics, transverse abdominis plane block, epidural, cesarean section, pain
Introduction
The Caesarean Section (CS) is the most commonly performed surgical procedure globally, involving a linear incision to deliver the baby.1,2 While it has significantly reduced maternal and perinatal morbidity and mortality in appropriate cases,1,3 its frequency has surged by over 30% in the past decades,1,2 surpassing the recommended rate of 10%-15%.4 Latin America witnesses over 850,000 unnecessary caesareans annually, whit Mexico alone having a 37.8% CS birth rate.2,5
With the increasing prevalence of CS births, managing potential complications is crucial. These births carry inherent risks, including uterine rupture, abnormal placentation, stillbirth, and preterm birth, impacting both mother and child.6 Among the various post-CS complications, postoperative pain is a significant concern.7 Studies from America, Europe, and Asia show an incidence of 78.4% to 92% for moderate-to-severe post-CS pain.3,8–10
Postoperative pain following a CS can impede recovery, patient satisfaction, successful breastfeeding, and mother-child bonding.3 In the long term, it may lead to hyperalgesia and chronic postoperative pain.11 Neglecting pain management can have adverse effects on various systems, including the neuroendocrine, cardiovascular, respiratory, digestive, and central nervous systems.9
Various treatments are available for post-CS pain management, but a standardized guideline remains elusive. These treatments include systemic and intrathecal opioid administration, patient-controlled analgesia, intramuscular injection of non-steroidal anti-inflammatories (NSAIDs), and regional nerve blocks. Often, these methods are part of multimodal analgesia, offering combined pain relief with potential side effects.12,13
Historically, the epidural block was a primary method for postoperative pain management. However, its associated with complications such as nausea, vomiting, and itching,14 necessitating the exploration of alternative techniques. Complications of neuroaxial blocks can include temporary or permanent neurological deficits, total spinal blocks, or local anesthetic toxicity.15–17 One promising alternative is the Transverse Abdominal Plane (TAP) Block, which is believed to have fewer complications.18 TAP offers benefits such as reduced medication reliance, faster thrombo-prophylaxis, earlier patient mobilization, and the elimination of motor blockade risks.14,18,19
Previous research comparing epidural and TAP block for post-CS pain relief has generated conflicting results. Some studies favor epidural due to its perceived ability to manage both somatic and visceral pain.3,18,20 However, the literature remains divided on the efficacy and clinical significance of postoperative pain management. Some argue that a 33% reduction in a pain score is necessary for meaningful clinical improvement.21
Despite the benefits observed with TAP, its consistent utilization remains a topic of debate,3,19,22–24 particularly in Latin America and the Caribbean. Limited evaluations and methodological inconsistencies, stemming from its recent introduction, have resulted in ambiguous findings.25,26 This knowledge gap is particularly significant in Mexico, where assessment of TAP’s impact on post-CS pain management are scarce.
Efficient post-CS analgesia is vital for comprehensive care, encouraging early mobilization, and enhancing patient satisfaction. While the literature provides various insights into TAP’s role in post-CS pain management, any approach directly influencing a laboring woman’s postoperative pain is crucial for both mother and child.3,6 This study aims to determine if the transverse abdominal block can reduce postoperative pain in CS patients compared to epidural analgesia.
Materials and Methods
The study was conceptualized as a pilot study conducted at a single center, the “Hospital General de Occidente” in Jalisco, Mexico, with pregnant women. It followed a parallel-group design and was conducted between August and December 2021. It adhered to the Consolidated Standards of Reporting Trials (CONSORT) guidelines27 and “Improving the Reporting Quality of Non-Randomized Evaluations of Behavioral and Public Health Interventions: The TREND Statement”.28 The protocol was not registered in clinical trials or other clinical trial platforms as this study is being established as a pilot. The TREND checklist can be found in Supplement 1.
Sample size determination utilized G*Power 3.1.9.7 for Windows 10.29 Estimations were based on results from a similar study,30 revealing markedly lower pain in the TAP group compared to the control group 2 hours post-caesarean (μ1 for TAP group: 0.5) and (μ2 for the control group: 1.9). Given four repeated measurements per group and an anticipated effect size exceeding 0.58, with a type I error (α) specified as two-sided 5% (Z1-α/2 = 1.96), and a type II error (β) set at 20% (Z1-β = 0.80) ensuring 90% study power, 22 participants were estimated for each group, however, 49 pregnant patients participated of study. Anesthesia methods were determined based on patient preference. Participants were divided into an Experimental Group (EG) comprising 25 women undergoing the TAP block, and a Control Group (CG) of 24 women receiving epidural analgesia.
The study included women aged ≥ 18, indicated for caesarean section, classified as ASA I or II (patients with coexisting pathology, compensated; patients over 65 years of age; obese patients; patients with a full stomach; pregnant patients); who provided informed consent to participate in the study. Exclusion criteria encompassed patients with a BMI exceeding 40 kg/m2 or those contraindicated for combined neuroaxial blockade anesthesia.
Interventions and Procedure
Data collection occurred in the gynecology department. All participants were escorted to the operating theatre equipped with type I monitoring (SpO2, heart rate, temperature, non-invasive blood pressure). A specialist proficient in the technique administered combined neuroaxial block anesthesia. A puncture was made in the L3-L4 lumbar space using a Tuohy needle to access the peridural space. Subsequently, a long Whitacre #27 spinal needle was inserted, through which hyperbaric bupivacaine 0.5% (10 mg) was administered. An inert peridural catheter was then positioned. The epidural catheter was placed before the cesarean section. All patients were monitored for 24 hours. All 49 patients received a 10 mg dose of hyoscine at the end of the surgery, administered regularly every 8 hours for visceral pain management. After cesarean, if VAS > 6, tramadol 50 mg was prescribed.
Experimental Group (TAP Block)
For 25 participants, post cesarean an ultrasound-guided (Sonosite S-Nerve Ultrasound System) bilateral medial block was performed post-surgery was performed using linear probes (6–15MHz). After asepsis and antisepsis using povidone iodine, an 80–100 mm echogenic needle (size dependent on the patient’s adipose tissue) was inserted in line with the ultrasound beam to identify the anesthetic site between the internal oblique and transverse abdominal muscles. There, 10 mL of ropivacaine 0.2% was injected both medially and subcostally. This process was replicated on the opposite side, totaling 20 mL.
Control Group (Epidural Analgesia)
For the 24 other participants, post-surgery, the correct positioning of the epidural catheter was verified. Post cesarean, ensuring no leakage of blood or cerebrospinal fluid, 5 cc of 0.2% ropivacaine was introduced. Thereafter, an infusion pump dispensed 0.2% ropivacaine at 4 mL/h for 24 hours.
Both groups, the primary study outcome was pain intensity. Using the VAS scale, pain was quantified at four distinct time points: 0, 6, 12, and 24 hours. Participants were instructed to assess their pain during both rest and movement on the VAS, where “0” symbolized no pain and “10” represented the most intense pain imaginable. Anesthesiologists not involved in the trial conducted this assessment. In addition to the primary outcome, surgical bleeding was analyzed post-operation. The gauze utilized was extracted, weighed, converted to milliliters, and the contents of the suction canister were quantified. The effect on residual motor activity was also assessed using the Aldrete scale.
Statistical Methods
All data analyses were executed using JASP (ver. 0.17.1).31 The Shapiro–Wilk test was employed to assess data normality. Descriptive statistics for pain were conveyed as mean ± standard deviation (SD), median, and interquartile range (IQR). Additionally, the U Mann–Whitney test was utilized to compare quantitative variables between the two study groups for data that did not follow a normal distribution. Categorical variables were depicted as frequencies and percentages, with hypothesis testing conducted using the chi-squared or Fisher’s exact test, as appropriate.
To evaluate differences in pain scores (VAS scores) at the four post-caesarean section time intervals (0, 6, 12, and 24 hours), the non-parametric Friedman test was applied. This test is tailored for repeated measures on data that are not approximating a normal distribution. Following this, Conover's post hoc test was adopted to determine specific VAS measures with disparities. Conover's test, being a fitting non-parametric test, offers both Bonferroni and Holm’s corrections for assessing significance levels.
Divergences in pain scores between the TAP block and the epidural (set as the reference category) for the four recurring measures over time were computed using Generalized Linear Models (GLM) with a Gaussian variance function and an identity link function. The effect size was estimated using Eta square (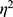 ).32 gauged the magnitude of pain reduction difference between the groups over time, categorizing effects: values below 0.1 as small, up to 0.25 as medium, and values ≥0.38 as large.
).32 gauged the magnitude of pain reduction difference between the groups over time, categorizing effects: values below 0.1 as small, up to 0.25 as medium, and values ≥0.38 as large.
Ethical Considerations
The study was meticulously adherent to principles of voluntariness, anonymity, privacy, and protection of personal information. Conducted in alignment with the Declaration of Helsinki,33 ethical clearances were procured from the “Hospital General de Occidente”, Mexico, documented on the 16th of August 2021 under the registration number 144/21. All pregnant women signed the informed consent before the procedure.
Results
Figure 1 delineates the flowchart for the study participants. The study encompassed 49 women; 24 in the control group who received epidural analgesia and 25 in the experimental group treated with a TAP block.
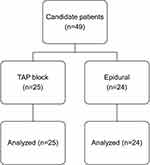 |
Figure 1 The CONSORT flow chart of study population. |
Table 1 provides a comparative analysis of demographic and clinical variables across the two groups. The mean age of pregnant women in both study groups ranged from 24 to 25 years. The horizontal Pfannenstiel technique was employed in more than 80% of the procedures. Cephalopelvic disproportion stood out as the predominant cause for caesarean sections in the epidural group, accounting for 37.5%. Less than 20% of pregnant women in both groups reported comorbidity. Parameters like age, weight, height, BMI, incision size, and hospital discharge remained comparable across both groups. However, notable differences in mean bleeding were observed: 450mL for the TAP block group and 386mL for the epidural group. Notably, only three adverse procedural incidents were reported in the epidural group, all attributable to motor block-induced extended immobilization.
 |
Table 1 The Patient’s Characteristics and Operative Data |
The Table 2 and Figure 2A depict the pain scores across time for both study groups. The Friedman test demonstrates significant variance in pain scores (X2=118.79, p < 0.001). Distinct statistical differences in the pain scores of the participating women observed (p < 0.01), the pain disparity was re-affirmed with superior outcomes for women administered the TAP block at the 0-hour and 6-hour post-caesarean checkpoints. The TAP block patients reported lower pain scores at both these intervals, registering mean values of 0.04 at 0 hours and 1.16 at 6 hours. This was in contrast to the epidural group that documented average scores of 0.5 and 2.0, respectively. The data underscored a pronounced clinical reduction in pain within the first 6 post-operative hours for the TAP group compared to the epidural group: a substantial 92% reduction at the 0-hour mark and 42% at 6 hours. This pronounced difference in pain perception between the two groups wanted by the 12 and 24-hour time frames. A high effect size was observed for the overall VAS score (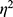 :0.77; p < 0.001), whereas the effect size for the interaction between the pain score and the intervention group was notably lower (VAS*group; =0.15; p-value<0.001).
:0.77; p < 0.001), whereas the effect size for the interaction between the pain score and the intervention group was notably lower (VAS*group; =0.15; p-value<0.001).
 |
Table 2 VAS Scores Among Different Study Groups |
The results from Conover's post hoc tests indicate that the pain score (VAS) initially remains low during the recovery phase but escalates over time, with significant upticks at 6 and 12 hours (p < 0.001 for both). However, the delta in pain scores becomes statistically insignificant when comparing the 12-hour and 24-hour intervals (p > 0.05) (Table 3). Indeed, the marginal estimate of the mean pain score, comparing both the TAP block and postoperative epidural in a single model between 0 and 24 hours, confirms significant differences at 0 and 6 hours (Figure 2B).
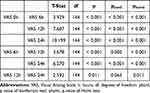 |
Table 3 Conover’s Post Hoc Comparisons - VAS |
Discussion
This study aimed to compare two analgesic techniques, TAP block and epidural analgesia, for postoperative pain management in caesarean sections patients at the “Hospital General de Occidente” in Mexico. Patients receiving TAP block reported lower VAS pain scores at 0- and 6-hours post-caesarean (p-value < 0.01). However, pain levels increased at 12 and 24 hours in both TAP block and epidural groups, with no statistically significant differences. Three adverse events related to motor blocks causing prolonged immobilization were observed in control group.
Transverse plane block for abdominal surgery is a relatively recent development, and its application in caesarean sections has been less common.20,34 The TAP block technique has proven effective not only in pain management but also in mitigating adverse effects like nausea and vomiting.34,35 Despite being a novel procedure in Latin America and the Caribbean, its impact in the region remains largely unexplored.25,26
Earlier systematic reviews suggested that epidural analgesia and TAP block are clinically comparable after abdominal surgery, especially when considering minimal clinically significant differences in postoperative pain scores.20 However, these assessments were conducted 12 hours after surgery, and the meta-analysis included only one study specifically focused on caesarean sections. In contrast, a 2021 review specifically addressing pain management for elective caesarean sections favored the TAP technique. Nearly all studies in this review concluded that TAP blocks improved pain relief, increased maternal satisfaction, and reduced the need for rescue analgesia.3 Our study’s findings further support the effectiveness of the transverse block, particularly in reducing pain during the initial post-delivery hours. Several studies investigating the efficacy of the transverse plane block in managing postoperative pain in women undergoing caesarean sections have noted pain reduction up to 24 hours after surgery.19,30,36–39 Similar to our findings, certain researchers observed changes in the initial hours. For example, Lee’s study reported pain reduction as early as 2 hours post-operation.19,30,36 Canakci et al18 documented comparable outcomes, identifying statistically significant differences in pain levels 2 hours after the caesarean procedure.
Unlike our findings, some studies advocate for the use of epidural analgesia for postoperative pain, however, the criteria to ascertain changes in pain perception in their study remained ambiguous.18 Currently, there does not seem to be definitive guidance on the preference between TAP and epidural techniques. However, regional variances in procedural methodologies and techniques should be taken into account. Advancements in ultrasound technology, the increased success rate of similar interventions, and the safety of TAP block have significantly improved this procedure, making it favorable over epidural.3,40 However, specific nuances in the TAP technique itself can impact outcomes. For example, a posterior TAP block provides longer-lasting analgesia compared to a lateral TAP block, and the addition of continuous TAP block to a single injection TAP block may further enhance and prolong the analgesic effect.40
Various local anesthetic techniques, including TAP blocks, quadratus lumborum blocks, and local anesthetic wound infiltration, have proven efficacious in reducing opioid requirements and attenuating pain scores.3 The transverse abdominal block, in particular, offers the benefit of circumventing neuroaxial complications.3,18,20 Notably, the posterior approach of this technique yields superior pain scores in comparison to its lateral counterpart.3,41 However, there’s a perspective in the literature that suggests the incremental benefits of these techniques, especially when integrated with intrathecal morphine, might be marginal.3 While there exists some degree of variability in the literature, the preponderance of evidence leans towards endorsing TAP as a crucial strategy to mitigate pain and associated complications post-caesarean section.19,20,24 Numerous studies underscore the premise that when executed proficiently and within the framework of a multimodal analgesic regimen, TAP blockade effectively addresses both acute somatic and visceral pain. Its merits extend to minimal adverse effects, cost-effectiveness, and enhanced maternal contentment regarding pain management.19,22,24
Pain relief for pregnant women is crucial. Managing pain during and after childbirth is a primary concern.42 However, postoperative pain’s implications go beyond immediate discomfort. Caesarean sections often result in significant postoperative pain, affecting post-recovery, patient satisfaction, breastfeeding, and mother-child bonding.24,42,43 Our study found a notable reduction in pain during the initial 6 hours after TAP intervention, which is crucial for early mother-child contact and breastfeeding initiation. Persistent pain can hinder maternal-fetal bonding and early breastfeeding.42,43 Our study focused solely on pain measurements and could not assess broader impacts. Postoperative pain can lead to short and long-term consequences, including postpartum depression, persistent pain, and various psychological, familial, social, and economic challenges.42,44 Given these stakes, efforts to alleviate labor pain are vital.
While our results support the use of TAP, it’s important to exercise caution when interpreting these findings. One primary limitation was our study’s design, which prevented participant randomization. However, our sample size allowed us to identify significant differences, with percentage change and effect size exceeding clinically relevant benchmarks established in prior research.21 Notably, our study is one of the pioneering efforts to assess postoperative pain in women undergoing caesareans in Latin America and the Caribbean.25,26,36
Pain scores and opioid consumption alone may not fully assess these interventions. Future studies should include maternal satisfaction and mother-child bonding as secondary outcomes. Our study focused on healthy, full-term parturients, limiting applicability to women with conditions like obesity, neuropathic pain, fibromyalgia, or those in preterm labor. We recommend future multi-centered research in the Americas, employing randomized clinical trials, and considering additional outcomes like hospital stay duration, initial mother-child interaction, breastfeeding initiation, and more. Evaluating TAP’s impact on non-clinical outcomes and quality of life as reported by patients is essential.
Conclusions
The TAP block contributes to pain relief during the crucial initial 6 hours postpartum after a cesarean section -a crucial period for mother-child interaction and the prompt initiation of breastfeeding. These findings offer an initial glimpse into the technique’s suitability, paving the way for potential adoption in the gyneco-obstetric field in the Latin American region.
Funding
This study was funded by the San Martin University Foundation (PYI-2022-012), Colombia.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6(6):e005671. doi:10.1136/bmjgh-2021-005671
2. Boerma T, Ronsmans C, Melesse DY, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392(10155):1341–1348. doi:10.1016/S0140-6736(18)31928-7
3. Roofthooft E, Joshi GP, Rawal N, et al.; PROSPECT Working Group* of the European Society of Regional Anaesthesia and Pain Therapy and supported by the Obstetric Anaesthetists’ Association. PROSPECT guideline for elective caesarean section: updated systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76(5):665–680. doi:10.1111/anae.15339
4. World Health Organization. WHO Statement on Cesarean Section Rates. Ginebra: World Health Organization; 2015.
5. Bernal-García C, Escobedo-Campos C. Cesarean section: current situation and associated factors in Mexico. Rev Salud Quintana Roo. 2018;11(40):28–33.
6. Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. 2018;392(10155):1349–1357. doi:10.1016/S0140-6736(18)31930-5
7. Cohen M, Quintner J, van Rysewyk S. Reconsidering the International Association for the Study of Pain definition of pain. Pain Rep. 2018;3(2):e634. doi:10.1097/PR9.0000000000000634
8. Wang L, Wei C, Xiao F, et al. Incidence and risk factors for chronic pain after elective caesarean delivery under spinal anesthesia in a Chinese cohort: a prospective study. Int J Obstet Anesth. 2018;2018(34):21–27. doi:10.1016/j.ijoa.2018.01.009
9. Borges NC, Pereira LV, de Moura LA, Silva TC, Pedroso CF. Predictors for moderate to severe acute postoperative pain after cesarean section. Pain Res Manag. 2016;2016:5783817. doi:10.1155/2016/5783817
10. Murray A, Retief F. Acute postoperative pain in 1 231 patients at a developing country referral hospital: incidence and risk factors. South Afr J Anaesth Analgesia. 2016;22(1):26–31 doi:10.1080/22201181.2015.1115608.
11. Kainu JP, Sarvela J, Tiippana E, Halmesmaki E, Korttila KT. Persistent pain after caesarean section and vaginal birth: a cohort study. Int J Obstet Anesth. 2010;19(1):4–9. doi:10.1016/j.ijoa.2009.03.013
12. Gamez BH, Habib AS. Predicting severity of acute pain after cesarean delivery: a narrative review. Anesth Analg. 2018;126(5):1606–1614. doi:10.1213/ANE.0000000000002658
13. Bimrew D, Misganaw A, Samuel H, Daniel T, Bayable S. Incidence and associated factors of acute postoperative pain within the first 24h in women undergoing cesarean delivery at a resource-limited setting in Addis Ababa, Ethiopia: a prospective observational study. Sage Open Med. 2022;10:1–10. doi:10.1177/20503121221133190
14. Fonseca R, Goncalves D, Bento S, Valente E. Postoperative epidural analgesia in cesarean section: comparison of therapeutic schemes. Cureus. 2020;12(12):e12166. doi:10.7759/cureus.12166
15. Lawrence H, Morton A. Postpartum complications following neuraxial anaesthesia for obstetric physicians. Obstet Med. 2023;16(3):142–150. doi:10.1177/1753495X221146329
16. Kulkarni SS, Tayade DN, Kane PP, et al. Major complications following central neuraxial block - A multi-centre observational study in Maharashtra (MGMM CNB Study). Indian J Anaesth. 2023;67(Suppl 1):S15–S28. doi:10.4103/ija.ija_747_22
17. Prior CH, Burlinson CEG, Chau A. Emergencies in obstetric anaesthesia: a narrative review. Anaesthesia. 2022;77(12):1416–1429. doi:10.1111/anae.15839
18. Canakci E, Gultekin A, Cebeci Z, Hanedan B, Kilinc A. The analgesic efficacy of transverse abdominis plane block versus epidural block after caesarean delivery: which one is effective? TAP block? Epidural block? Pain Res Manag. 2018;2018:3562701. doi:10.1155/2018/3562701
19. Kupiec A, Zwierzchowski J, Kowal-Janicka J, et al. The analgesic efficiency of transversus abdominis plane (TAP) block after caesarean delivery. Ginekol Pol. 2018;89(8):421–424. doi:10.5603/GP.a2018.0072
20. Desai N, El-Boghdadly K, Albrecht E. Epidural vs. transversus abdominis plane block for abdominal surgery - a systematic review, meta-analysis and trial sequential analysis. Anaesthesia. 2021;76(1):101–117. doi:10.1111/anae.15068
21. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424–429. doi:10.1093/bja/aew466
22. Fusco P, Cofini V, Petrucci E, et al. Transversus abdominis plane block in the management of acute postoperative pain syndrome after caesarean section: a randomized controlled clinical trial. Pain Physician. 2016;19(8):583–591.
23. Jadon A, Jain P, Chakraborty S, et al. Role of ultrasound guided transversus abdominis plane block as a component of multimodal analgesic regimen for lower segment caesarean section: a randomized double blind clinical study. BMC Aesthesiol. 2018;14(18):53. doi:10.1186/s12871-018-0512-x
24. Kakade A, Wagh G. Evaluate the feasibility of surgical transversus abdominis plane block for postoperative analgesia after cesarean section. J Obstet Gynaecol India. 2019;69(4):330–333. doi:10.1007/s13224-019-01241-3
25. Jadon A, Amir M, Sinha N, Chakraborty S, Ahmad A, Mukherjee S. Bloqueio do plano do quadrado lombar ou transverso do abdome para analgesia pós-operatória após cesariana: um estudo randomizado duplo-cego. Braz J Aneshesiol. 2022;72(4):472–478 doi:10.1016/j.bjane.2021.06.014.
26. Barrios-Caro C, Morales-Tuesca J, Ramos-Clason E, Bellini-Pardo S. Analgesic effectiveness of ultrasonography-guided transverse abdomen block for postoperative pain management in patients undergoing cesarean section under spinal anesthesia with intratecal morphine as adjuvant, a cohort study. Archivos de medicina. 2022;18(7):1–5 doi:10.36648/1698-9465.22.18.1549.
27. Jayaraman J. Guidelines for reporting randomized controlled trials in paediatric dentistry based on the CONSORT statement. Int J Paediatr Dent. 2020;31 Suppl 1(S1):38–55. doi:10.1111/ipd.12733
28. Des Jarlais DC, Lyles C, Crepaz N, Group T. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. 2004;94(3):361–366. doi:10.2105/AJPH.94.3.361
29. Faul F, Erdfelder E, Lang A, Buchner A. Statistical power analyses using G*Power 3.1: test for correlation and regression analyses. Behavior Research Methods. 2009;41(4):1149–1160. doi:10.3758/BRM.41.4.1149
30. Lee AJ, Palte HD, Chehade JM, Arheart KL, Ranasinghe JS, Penning DH. Ultrasound-guided bilateral transversus abdominis plane blocks in conjunction with intrathecal morphine for postcesarean analgesia. J Clin Anesth. 2013;25(6):475–482. doi:10.1016/j.jclinane.2013.05.004
31. JASP. JASP (versión 0.17.3) [Computer software]; 2023.
32. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141(1):2–18. doi:10.1037/a0024338
33. Asociación Médica Mundial. Declaración de Helsinki de la AMM-Principios éticos para las investigaciones médicas en seres humanos. 64ª Asamblea General. Fortaleza, Brasil: Asociación Médica Mundial; 2019.
34. Escudero-Fung M, Lehman EB, Karamchandani K. Timing of transversus abdominis plane block and postoperative pain management. Local Reg Anesth. 2020;13:185–193. doi:10.2147/LRA.S278372
35. Jelting Y, Klein C, Harlander T, Eberhart L, Roewer N, Kranke P. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth. 2017;10:83–90. doi:10.2147/LRA.S111459
36. Ripollés J, Marmaña S, Abad A, Calvo J. Eficacia analgésica del bloqueo del plano transverso del abdomen ecoguiado-revisión sistemática. Rev Bras Anestsiol. 2015;65(4):255–280. doi:10.1016/j.bjan.2013.10.014
37. Costello J, Moore A, Wieczorek P, et al. The transversus abdominis plane block, when used as part of a multimodal regimen inclusive of intrathecal morphine, does not improve analgesia after cesarean delivery. Reg Anesth Pain Med. 2009;34(6):586–589. doi:10.1097/AAP.0b013e3181b4c922
38. Eslamian L, Jalili Z, Jamal A, Marsoosi V, Movafegh A. Transversus abdominis plane block reduces postoperative pain intensity and analgesic consumption in elective cesarean delivery under general anesthesia. J Anesth. 2012;26(3):334–338. doi:10.1007/s00540-012-1336-3
39. Canovas L, Lopez C, Castro M, Rodriguez AB, Perez L. Contribución del bloqueo del plano transverso abdominal guiado por ultrasonidos a la analgesia postoperatoria tras la cesárea [Contribution to post-caesarean analgesia of ultrasound-guided transversus abdominis plane block]. Rev Esp Anestesiol Reanim. 2013;60(3):124–128. Spanish. doi:10.1016/j.redar.2012.09.024
40. Tsai HC, Yoshida T, Chuang TY, et al. Transversus abdominis plane block: an updated review of anatomy and techniques. Biomed Res Int. 2017;2017:8284363. doi:10.1155/2017/8284363
41. Faiz SHR, Alebouyeh MR, Derakhshan P, Imani F, Rahimzadeh P, Ghaderi Ashtiani M. Comparison of ultrasound-guided posterior transversus abdominis plane block and lateral transversus abdominis plane block for postoperative pain management in patients undergoing cesarean section: a randomized double-blind clinical trial study. J Pain Res. 2018;11:5–9. doi:10.2147/JPR.S146970
42. Sangkum L, Thamjamrassri T, Arnuntasupakul V, Chalacheewa T. The current consideration, approach, and management in postcesarean delivery pain control: a narrative review. Anesthesiol Res Pract. 2021;2021:2156918. doi:10.1155/2021/2156918
43. Karlstrom A, Engstrom-Olofsson R, Norbergh KG, Sjoling M, Hildingsson I. Postoperative pain after cesarean birth affects breastfeeding and infant care. J Obstet Gynecol Neonatal Nurs. 2007;36(5):430–440. doi:10.1111/j.1552-6909.2007.00160.x
44. Weibel S, Neubert K, Jelting Y, et al. Incidence and severity of chronic pain after caesarean section: a systematic review with meta-analysis. Eur J Anaesthesiol. 2016;33(11):853–865. doi:10.1097/EJA.0000000000000535
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

