Back to Journals » Cancer Management and Research » Volume 14
Transrectal Ultrasound in Prostate Cancer: Current Utilization, Integration with mpMRI, HIFU and Other Emerging Applications
Authors Panzone J , Byler T, Bratslavsky G, Goldberg H
Received 13 October 2021
Accepted for publication 14 March 2022
Published 22 March 2022 Volume 2022:14 Pages 1209—1228
DOI https://doi.org/10.2147/CMAR.S265058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
John Panzone, Timothy Byler, Gennady Bratslavsky, Hanan Goldberg
Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA
Correspondence: Hanan Goldberg, Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, Tel +1-315-271-4173, Email [email protected]
Abstract: Transrectal ultrasound (TRUS) has been an invaluable tool in the assessment of prostate size, anatomy and aiding in prostate cancer (PCa) diagnosis for decades. Emerging techniques warrant an investigation into the efficacy of TRUS, how it compares to new techniques, and options to increase the accuracy of prostate cancer diagnosis. Currently, TRUS is used to guide both transrectal and transperineal biopsy approaches with similar cancer detection rates, but lower rates of infection have been reported with the transperineal approach, while lower rates of urinary retention are often reported with the transrectal approach. Multiparametric MRI has substantial benefits for prostate cancer diagnosis and triage such as lesion location, grading, and can be combined with TRUS to perform fusion biopsies targeting specific lesions. Micro-ultrasound generates higher resolution images that traditional ultrasound and has been shown effective at diagnosing PCa, giving it the potential to become a future standard of care. Finally, high-intensity focused ultrasound focal therapy administered via TRUS has been shown to offer safe and effective short-term oncological control for localized disease with low morbidity, and the precise nature makes it a viable option for salvage and repeat therapy.
Keywords: transrectal ultrasound, prostate cancer, transperineal biopsy
Introduction
Prostate cancer (PCa) is the second most common and second most lethal cancer among American men.1 In 2021, it was predicted that nearly 250,000 men in the United States will be diagnosed with prostate cancer, with the majority of patients aged 65 or older.2 The early detection of prostate cancer is crucial, as the 5-year survival rate drops from nearly 100% for patients diagnosed with local or regional prostate cancer to 30% for patients whose cancer has spread to other organs.2 Various biomarkers, with prostate-specific antigen (PSA) being the most common, can assist in screening and diagnosing patients with prostate cancer,3 but prostate biopsies are still required for definitive cancer diagnosis. Due to the unique anatomical position of the prostate, specialized techniques have been developed to accurately obtain biopsy samples without damaging surrounding structures. With the advancement of imaging technology, several techniques, including the use of ultrasound and magnetic resonance imaging (MRI), have become viable options for physicians to implement while collecting prostate biopsies. However, each technique carries inherent advantages, disadvantages, and risks, which Urologists and their patients should be aware of. The most common and longest standing guided biopsy technique is the transrectal-ultrasound (TRUS)-guided biopsy. While this method has been utilized for decades, the development of new techniques warrants an evaluation of the efficacy of TRUS-guided biopsy and how it compares to more modern techniques. The objective of this review was to assess the current state of TRUS for prostate biopsies, in its various forms, understand its role in focal treatment of prostate cancer, and compare it to other available imaging modalities.
Methods
This narrative review was conducted with the aim of providing an overview of the broad topic of TRUS use in prostate cancer. We sought to highlight the most common clinical applications, the advantages, and disadvantages of various techniques, and provide insight as to future uses and developments. The PubMed database was searched, and articles were selected based upon the discretion and expertise of the authors and relevance to the topics being addressed. Appropriate articles as determined by the authors are summarized and included in the tables.
History of Transrectal Ultrasound
The transrectal route of prostate biopsy was first described in scientific literature by Grabstald and Elliot in 1953.4 While this early technique was rudimentary, lacking any form of external guidance and using simple clamps to take bites of the prostate, it laid the foundation for the more advanced techniques practiced today. In the late 1980s, Lee and Cooner introduced the use of TRUS for guiding prostate biopsy5,6 which has been the cornerstone of prostate cancer diagnosis since. However, advancements in the field of Urology over the last several years have led physicians to consider new alternatives for increased accuracy in performing prostate biopsies and detecting clinically significant prostate cancers (csPCa).
Use of Transrectal Ultrasound for Prostate Biopsy
Transrectal Approach
TRUS-guided prostate biopsy through the transrectal approach has been the main prostate cancer detection pathway for decades. In performing a TRUS-guided transrectal biopsy, the physician uses an ultrasound probe placed in the rectum to assess and visualize the prostate and guide biopsy needles transrectally, to collect tissue samples.7 Transrectal prostate biopsy is an outpatient procedure and can safely and comfortably be performed under local anesthesia.8
This technique used to perform TRUS prostate biopsy has remained largely consistent since its conception, with the most notable alterations manifesting in the number and location of biopsy cores collected. The sextant mapping technique, which involves extracting six sample cores, was the initial standard baseline practice but gradually emerging data suggested that collecting more cores may be associated with higher prostate cancer detection rates. Collecting a minimum of eight prostate cores, with at least three targeted at the lateral aspect of the peripheral zone, has been shown to increase PCa detection by approximately 15%.9 Phillip et al furthered this conclusion, reporting a 16.9% increase in detection rate of PCa from parasagittal sextant biopsies to eight core biopsies including peripheral basal body.10 This detection rate further increased by another 9.9% with a 10-core biopsy strategy.10 However, the increase in detection rates between 10 and 12 core biopsies was only 1.4%, indicating a significantly diminishing return after a certain number of cores.10 Other studies suggest that the addition of 4 cores in the lateral peripheral zone of the prostate (10 cores total) could increase detection rates between 23% and 105%.11 Research from Eskicorapci et al found a 25.5% increase in cancer detection rate when using a 10 core sampling method when compared to sextant sampling,12 while Guichard et al report a 22% improvement in cancer detection using a 12-core sampling method compared to a 6-core sampling method.13
One limitation of TRUS-guided prostate biopsy is the lack of a constant visual reference to ensure an even distribution of biopsy cores.14 This, combined with variable operators’ experience,15 contributes to the high false-negative rate associated with TRUS-guided prostate biopsy with studies reporting a csPCa detection rate between 14% and 27% upon repeat transperineal biopsy among men with prior negative TRUS biopsy.16 However, even biopsies performed by experienced urologists resulted in significant biopsy template deviations, leading to clustered patterns and under-sampling of a sizeable portion of the prostate.17 The use of simulators has been suggested to help achieve practical experience in a safe environment to address this limitation.18
The greatest drawback of transrectal prostate biopsy is the associated infection rate. The infection rate from transrectal biopsy has been reported as high as 7%,19 and is responsible for up to 72% of prostate biopsy complication-related hospitalizations.20 Life-threatening sepsis stemming from infection occurs in 2–5% of the cases and the associated costs for patients range from approximately $9000 to $19,000.21 The administration of antibiotic prophylaxis and cleaning the anus and lower rectum prior to conducting the procedure have been shown to reduce infection rates from TRUS biopsies.22 However, the rate of infection following TRUS biopsies has been increasing, likely due to increasing antibiotic resistance of bacteria frequently found in the rectum.23 Therefore, the risk of antibiotic prophylaxis contributing to the development of antibiotic-resistant bacteria must be considered if it is to be implemented as a long-term practice. Currently, utilizing the transperineal approach is a recommend strategy to minimize the likelihood of infections for patients who are considered as high risk for this complication.19 Commonly reported complications of transrectal and transperineal prostate biopsies are presented in Table 1. Among transrectal biopsies, potential non-infectious complications include rectal bleeding, hematuria, hematospermia, vasovagal episodes, and persistent dysuria.24
 |
Table 1 Commonly Reported Complication Rates Following Transrectal and Transperineal Prostate Biopsy |
Transperineal Approach
The transperineal approach for prostate biopsy was first described in scientific literature in the 1950s25 and has become an increasingly popular choice among physicians. This approach also uses TRUS to for guidance, but passes biopsy needles through the perineum, rather than the rectum, to access the prostate. This approach avoids puncturing the transrectal mucosa, reducing the transfer of bacteria and resulting in lower rates of infection compared to the transrectal approach. A recent meta-analysis revealed a 76% risk reduction in fever caused by infection following transperineal biopsy compared to the transrectal approach.26
Overall PCa detection rates are similar between the transrectal and transperineal approaches, with numerous studies reporting no significant difference between them.27 Huang et al report detection rates of 45% and 49% for the transperineal and transrectal approach, respectively.28 Takenaka et al published similar findings with non-significantly different detection rates of 47% for transperineal biopsy and 53% for transrectal biopsy.29 Other studies report even closer, non-significant difference in the detection rates of less than a 4% between the techniques.30,31 Reported detection rates for transrectal and transperineal approaches are displayed in Table 2.
 |
Table 2 Cancer Detection Rates of Transrectal and Transperineal Prostate Biopsy |
Despite similar overall detection rates, the transperineal approach has been shown to be superior at detecting anteriorly located prostate tumors when compared to the transrectal approach.32,33 This is significant, as anterior tumors account for approximately 20% of all prostate tumors34 and are often larger, more likely to display positive margins, manifest with lower PSA levels and are less readily palpable.35
While associated with lower rates of infection, transperineal biopsies have been associated with increased risk of urinary retention compared to transrectal biopsy (Table 1). Berry et al report a slightly higher incidence using the transperineal approach compared to the transrectal approach (1.9% vs 1.0%),36 but other research has found an increase in urine retention by as much as 7.9% in transperineal biopsy.37 Patients undergoing transperineal biopsy are susceptible to other complications as well, including hematuria, urethrorrhagia, and hematospermia, prostatitis, and perineal hematoma (Table 1). Other research from Symons et al observed 409 men who underwent transperineal prostate biopsy and found 49.3% experienced minor hematuria, 2.4% experienced major hematuria, 16.4% experienced dysuria, and 4.2% experienced urinary retention.38 It is important to note that while 10 or 12 core biopsies are most commonly used during transrectal biopsy, transperineal biopsy can often be conducted with as many as 20 cores.39 Therefore, number of cores must be considered when assessing complication rates in addition to route of administration.
Previously, transperineal biopsies had to be performed under general anesthesia and in the operating room, increasing cost and adding significant inconvenience for physicians, patients, and healthcare facilities. However, this procedure has evolved and is now performed under local anesthesia in the office using various transperineal access systems such as the PrecisionPoint Transperineal Access System (Perineologic, Cumberland, MD).40 This device employs a single access needle that minimizes the number of punctures to the perineal skin and serves to stabilize the biopsy needle in-plane with the ultrasound probe, thereby overcoming the limitations of freehand approaches, and avoiding the use of a stepper/grid and its need for multiple puncture sites. Additionally, simple and accurate techniques for administering transperineal biopsy have been described41 which could increase the accessibility for low tech centers both domestically and internationally, helping further establish transperineal biopsy as a standard outpatient procedure. One such study conducted by Wetterauer et al assessed 400 patients who underwent freehand fusion transperineal prostate biopsy between 2015 and 2019 and report 0% rates of infections or periprocedural complications with an overall cancer detection rate of 64.5%.42 Overall, these transperineal access systems enable the successful performance of in-office transperineal prostate biopsies under local anesthesia without the need for periprocedural antibiotics, with similar cancer detection rates as in the transrectal approach with minimal complications.43
The low cost,44 familiarity among physicians, and similar overall detection rates45 indicate that transrectal biopsies are unlikely to disappear anytime soon. However, the lower infection rate associated with the transperineal approach may justify its use as a future standard for prostate cancer biopsy.33 Currently, the European Association of Urology (EAU) strongly recommends performing transperineal biopsy with proper surgical preparation of the skin due to the lower risk of infection compared to the transrectal approach.46
If performing a transrectal biopsy, the EAU strongly recommends cleaning the rectum with povidone-iodine prior to the procedure.46 The American Urological Association (AUA) considers TRUS-guided transrectal biopsy using 12-core systematic sampling as the optimal approach47 but recognizes the use of transperineal biopsy approach to reduce the risk of infection and avoid antibiotic use.19
TRUS for Assessing Prostate Size and Anatomy
TRUS is also useful for assessing prostate size and anatomy. Research has shown that TRUS measurements of prostate volume are highly accurate, showing no significant difference from the actual volume of the removed specimen.48 The accuracy of TRUS in estimating prostatic volume has been shown to be comparable to that of MRI,48 and superior to that of digital rectal exams, which often underestimate prostate size, especially if the volume is greater than 30 milliliters.49 The ability to accurately assess prostate volume make TRUS a useful tool in the diagnosis and treatment planning of benign prostatic hyperplasia. The degree of prostate enlargement can affect the severity of symptoms, response to nonsurgical therapy, need for surgical intervention and decision on the type of surgical intervention to be used.50 TRUS also enables physicians to elucidate prostate and bladder neck anatomy, including the extent and size of a prostate median lobe. While research suggests that MRI cross-sectional imaging may offer slightly greater accuracy in measuring prostate size than TRUS,51 the decreased cost and increased availability of TRUS solidify it as the preferred method of prostate size and shape mapping for many urologists.52 Current AUA guidelines recommend clinicians consider preoperative prostate size and shape assessment via either TRUS or MRI, among other imaging modalities.53
Comparison of TRUS to Multiparametric MRI
mpMRI Background
Multiparametric magnetic resonance imaging (mpMRI) is a relatively new technique generating significant enthusiasm among the urologic community. First described in scientific literature circa 2008,54–56 mpMRI has become widely recognized as a useful tool in the detection and diagnosis of prostate cancer. mpMRI uses three modes of observation to assess if tissue may be cancerous: T2 weighted imaging to assess tissue structures, diffusion weighted imaging to assess cell density, and dynamic contrast imaging to assess vascularity.56 mpMRI imaging is standardized using the Prostate Imaging-Reporting and Data System (PI-RADS), which provides assessment criteria to categorize imaging that represents suspicious lesions or areas of the prostate at high risk for csPCa.57 PI-RADS, now in its second version, uses a combination of the mpMRI modes of observation to rate the likelihood of csPCa being present on a scale from 1 to 5. (Figure 1). PI-RADS 1 indicates there is a very low likelihood of csPCa being present, while PI-RADS 5 means there is a very high likelihood that csPCa is present in the prostate.57 Among the applications of mpMRI being explored are use as a risk stratification tool among patients with elevated serum PSA, integration with traditional TRUS for enhanced cancer identification and biopsy accuracy, and as a potential substitute for biopsy altogether.58 While some literature suggests negative mpMRI analyzed by experienced radiologists may be implemented among the general patient population to safely avoid biopsy,59 most studies indicate that imaging alone is currently insufficient unless the patient is regarded as low risk. Current AUA and EAU guidelines support the use of mpMRI imaging in men at risk for prostate cancer without a previous biopsy or with increasing PSA levels following a negative biopsy.60,61
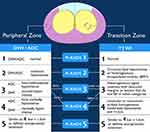 |
Figure 1 PIRADS VERSION 2 (American College of Radiology. Prostate Imaging – Reporting and Data System. 2019. Version 2.1. PIRADS). Abbreviations: DWI, diffusion weighted imaging; DCE, dynamic contrast enhanced; WI, weighted imaging; ADC, apparent diffusion coefficient; BPH, benign prostatic hyperplasia. Notes: Reproduced from the Radiology Assistant.146 |
mpMRI Use in Biopsy-Naïve Patients
Biopsy-naïve patients are of particular interest regarding the use of mpMRI prior to prostate biopsy. A lack of consensus currently exists on whether biopsy-naïve patients suspected of PCa can safely avoid biopsy based on the results of mpMRI imaging alone. Oshi et al suggest that biopsy-naïve men should be considered for biopsy regardless of mpMRI findings, especially if their PSA density is greater than 0.15 ng/mL/cc.62 A review conducted by the EAU Prostate Cancer Guidelines Panel also suggests that the NPV is too variable for all patients to safely avoid biopsy.63
Despite this, some literature suggests that mpMRI can be used to safely avoid biopsy in biopsy-naïve patients deemed low risk for PCa. The previously mentioned EAU review suggests that, in patients determined to be low risk through other reliable tools, negative mpMRI could be sufficient to avoid unnecessary biopsy.63 Ryoo et al assessed 1098 patients who underwent mpMRI before prostate biopsy and found that csPCa was only detected in 4% of biopsy-naïve patients with a PI-RADS score of 1, and was only detected in 2% of biopsy-naïve patients with PSA density less than 0.15 ng/mL and a PI-RADS score of 3.64 In addition, only 4% of the patients with a PI-RADS score of 3 and a PSA density between 0.15 and 0.3 ng/mL were found to have csPCa.64 Thus, it was concluded that biopsy-naïve patients with a PI-RADS score of 2 or less can safely avoid unnecessary biopsy, while patients with a PI-RADS score of 3 may avoid biopsy based on PSA density.64
mpMRI in Patients with Previous Negative Biopsy
Patients with a previous, negative biopsy may also benefit from mpMRI as a way to avoid unnecessary repeat biopsy. Oshi et al suggest that men with negative mpMRI, a previously negative biopsy, and a PSA density below 0.15 ng/mL/cc can safely avoid rebiopsy.62 Other research indicates that men who have a negative MRI following a negative biopsy are likely safe to avoid repeat biopsy, but repeat biopsy is warranted among those who have a positive MRI following initial negative biopsy.65 Current AUA guidelines recommend obtaining high-quality MRI in patients with prior negative biopsy but showing persistent suspicious signs of PCa.66 The AUA also recommends that if repeat biopsy is deferred based on MRI findings then the patient should continue clinical and PSA follow-ups and repeat MRI as part of the surveillance protocol should be considered.66 Wang et al retrospectively applied the PRECISION trial strategy to patients who received mpMRI imaging before systematic and targeted biopsy and found that, among patients with previous negative biopsy, the PRECISION approach would avoid 21% of repeat biopsies while detecting 1.5% more csPCa.67 Salami et al assessed 140 men with previous negative biopsy and found that fusion biopsy was significantly more likely to detect csPCa when compared to 12-core systematic biopsy (47.9% vs 30.7%) and that only using a fusion biopsy among men highly suspected of PCa would have detected all but 3.5% csPCa.68 Stonier et al assessed 2642 men, including both biopsy-naïve and previous negative biopsy patients, with either rising PSA density or abnormal digital rectal examination and concluded that approximately one-third of men may avoid immediate biopsy based on mpMRI ruling out csPCa.69 However, relying on mpMRI and PSA density of 0.12 ng/mL, with a reported NPV of 91.2%, could still miss nearly 10% of csPCa.69
mpMRI Combined with TRUS in Fusion-Targeted Biopsy
mpMRI has also shown significant promise when combined with TRUS through fusion biopsy. Fusion biopsy combines mpMRI imaging with ultrasound during the biopsy procedure, enabling live visualization of suspicious lesions. The additional imaging provided by mpMRI enables targeted biopsies, which refer to intentionally sampling a suspicious lesion suspected to contain csPCa, to be conducted.
Substantial research indicates that fusion biopsies have higher rates of csPCa detection compared to standard TRUS biopsy. Siddiqui et al report that fusion-targeted biopsy increases detection of higher grade tumors by as much as 67% while reducing detection of lower grade tumors by 36% compared to traditional systematic sampling.70 Son et al report that fusion-targeted biopsy detects 3 times more cancer (21% vs 7%) than systematic biopsy without fusion guidance.71 Borkowetz et al compared systematic TRUS biopsy with transperineal fusion biopsy and found fusion biopsy has a significantly higher overall cancer detection rate (44% vs 35%) and detected 44% more csPCa than systematic biopsy alone.72
Furthermore, a study assessing biopsy-naïve men from Japan found that fusion-targeted biopsy detection rates for csPCa and insignificant PCa were 43.5% and 17.6%, respectively, compared to 35.9% and 25.2% for systematic biopsy.73 In addition, TRUS fusion biopsy has been associated with a significantly lower rate of disease state upgrading during subsequent radical prostatectomy compared to patients who received standard biopsy (1.8% vs 38.8%, respectively).74
Fusion biopsy may also play a key role in cancer detection among specific populations of patients. For example, literature has shown that fusion-targeted biopsies improve detection of csPCa in enlarged prostates, with detection rates ranging between 57.5% and 30.4% for prostates between 40cc and 115 cc or greater, whereas typical TRUS-guided detection rates are usually 30% or less.75 Additionally, a study comparing fusion-targeted biopsy and systematic biopsy in patients with a previous negative biopsy and patients under active surveillance (AS) found that fusion-targeted biopsy detected a significantly higher rate of csPCa than systematic biopsy among patients with a previous negative biopsy (41.3% vs 27%, p=0.038) but report no significant difference in overall PCa (50% vs 73.1%) or csPCa (30.8% vs 26.9%, p=0.705) detection for patients under AS.76
mpMRI Use in Robotic-Assisted Fusion Biopsy
mpMRI also serves as a vital component of technologically advanced robotic-assisted biopsy systems. These systems utilize mpMRI and TRUS fusion biopsies to provide real-time imaging of the prostate while using robotic assistance to help guide and perform the biopsy. The use of fusion biopsy with robotic assistance enables accurate needle placement, precise techniques targeting lesions detected on MRI,77 and can provide wide coverage of the prostate while reducing the number of necessary entry points and deformation of the prostate due to the ultrasound probe.78 Importantly, robotic-assisted prostate biopsy is not as widely used as traditional techniques and evidence of its clinical efficacy is limited. Still, numerous studies have indicated that robotic-assisted prostatectomy can be both effective and safe for patients. Wetterauer et al, for example, assessed 118 patients who received robotic-assisted transperineal prostate biopsy and found a csPCa detection rate of 78.3% when saturation biopsy was performed.79 Additionally, Vilanova et al assessed 30 patients with cancer suspicious lesions who underwent robotic remote controlled transrectal prostate biopsy and report an overall cancer detection rate of 73%, a csPCa detection rate of 86% and only a single complication of rectal bleeding.80
Despite these advantages, limited availability of advanced robotic systems, learning curves for users, frequent reliance on general anesthesia, and potential cost compared to effective traditional procedures will likely prevent robotic-assisted biopsy from becoming a standard of care in the near future. However, robotic assistance may currently play a useful role in complicated situations like rebiopsy or for targeting lesions in difficult to reach locations and it’s utilization is likely to grow.
Combination Biopsy vs mpMRI-Targeted or Systematic Biopsy
A substantial body of literature has also shown that mpMRI-targeted biopsy, when combined with traditional systematic biopsy, is superior to either targeted or systematic biopsy alone.81 Areas identified with PI-RADS scores of 3, 4 and 5 on MRI are of particular interest, as studies have reported csPCa detection rates as high as 29.7%, 42.3%, and 82.4% in these regions, respectively.82 Numerous studies have indicated that MRI-guided biopsies can detect higher rates of csPCa and lower rates of clinically insignificant PCa compared to standard systematic TRUS biopsies.83 Kasivisvanathan et al in the PRECISION trial report detection rates of 38% of clinically significant prostate cancer in the MRI-targeted biopsy group compared to 26% in the standard biopsy group, but claim MRI with or without biopsy is noninferior to standard biopsy.84 In addition, the rate of clinically insignificant prostate cancer in the mpMRI group was 9% compared to the rate of 22% in the standard biopsy group.84 When broadly assessing diagnostic accuracy of either csPCa or insignificant PCa, Drost et al found that the MRI pathway is 44% more likely to make the correct diagnosis among men with a previously negative biopsy, 12% more likely to make the correct diagnosis among men who are either biopsy naive or have had a previous negative biopsy, and 5% more likely among men who are biopsy naïve compared to TRUS systematic sampling.85 Klotz et al report a 30% detection rate of cancer Gleason Grade 2 or higher for patients undergoing TRUS biopsy compared to a 35% detection rate for those who received mpMRI-targeted biopsy.86 Concurrently, grade 1 cancer detection was reduced from 22% in the TRUS group to 10% in the MRI group.86 Van der Leest et al. report identical detection rates of csPCa between TRUS- and MRI-guided biopsy, but a substantial decrease in insignificant PCa detection rate for MRI pathway biopsy compared to TRUS-guided biopsy (14% and 25%, respectively).87 Siddiqui et al found that targeted biopsy diagnosed 30% more high-risk cancers and 17% less low-risk cancers when compared to standard biopsy.88
Rouviere et al report similar detection rates for standard systematic (29.9%) and mpMRI-targeted biopsy (32.3%), but higher efficacy if the techniques are combined.89 Using both techniques in conjunction detected an additional 5.2% of csPCa over targeted biopsy alone and 7.6% over systematic biopsy alone.89 Ahdoot et al report combined biopsy led to 10% higher PCa diagnosis rate than either systematic or targeted biopsy alone and detected higher grade cancer than previously identified in 21.8% of the patients.90 A study by Oderda et al found using a combined biopsy technique increased overall cancer detection rate by 15% and the detection of csPCa by 12% over targeted biopsy alone.91 Research by Elkhoury et al further support these data, reporting a 23% increase in cancer detection over targeted biopsy alone and a 10% increase over systematic alone when using a combined technique.92 Fourcade et al report similar findings, suggesting combined biopsy increases PCa detection rate by 33% and 11.5% over targeted biopsy and systematic biopsy, respectively.93
Other literature suggests that that rates of missing clinically significant cancers can increase by as much as 10% when using exclusively systematic and 13% when using exclusively targeted biopsies.94 Utilizing mpMRI-targeted and systematic biopsies in conjunction has also been shown to provide sufficient information to increase the accuracy of predicting patients at high risk for adverse pathology should they undergo radical prostatectomy.95 Furthermore, research suggests that targeted biopsy is associated with lower rates of complications such as hematuria than standard systematic biopsy, but the number of cores must also be considered as an additional factor that may vary between the techniques.96
Despite the evidence favoring a combination approach over either mpMRI-targeted or systematic biopsy, some research indicates mpMRI alone may be sufficient. A 2019 meta-analysis analyzing 29 studies determined that mpMRI-targeted biopsy, when compared to systematic biopsy alone, demonstrated a 15% higher detection rate of all PCa, with a higher detection rate of csPCa and no significant difference in the detection of non-clinically significant PCa.97 Moreover, the study concluded that excluding systematic biopsy from mpMRI-targeted biopsy reduced the detection rate of insignificant PCa without influencing the detection rate of csPCa.97
mpMRI Drawbacks
Despite the numerous advantages of utilizing mpMRI in PCa risk stratification and diagnosis, there are some potential drawbacks to consider. Studies have indicated that, while MRI can provide accurate mapping of lesions, it consistently underestimates the size of cancerous lesions by an average of 11 millimeters in length and 3 times in volume.83 This disparity can affect treatment decisions and dissuade the decision to only biopsy MRI identified lesions. When compared to TRUS, MRI imaging also lacks real-time perspective meaning the view is provided via a 3D scan rather than showing what is happening. Additionally, discrepancies in the experience of the physician interpreting the MRI findings and potential learning curve for individuals adapting to MRI-guided biopsies create a risk of inconsistency between physicians. Furthermore, lesions identified as a PI-RADS score of 3, indicating equivocal chance of cancer, pose significant clinical management challenges. MRI involving sedation is also significantly more expensive than traditional methods, with MRI fusion biopsy and In-bore MRI costing 150% and 125% more than standard TRUS biopsy, respectively.44 However, the growing use of local anesthesia for MRI-guided biopsies is helping to attenuate these disparities.
Comparison of Micro-Ultrasound to Standard Ultrasound Used in Traditional TRUS
Micro-ultrasound (mUS) is another rapidly advancing technique garnering substantial enthusiasm from the urologic community. Compared to standard ultrasound, which uses sound waves in the range of 2–12-megahertz, micro-ultrasound imaging devices like the ExactVu Micro-Ultrasound Platform (Exact Imaging, Markham, Ontario) utilize frequencies as high as 29 megahertz and provide a reported 300% improvement in image resolution.98 mUS imaging is standardized using the Prostate Risk Identification Using Micro-Ultrasound (PRI-MUS) system, which uses a 5-point scale to assess the likely degree of disease in prostate tissue. Similar to the PI-RADS system for MRI, higher scores in the PRI-MUS system suggest a more severe disease. Research has shown that each increase in PRI-MUS score is correlated with a 10.1% increase in the probability of csPCa presence.99 The PRI-MUS scores obtained through mUS help guide risk stratification, biopsy technique, and the patient’s course of treatment. Due to the recency of mUS integration as a novel technique for prostate imaging, the body of literature describing its effectiveness is limited. However, studies indicate significant potential for mUS as a diagnostic tool for prostate cancer.
Given the long history of standard TRUS use for prostate diagnosis, many physicians would likely appreciate the familiarity they had experienced using mUS. mUS provides a convenient and cost-effective technique which is effective at diagnosing csPCa and provides imaging in real time.100 Some literature suggests the visualizing capability of mUS, which can distinguish ductal anatomy and cellular density at a resolution as fine as 70 micrometers, make it comparable or perhaps even superior to mpMRI at diagnosing csPca.101 The clinical effectiveness of micro-ultrasound is summarized in Table 3. Research comparing mUS and mpMRI for prostate cancer diagnosis found mUS exhibited superior sensitivity (94% vs 90%), a stronger NPV (85% vs 77%), identical specificity (22% for both) and similar PPV (44% for mUS and 43% for mpMRI).102 Other papers have concluded that the reported sensitivity (89.7%), NPV (81.5%), specificity (26.0%), and PPV (40.8%) place it’s detection rate, when combined with randomized biopsies, on par with the detection rate of MRI-targeted biopsies.103 While larger studies should be conducted to further substantiate these findings, the prospects for higher detection rates while maintaining the cost-effectiveness and ease of traditional ultrasound102,104 make mUS an extremely promising technique. It has also been reported that mUS could exceed standard TRUS performance when combined with MRI imaging or in fusion biopsy, as studies have shown detection of higher-grade cancers in mUS-targeted biopsies (26%) than nontargeted and mpMRI-targeted biopsies (16%).105 Broadly speaking, mUS has shown potential for enhanced risk stratification and monitoring in a convenient and cost-effective manner.106
 |
Table 3 Summary of Studies from the Last 5 Years Assessing Clinical Effectiveness of Micro-Ultrasound for Prostate Cancer Detection |
TRUS for Focal Therapy of Prostate Cancer
Focal therapy of prostate cancer is still an investigational modality being studied with various available techniques. Although controversial and not currently considered a standard of care, the hypothetical advantages of focal therapy compared to the definitive treatment of prostate cancer, considered standard of care, are quite numerous. The standard definitive treatment of csPCa to date includes radical prostatectomy or radiotherapy with or without the addition of androgen deprivation therapy.107 Although these treatments have been shown to be oncologically effective for localized prostate cancer,108–112 they do entail significant potential adverse effects, including bleeding, intestinal and rectal injury, infection, incontinence, erectile dysfunction and others.113,114
Focal therapy refers to destroying a specific part of tissue while sparing the rest of the gland. Rather than targeting the entire gland, focal therapy concentrates on treating “the index lesion,” which is defined as the dominant tumor visible on MRI.115 Treating the index lesions helps to attack the most prominent locus of tumor growth, which can disrupt tumor growth, cellular proliferation, and cancer progression.116 Ideally, focal therapy will avoid the many associated adverse effects of the standard definitive radical treatments for prostate cancer, while still treating the cancer effectively, as it only potentially targets the areas with cancer within the gland.116 Common focal ablation techniques that utilize extreme temperature to ablate tissue include cryotherapy, photodynamic therapy, and laser ablation.
Another commonly used focal ablation modality is high-intensity focused ultrasound (HIFU), which utilizes high-frequency sound waves to heat and kill tumors or suspected cancerous tissue through a process known as ablation. In HIFU therapy, the TRUS probe is used both for imaging of the prostate and as the source of the ultrasound waves used during ablation. Rosette et al established a consensus on criteria for patients eligible to receive HIFU, including patients with low to intermediate risk disease, and specific parameters for prostate size and tumor volume.117 Although still limited, there are some early indications to suggest benefit in treating selected prostate cancer patients with this modality, if they meet the appropriate criteria.118 Table 4 displays cancer control rates and morbidities associated with focal therapy. Studies report failure free survival rates at 99%, 92%, and 88% at 1 year, 3 years, and 5 years, respectively, with a 99% overall survival rate 5 years following HIFU treatment in appropriately selected patients.119
 |
Table 4 Summary of Studies Assessing Clinical Outcomes Following HIFU Focal Therapy for Prostate Cancer |
While the limited supply of long-term follow-ups for HIFU therapy make a direct comparison to traditional treatments difficult, these findings show promise and should be further investigated for the appropriately selected patients. The precise nature of HIFU focal therapy and avoidance of damage to surrounding tissue could also make it effective in repeated treatments,120 potentially avoiding or delaying the need for more invasive procedures. Additionally, literature has shown that HIFU can be utilized as an effective form of salvage therapy for local relapses following other forms of treatment.121
In addition to promising oncological control, numerous studies have reported low morbidity following the HIFU therapy. Rischmann et al report preservation of continence in 97% of the patients and erectile function in 78% of the patients 12 months following HIFU hemiablation with no significant decrease in quality of life.122 Checcucci et al also report limited complications among 20 patients who received HIFU, with 0 Clavien-Dindo grade 3 complications reported, 8 patients experiencing urgency at 3-month follow-up, and 4 cases of urinary tract infection while noting no significant decline in sexual function or quality of life.123
However, HIFU therapy has been associated with other adverse effects, such as urethral stricture and hematuria.124
Importantly, there are several caveats when using HIFU. These include lack of treatment of all cancerous lesions, inadequate ablation of anterior tumors,125 lack of standardization of how to follow these patients,126 and to-date, lacking long-term follow-up data.127
Future Directions
Standard TRUS in PCa diagnosis and biopsy may be upgraded with the use of mUS in the future. The ease of use, cost-effectiveness, and high resolution, combined with detection capabilities comparable or potentially superior to mpMRI, give mUS the potential to become a standard of care in PCa diagnosis and biopsy. The use of mpMRI, and the adoption of focal treatment through HIFU, will likely continue to grow in usage as well, providing physicians more tools to aid PCa diagnosis and management.
Conclusions
Standard TRUS is still an effective tool in measuring and assessing prostate volume and anatomy and is essential in guiding various prostate biopsy techniques including transrectal and transperineal approaches. The transrectal and transperineal biopsy techniques have similar cancer detection rates but different complication panels with transrectal biopsy being associated with higher rates of infection and sepsis while transperineal biopsy is associated with higher rates of urinary retention. TRUS is also an essential component of mpMRI fusion biopsies, which allow targeting of suspicious lesions and have been shown to offer greater cancer detection rates than systematic TRUS-guided biopsy alone. Micro-ultrasound offers higher resolution imaging compared to traditional TRUS and although data on its efficacy is limited, this modality shows tremendous potential in cancer detection and guiding biopsies. Finally, HIFU focal therapy administered via TRUS has been shown both safe and effective for short-term control of localized cancer with low morbidity for patients and particularly high potential in repeat or salvage therapies.
Summary
The use of transrectal ultrasound in prostatic disease and prostate cancer will most likely not dissipate anytime soon, due to familiarity among physicians, ease of use, and low cost. However, other techniques may demonstrate superiority and should be further refined and studied.
Abbreviations
PCa, prostate cancer; PSA, prostate-specific antigen; TRUS, transrectal ultrasound; MRI, magnetic resonance imaging; csPCa, clinically significant prostate cancer; mpMRI, multiparametric magnetic resonance imaging; mUS, micro-ultrasound; HIFU, high-intensity focused ultrasound; NPV, negative predictive value; PPV, positive predictive value; AS, active surveillance; NCCN, National Comprehensive Cancer Network.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Dr Gennady Bratslavsky owns stock from Taurus Diagnostics and is a consultant for Merck and Johnson & Johnson, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. American Cancer Society. Key statistics for prostate cancer; 2021. Available from: https://www.cancer.org/cancer/prostate-cancer/about/key-statistics.html.
2. Cancer.Net. Prostate cancer: statistics; 2021. Available from: https://www.cancer.net/cancer-types/prostate-cancer/statistics.
3. Catalona WJ. Prostate cancer screening. Med Clin North Am. 2018;102(2):199–214. doi:10.1016/j.mcna.2017.11.001
4. Grabstald H, Elliott JL. Transrectal biopsy of the prostate. J Am Med Assoc. 1953;153(6):563–565. doi:10.1001/jama.1953.02940230035006k
5. Cooner WH, Mosley BR, Rutherford CL, et al. Clinical application of transrectal ultrasonography and prostate specific antigen in the search for prostate cancer. J Urol. 1988;139(4):758–761. doi:10.1016/s0022-5347(17)42624-3
6. Lee F, Torp-Pedersen ST, Siders DB. The role of transrectal ultrasound in the early detection of prostate cancer. CA Cancer J Clin. 1989;39(6):337–360. doi:10.3322/canjclin.39.6.337
7. Cancer Research UK. Transrectal ultrasound guided biopsy; 2019. Available from: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/getting-diagnosed/tests/transrectal-ultrasound-guided-trus-biopsy.
8. Li M, Wang Z, Li H, et al. Local anesthesia for transrectal ultrasound-guided biopsy of the prostate: a meta-analysis. Sci Rep. 2017;7:40421. doi:10.1038/srep40421
9. Cookson MS. Update on transrectal ultrasound-guided needle biopsy of the prostate. Mol Urol. 2000;4(3):
10. Philip J, Ragavan N, Desouza J, Foster CS, Javle P. Effect of peripheral biopsies in maximising early prostate cancer detection in 8-, 10- or 12-core biopsy regimens. BJU Int. 2004;93(9):1218–1220. doi:10.1111/j.1464-410X.2004.04857.x
11. Stamatiou K, Alevizos A, Karanasiou V, et al. Impact of additional sampling in the TRUS-guided biopsy for the diagnosis of prostate cancer. Urol Int. 2007;78(4):313–317. doi:10.1159/000100834
12. Eskicorapci SY, Baydar DE, Akbal C, et al. An extended 10-core transrectal ultrasonography guided prostate biopsy protocol improves the detection of prostate cancer. Eur Urol. 2004;45(4):
13. Guichard G, Larre S, Gallina A, et al. Extended 21-sample needle biopsy protocol for diagnosis of prostate cancer in 1000 consecutive patients. Eur Urol. 2007;52(2):430–435. doi:10.1016/j.eururo.2007.02.062
14. Long JA, Daanen V, Moreau-Gaudry A, Troccaz J, Rambeaud JJ, Descotes JL. Prostate biopsies guided by three-dimensional real-time (4-D) transrectal ultrasonography on a phantom: comparative study versus two-dimensional transrectal ultrasound-guided biopsies. Eur Urol. 2007;52(4):1097–1104. doi:10.1016/j.eururo.2006.11.034
15. Benchikh El Fegoun A, El Atat R, Choudat L, et al. The learning curve of transrectal ultrasound-guided prostate biopsies: implications for training programs. Urology. 2013;81(1):12–15. doi:10.1016/j.urology.2012.06.084
16. Valerio M, Anele C, Bott SRJ, et al. The prevalence of clinically significant prostate cancer according to commonly used histological thresholds in men undergoing template prostate mapping biopsies. J Urol. 2016;195(5):1403–1408. doi:10.1016/j.juro.2015.11.047
17. Han M, Chang D, Kim C, et al. Geometric evaluation of systematic transrectal ultrasound guided prostate biopsy. J Urol. 2012;188(6):2404–2409. doi:10.1016/j.juro.2012.07.107
18. Zhang Z, Lampotang S, Yu Y, et al. Attitude is everything: keep probe pitch neutral during side-fire prostate biopsy. A simulator study. BJU Int. 2021;128:615–624. doi:10.1111/bju.15445
19. Liss MA, Ehdaie B, Loeb S, et al. An update of the American Urological Association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol. 2017;198(2):329–334. doi:10.1016/j.juro.2017.01.103
20. Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183(3):963–968. doi:10.1016/j.juro.2009.11.043
21. Gross MD, Alshak MN, Shoag JE, et al. Healthcare costs of post-prostate biopsy sepsis. Urology. 2019;133:11–15. doi:10.1016/j.urology.2019.06.011
22. Lee JE, Shin SS, Kang TW, Kim JW, Heo SH, Jeong YY. Comparison of different rectal cleansing methods for reducing post-procedural infectious complications after transrectal ultrasound-guided prostate biopsy. Urol J. 2020;17(1):36–41. doi:10.22037/uj.v0i0.4583
23. Holmes M, Littler R, Lyons M, et al. MP11-13 risk factor assessment for fluoroquinolone resistant E. coli (FRE) in bowel flora is not sufficiently discriminatory: the case for a pre-biopsy rectal swab in all patients. J Urol. 2017;197(4S):e141. doi:10.1016/j.juro.2017.02.414
24. Djavan B, Waldert M, Zlotta A, et al. Safety and morbidity of first and repeat transrectal ultrasound guided prostate needle biopsies: results of a prospective European prostate cancer detection study. J Urol. 2001;166(3):856–860. doi:10.1016/S0022-5347(05)65851-X
25. Bauer KM. [Transperineal trial excision of prostate (prostate biopsy)]. Die transperineale Probeexzision der Vorsteherdruse (Prostatabiopsie). Medizinische. 1955;1955(33–34):1129–1131.
26. Xiang J, Yan H, Li J, Wang X, Chen H, Zheng X. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):31. doi:10.1186/s12957-019-1573-0
27. Shen PF, Zhu YC, Wei WR, et al. The results of transperineal versus transrectal prostate biopsy: a systematic review and meta-analysis. Asian J Androl. 2012;14(2):310–315. doi:10.1038/aja.2011.130
28. Huang GL, Kang CH, Lee WC, Chiang PH. Comparisons of cancer detection rate and complications between transrectal and transperineal prostate biopsy approaches - a single center preliminary study. BMC Urol. 2019;19(1):101. doi:10.1186/s12894-019-0539-4
29. Takenaka A, Hara R, Ishimura T, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis. 2008;11(2):134–138. doi:10.1038/sj.pcan.4500985
30. Cerruto MA, Vianello F, D’Elia C, Artibani W, Novella G. Transrectal versus transperineal 14-core prostate biopsy in detection of prostate cancer: a comparative evaluation at the same institution. Arch Ital Urol Androl. 2014;86(4):284–287. doi:10.4081/aiua.2014.4.284
31. Guo LH, Wu R, Xu HX, et al. Comparison between ultrasound guided transperineal and transrectal prostate biopsy: a prospective, randomized, and controlled trial. Sci Rep. 2015;5:16089. doi:10.1038/srep16089
32. Cowan T, Baker E, McCray G, Reeves F, Houlihan K, Johns-Putra L. Detection of clinically significant cancer in the anterior prostate by transperineal biopsy. BJU Int. 2020;126(Suppl 1):33–37. doi:10.1111/bju.15124
33. Stefanova V, Buckley R, Flax S, et al. Transperineal prostate biopsies using local anesthesia: experience with 1287 patients. Prostate cancer detection rate, complications and patient tolerability. J Urol. 2019;201(6):1121–1126. doi:10.1097/ju.0000000000000156
34. Abdelsayed GA, Danial T, Kaswick JA, Finley DS. Tumors of the anterior prostate: implications for diagnosis and treatment. Urology. 2015;85(6):1224–1228. doi:10.1016/j.urology.2014.12.035
35. Mygatt J, Sesterhenn I, Rosner I, et al. Anterior tumors of the prostate: clinicopathological features and outcomes. Prostate Cancer Prostatic Dis. 2014;17(1):75–80. doi:10.1038/pcan.2013.54
36. Berry B, Parry MG, Sujenthiran A, et al. Comparison of complications after transrectal and transperineal prostate biopsy: a national population-based study. BJU Int. 2020;126(1):97–103. doi:10.1111/bju.15039
37. Skouteris VM, Crawford ED, Mouraviev V, et al. Transrectal ultrasound-guided versus transperineal mapping prostate biopsy: complication comparison. Rev Urol. 2018;20(1):19–25. doi:10.3909/riu0785
38. Symons JL, Huo A, Yuen CL, et al. Outcomes of transperineal template-guided prostate biopsy in 409 patients. BJU Int. 2013;112(5):585–593. doi:10.1111/j.1464-410X.2012.11657.x
39. Schaufler C, Daigle R, Singhaviranon S, Gjertson CK, Albertsen PC, Ristau BT. How many cores are enough? Optimizing the transperineal prostate biopsy template. Urol Oncol. 2022. doi:10.1016/j.urolonc.2021.11.026
40. United States Food and Drug Administration. Approval of the PrecisionPoint transperineal access system. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K160414.pdf.
41. Zimmerman ME, Meyer AR, Carter HB, Allaf ME, Gorin MA. In-office transperineal prostate biopsy using biplanar ultrasound guidance: a step-by-step guide. Urology. 2019;133:247. doi:10.1016/j.urology.2019.07.021
42. Wetterauer C, Shahin O, Federer-Gsponer JR, et al. Feasibility of freehand MRI/US cognitive fusion transperineal biopsy of the prostate in local anaesthesia as in-office procedure-experience with 400 patients. Prostate Cancer Prostatic Dis. 2020;23(3):429–434. doi:10.1038/s41391-019-0201-y
43. Meyer AR, Joice GA, Schwen ZR, Partin AW, Allaf ME, Gorin MA. Initial experience performing in-office ultrasound-guided transperineal prostate biopsy under local anesthesia using the precisionpoint transperineal access system. Urology. 2018;115:8–13. doi:10.1016/j.urology.2018.01.021
44. Altok M, Kim B, Patel BB, et al. Cost and efficacy comparison of five prostate biopsy modalities: a platform for integrating cost into novel-platform comparative research. Prostate Cancer Prostatic Dis. 2018;21(4):524–532. doi:10.1038/s41391-018-0056-7
45. Hara R, Jo Y, Fujii T, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71(2):191–195. doi:10.1016/j.urology.2007.09.029
46. European Association of Urology. Prostate cancer. Available from: https://uroweb.org/guideline/prostate-cancer/.
47. American Urological Associaiton. Optimal techniques of prostate biopsy and specimen handling. Available from: https://www.auanet.org/guidelines/guidelines/prostate-biopsy-and-specimen-handling.
48. Martins T, Mussi TC, Baroni RH. Prostate volume measurement by multiparametric magnetic resonance and transrectal ultrasound: comparison with surgical specimen weight. Einstein (Sao Paulo). 2020;18:eAO4662. doi:10.31744/einstein_journal/2020AO4662
49. Roehrborn CG, Girman CJ, Rhodes T, et al. Correlation between prostate size estimated by digital rectal examination and measured by transrectal ultrasound. Urology. 1997;49(4):548–557. doi:10.1016/s0090-4295(97)00031-9
50. Nickel JC. Benign prostatic hyperplasia: does prostate size matter? Rev Urol. 2003;5(Suppl 4):S12–7.
51. Christie DRH, Sharpley CF. How accurately can prostate gland imaging measure the prostate gland volume? Results of a systematic review. Prostate Cancer. 2019;2019:6932572. doi:10.1155/2019/6932572
52. Lee JS, Chung BH. Transrectal ultrasound versus magnetic resonance imaging in the estimation of prostate volume as compared with radical prostatectomy specimens. Urol Int. 2007;78(4):323–327. doi:10.1159/000100836
53. Lerner LB, McVary KT, Barry MJ, et al. Management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA GUIDELINE PART I-initial work-up and medical management. J Urol. 2021;206(4):806–817. doi:10.1097/ju.0000000000002183
54. Macura KJ. Multiparametric magnetic resonance imaging of the prostate: current status in prostate cancer detection, localization, and staging. Semin Roentgenol. 2008;43(4):303–313. doi:10.1053/j.ro.2008.06.002
55. Kurhanewicz J, Vigneron D, Carroll P, Coakley F. Multiparametric magnetic resonance imaging in prostate cancer: present and future. Curr Opin Urol. 2008;18(1):71–77. doi:10.1097/MOU.0b013e3282f19d01
56. Jacobs MA, Ouwerkerk R, Petrowski K, Macura KJ. Diffusion-weighted imaging with apparent diffusion coefficient mapping and spectroscopy in prostate cancer. Top Magn Reson Imaging. 2008;19(6):261–272. doi:10.1097/RMR.0b013e3181aa6b50
57. Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016;69(1):16–40. doi:10.1016/j.eururo.2015.08.052
58. Wallis CJD, Haider MA, Nam RK. Role of mpMRI of the prostate in screening for prostate cancer. Transl Androl Urol. 2017;6(3):464–471. doi:10.21037/tau.2017.04.31
59. An JY, Sidana A, Holzman SA, et al. Ruling out clinically significant prostate cancer with negative multi-parametric MRI. Int Urol Nephrol. 2018;50(1):7–12. doi:10.1007/s11255-017-1715-7
60. Bjurlin MA, Carroll PR, Eggener S, et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J Urol. 2020;203(4):706–712. doi:10.1097/ju.0000000000000617
61. European Association of Urology. Prostate Cancer. Available from: https://uroweb.org/guideline/prostate-cancer/?type=summary-of-changes.
62. Oishi M, Shin T, Ohe C, et al. Which patients with negative magnetic resonance imaging can safely avoid biopsy for prostate cancer? J Urol. 2019;201(2):268–276. doi:10.1016/j.juro.2018.08.046
63. Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol. 2017;72(2):250–266. doi:10.1016/j.eururo.2017.02.026
64. Ryoo H, Kang MY, Sung HH, et al. Detection of prostate cancer using prostate imaging reporting and data system score and prostate-specific antigen density in biopsy-naive and prior biopsy-negative patients. Prostate Int. 2020;8(3):125–129. doi:10.1016/j.prnil.2020.03.003
65. Kinnaird A, Sharma V, Chuang R, et al. Risk of prostate cancer after a negative magnetic resonance imaging guided biopsy. J Urol. 2020;204(6):1180–1186. doi:10.1097/JU.0000000000001232
66. American Urological Associaiton. Prostate MRI and MRI-targeted biopsy in patients with prior negative biopsy. Available from: https://www.auanet.org/guidelines/guidelines/prostate-mri-and-mri-targeted-biopsy.
67. Wang NN, Teslovich NC, Fan RE, et al. Applying the PRECISION approach in biopsy naive and previously negative prostate biopsy patients. Urol Oncol. 2019;37(8):530e19–530 e24. doi:10.1016/j.urolonc.2019.05.002
68. Salami SS, Ben-Levi E, Yaskiv O, et al. In patients with a previous negative prostate biopsy and a suspicious lesion on magnetic resonance imaging, is a 12-core biopsy still necessary in addition to a targeted biopsy? BJU Int. 2015;115(4):562–570. doi:10.1111/bju.12938
69. Sklinda K, Mruk B, Walecki J. Active surveillance of prostate cancer using multiparametric magnetic resonance imaging: a review of the current role and future perspectives. Med Sci Monit. 2020;26:e920252. doi:10.12659/msm.920252
70. Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64(5):713–719. doi:10.1016/j.eururo.2013.05.059
71. Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189(1):86–91. doi:10.1016/j.juro.2012.08.095
72. Borkowetz A, Platzek I, Toma M, et al. Comparison of systematic transrectal biopsy to transperineal magnetic resonance imaging/ultrasound-fusion biopsy for the diagnosis of prostate cancer. BJU Int. 2015;116(6):873–879. doi:10.1111/bju.13023
73. Fujii S, Hayashi T, Honda Y, et al. Magnetic resonance imaging/transrectal ultrasonography fusion targeted prostate biopsy finds more significant prostate cancer in biopsy-naive Japanese men compared with the standard biopsy. Int J Urol. 2020;27(2):140–146. doi:10.1111/iju.14149
74. De Luca S, Fiori C, Bollito E, et al. Risk of Gleason Score 3+4=7 prostate cancer upgrading at radical prostatectomy is significantly reduced by targeted versus standard biopsy. Minerva Urol Nefrol. 2020;72(3):360–368. doi:10.23736/s0393-2249.19.03367-8
75. Walton Diaz A, Hoang AN, Turkbey B, et al. Can magnetic resonance-ultrasound fusion biopsy improve cancer detection in enlarged prostates? J Urol. 2013;190(6):2020–2025. doi:10.1016/j.juro.2013.05.118
76. Borghesi M, Bianchi L, Barbaresi U, et al. Diagnostic performance of MRI/TRUS fusion-guided biopsies vs. systematic prostate biopsies in biopsy-naïve, previous negative biopsy patients and men undergoing active surveillance. Minerva Urol Nephrol. 2021;73(3):357–366. doi:10.23736/s2724-6051.20.03758-3
77. Franklin A, Gianduzzo T, Yaxley J, et al. Use of a trizonal schema to assess targeting accuracy in prostatic fusion biopsy. BJU Int. 2020;126(Suppl 1):6–11. doi:10.1111/bju.14974
78. Lim S, Jun C, Chang D, Petrisor D, Han M, Stoianovici D. Robotic transrectal ultrasound guided prostate biopsy. IEEE Trans Biomed Eng. 2019;66(9):2527–2537. doi:10.1109/tbme.2019.2891240
79. Wetterauer C, Trotsenko P, Matthias MO, et al. Diagnostic accuracy and clinical implications of robotic assisted MRI-US fusion guided target saturation biopsy of the prostate. Sci Rep. 2021;11(1):20250. doi:10.1038/s41598-021-99854-0
80. Vilanova JC, Pérez de Tudela A, Puig J, et al. Robotic-assisted transrectal MRI-guided biopsy. Technical feasibility and role in the current diagnosis of prostate cancer: an initial single-center experience. Abdom Radiol (NY). 2020;45(12):4150–4159. doi:10.1007/s00261-020-02665-6
81. Dell’Oglio P, Stabile A, Soligo M, et al. There is no way to avoid systematic prostate biopsies in addition to multiparametric magnetic resonance imaging targeted biopsies. Eur Urol Oncol. 2020;3(1):112–118. doi:10.1016/j.euo.2019.03.002
82. Sathianathen NJ, Konety BR, Soubra A, et al. Which scores need a core? An evaluation of MR-targeted biopsy yield by PIRADS score across different biopsy indications. Prostate Cancer Prostatic Dis. 2018;21(4):573–578. doi:10.1038/s41391-018-0065-6
83. Priester A, Natarajan S, Khoshnoodi P, et al. Magnetic resonance imaging underestimation of prostate cancer geometry: use of patient specific molds to correlate images with whole mount pathology. J Urol. 2017;197(2):320–326. doi:10.1016/j.juro.2016.07.084
84. Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777. doi:10.1056/NEJMoa1801993
85. Drost FH, Osses DF, Nieboer D, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4(4):Cd012663. doi:10.1002/14651858.CD012663.pub2
86. Klotz L, Chin J, Black PC, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):534–542. doi:10.1001/jamaoncol.2020.7589
87. van der Leest M, Cornel E, Israel B, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: a large prospective multicenter clinical study. Eur Urol. 2019;75(4):570–578. doi:10.1016/j.eururo.2018.11.023
88. Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–397. doi:10.1001/jama.2014.17942
89. Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20(1):100–109. doi:10.1016/s1470-2045(18)30569-2
90. Ahdoot M, Wilbur AR, Reese SE, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med. 2020;382(10):917–928. doi:10.1056/NEJMoa1910038
91. Oderda M, Marra G, Albisinni S, et al. Elastic fusion biopsy versus systematic biopsy for prostate cancer detection: results of a multicentric study on 1119 patients. Evaluacion de la biopsia de fusion elastica vs. biopsia sistematica para la deteccion del cancer de prostata: resultados de un estudio multicentrico en 1.119 pacientes. Actas Urol Esp (Engl Ed). 2019;43(8):431–438. doi:10.1016/j.acuro.2019.01.009
92. Elkhoury FF, Felker ER, Kwan L, et al. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: the Prospective Assessment of Image Registration in the Diagnosis of Prostate Cancer (PAIREDCAP) study. JAMA Surg. 2019;154(9):811–818. doi:10.1001/jamasurg.2019.1734
93. Fourcade A, Payrard C, Tissot V, et al. The combination of targeted and systematic prostate biopsies is the best protocol for the detection of clinically significant prostate cancer. Scand J Urol. 2018;52(3):174–179. doi:10.1080/21681805.2018.1438509
94. Mannaerts CK, Kajtazovic A, Lodeizen OAP, et al. The added value of systematic biopsy in men with suspicion of prostate cancer undergoing multiparametric MRI-targeted biopsy. Urol Oncol. 2019;37(5):298e1–298 e9. doi:10.1016/j.urolonc.2019.01.005
95. Gandaglia G, Ploussard G, Valerio M, et al. The key combined value of multiparametric magnetic resonance imaging, and magnetic resonance imaging-targeted and concomitant systematic biopsies for the prediction of adverse pathological features in prostate cancer patients undergoing radical prostatectomy. Eur Urol. 2020;77(6):733–741. doi:10.1016/j.eururo.2019.09.005
96. Borghesi M, Ahmed H, Nam R, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71(3):353–365. doi:10.1016/j.eururo.2016.08.004
97. Goldberg H, Ahmad AE, Chandrasekar T, et al. Comparison of magnetic resonance imaging and transrectal ultrasound informed prostate biopsy for prostate cancer diagnosis in biopsy naive men: a systematic review and meta-analysis. J Urol. 2020;203(6):1085–1093. doi:10.1097/JU.0000000000000595
98. Exact Imaging. The practical Solution. Available from: https://www.exactimaging.com.
99. Ghai S, Eure G, Fradet V, et al. Assessing cancer risk on novel 29 MHz micro-ultrasound images of the prostate: creation of the micro-ultrasound protocol for prostate risk identification. J Urol. 2016;196(2):562–569. doi:10.1016/j.juro.2015.12.093
100. Zhang M, Wang R, Wu Y, et al. Micro-ultrasound imaging for accuracy of diagnosis in clinically significant prostate cancer: a meta-analysis. Front Oncol. 2019;9:1368. doi:10.3389/fonc.2019.01368
101. Laurence Klotz CM. Can high resolution micro-ultrasound replace MRI in the diagnosis of prostate cancer? Eur Urol Focus. 2020;6(2):419–423. doi:10.1016/j.euf.2019.11.006
102. Klotz L, Lughezzani G, Maffei D, et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: a multicenter, prospective analysis. Can Urol Assoc J. 2021;15(1):E11–E16. doi:10.5489/cuaj.6712
103. Lughezzani G, Maffei D, Saita A, et al. Diagnostic accuracy of microultrasound in patients with a suspicion of prostate cancer at magnetic resonance imaging: a single-institutional prospective study. Eur Urol Focus. 2020. doi:10.1016/j.euf.2020.09.013
104. Eure G, Fanney D, Lin J, Wodlinger B, Ghai S. Comparison of conventional transrectal ultrasound, magnetic resonance imaging, and micro-ultrasound for visualizing prostate cancer in an active surveillance population: a feasibility study. Can Urol Assoc J. 2019;13(3):E70–E77. doi:10.5489/cuaj.5361
105. Wiemer L, Hollenbach M, Heckmann R, et al. Evolution of targeted prostate biopsy by adding micro-ultrasound to the magnetic resonance imaging pathway. Eur Urol Focus. 2020;7:1292–1299. doi:10.1016/j.euf.2020.06.022
106. Rohrbach D, Wodlinger B, Wen J, Mamou J, Feleppa E. High-frequency quantitative ultrasound for imaging prostate cancer using a novel micro-ultrasound scanner. Ultrasound Med Biol. 2018;44(7):1341–1354. doi:10.1016/j.ultrasmedbio.2018.02.014
107. Groeben C, Wirth MP. Prostate cancer: basics on clinical appearance, diagnostics and treatment. Prostatakarzinom Grundzuge von Klinik, Diagnostik und Therapie. Med Monatsschr Pharm. 2017;40(5):192–201.
108. van Son MJ, Peters M, Reddy D, et al. Conventional radical versus focal treatment for localised prostate cancer: a propensity score weighted comparison of 6-year tumour control. Prostate Cancer Prostatic Dis. 2021;24:1120–1128. doi:10.1038/s41391-021-00369-6
109. Tewari A, Raman JD, Chang P, Rao S, Divine G, Menon M. Long-term survival probability in men with clinically localized prostate cancer treated either conservatively or with definitive treatment (radiotherapy or radical prostatectomy). Urology. 2006;68(6):1268–1274. doi:10.1016/j.urology.2006.08.1059
110. Albertsen PC, Hanley JA, Penson DF, Barrows G, Fine J. 13-year outcomes following treatment for clinically localized prostate cancer in a population based cohort. J Urol. 2007;177(3):932–936. doi:10.1016/j.juro.2006.10.051
111. Merglen A, Schmidlin F, Fioretta G, et al. Short- and long-term mortality with localized prostate cancer. Arch Intern Med. 2007;167(18):1944–1950. doi:10.1001/archinte.167.18.1944
112. Vernooij RW, Lancee M, Cleves A, Dahm P, Bangma CH, Aben KK. Radical prostatectomy versus deferred treatment for localised prostate cancer. Cochrane Database Syst Rev. 2020;6:CD006590. doi:10.1002/14651858.CD006590.pub3
113. Redondo C, Rozet F, Velilla G, Sánchez-Salas R, Cathelineau X. [Complications of radical prostatectomy]. Complicaciones de la prostatectomía radical. Arch Esp Urol. 2017;70(9):766–776.
114. Mirza M, Griebling TL, Kazer MW. Erectile dysfunction and urinary incontinence after prostate cancer treatment. Semin Oncol Nurs. 2011;27(4):278–289. doi:10.1016/j.soncn.2011.07.006
115. Stabile A, Moschini M, Montorsi F, Cathelineau X, Sanchez-Salas R. Focal therapy for prostate cancer - index lesion treatment vs. hemiablation. A matter of definition. Int Braz J Urol. 2019;45(5):873–876. doi:10.1590/S1677-5538.IBJU.2019.05.02
116. Tourinho-Barbosa RR, de la Rosette J, Sanchez-Salas R. Prostate cancer multifocality, the index lesion, and the microenvironment. Curr Opin Urol. 2018;28(6):499–505. doi:10.1097/MOU.0000000000000537
117. de la Rosette J, Ahmed H, Barentsz J, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24(5):775–780. doi:10.1089/end.2009.0596
118. Barkin J. High intensity focused ultrasound (HIFU). Can J Urol. 2011;18(2):5634–5643.
119. Guillaumier S, Peters M, Arya M, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol. 2018;74(4):422–429. doi:10.1016/j.eururo.2018.06.006
120. Kuru TH, van Essen J, Pfister D, Porres D. Role of focal therapy with high-intensity focused ultrasound in the management of clinically localized prostate cancer. Oncol Res Treat. 2015;38(12):634–638. doi:10.1159/000441600
121. Mantica G, Chierigo F, Suardi N, et al. Minimally invasive strategies for the treatment of prostate cancer recurrence after radiation therapy: a systematic review. Minerva Urol Nefrol. 2020;72(5):563–578. doi:10.23736/s0393-2249.20.03783-2
122. Rischmann P, Gelet A, Riche B, et al. Focal high intensity focused ultrasound of unilateral localized prostate cancer: a prospective multicentric hemiablation study of 111 patients. Eur Urol. 2017;71(2):267–273. doi:10.1016/j.eururo.2016.09.039
123. Checcucci E, De Luca S, Piramide F, et al. The real-time intraoperative guidance of the new HIFU Focal-One(®) platform allows to minimize the perioperative adverse events in salvage setting. J Ultrasound. 2021. doi:10.1007/s40477-021-00594-8
124. Bakavicius A, Sanchez-Salas R, Muttin F, et al. Comprehensive evaluation of focal therapy complications in prostate cancer: a standardized methodology. J Endourol. 2019;33(7):509–515. doi:10.1089/end.2018.0809
125. Dellabella M, Branchi A, Di Rosa M, et al. Oncological and functional outcome after partial prostate HIFU ablation with Focal-One((R)): a prospective single-center study. Prostate Cancer Prostatic Dis. 2021;24:1189–1197. doi:10.1038/s41391-021-00390-9
126. Lebastchi AH, George AK, Polascik TJ, et al. Standardized nomenclature and surveillance methodologies after focal therapy and partial gland ablation for localized prostate cancer: an international multidisciplinary consensus. Eur Urol. 2020;78(3):371–378. doi:10.1016/j.eururo.2020.05.018
127. Baumunk D, Schostak M. [Treatment of localized prostate cancer with high-intensity focused ultrasound]. Therapie des lokalisierten Prostatakarzinoms mit hochintensivem fokussierten Ultraschall. Urologe A. 2015;54(2):183–190. doi:10.1007/s00120-014-3666-2
128. Ecke TH, Gerullis H, Heuck CJ, et al. Does a new ultrasound probe change the complication rates of transrectal ultrasound-guided needle biopsies of the prostate? Anticancer Res. 2010;30(7):3071–3076.
129. Chowdhury R, Abbas A, Idriz S, Hoy A, Rutherford EE, Smart JM. Should warfarin or aspirin be stopped prior to prostate biopsy? An analysis of bleeding complications related to increasing sample number regimes. Clin Radiol. 2012;67(12):e64–70. doi:10.1016/j.crad.2012.08.005
130. Kariotis I, Philippou P, Volanis D, Serafetinides E, Delakas D. Safety of ultrasound-guided transrectal extended prostate biopsy in patients receiving low-dose aspirin. Int Braz J Urol. 2010;36(3):308–316. doi:10.1590/s1677-55382010000300007
131. Raheem OA, Casey RG, Galvin DJ, et al. Discontinuation of anticoagulant or antiplatelet therapy for transrectal ultrasound-guided prostate biopsies: a single-center experience. Korean J Urol. 2012;53(4):234–239. doi:10.4111/kju.2012.53.4.234
132. Joshi R. Transrectal ultrasound guided prostatic biopsy and its complications: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2020;58(221):44–47. doi:10.31729/jnma.4820
133. Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology. 2013;81(6):1142–1146. doi:10.1016/j.urology.2013.02.019
134. Winoker JS, Wajswol E, Falagario U, et al. Transperineal versus transrectal targeted biopsy with use of electromagnetically-tracked MR/US fusion guidance platform for the detection of clinically significant prostate cancer. Urology. 2020;146:278–286. doi:10.1016/j.urology.2020.07.072
135. Lo KL, Chui KL, Leung CH, et al. Outcomes of transperineal and transrectal ultrasound-guided prostate biopsy. Hong Kong Med J. 2019;25(3):209–215. doi:10.12809/hkmj187599
136. Di Franco CA, Jallous H, Porru D, et al. A retrospective comparison between transrectal and transperineal prostate biopsy in the detection of prostate cancer. Arch Ital Urol Androl. 2017;89(1):55–59. doi:10.4081/aiua.2017.1.55
137. Rodriguez Socarras ME, Gomez Rivas J, Cuadros Rivera V, et al. Prostate mapping for cancer diagnosis: the Madrid protocol. transperineal prostate biopsies using multiparametric magnetic resonance imaging fusion and micro-ultrasound guided biopsies. J Urol. 2020;204(4):726–733. doi:10.1097/ju.0000000000001083
138. Claros OR, Tourinho-Barbosa RR, Fregeville A, et al. Comparison of initial experience with transrectal magnetic resonance imaging cognitive guided micro-ultrasound biopsies versus established transperineal robotic ultrasound magnetic resonance imaging fusion biopsies for prostate cancer. J Urol. 2020;203(5):918–925. doi:10.1097/ju.0000000000000692
139. Cornud F, Lefevre A, Flam T, et al. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: initial results of a single-center study. Eur Radiol. 2020;30(9):4838–4846. doi:10.1007/s00330-020-06882-x
140. Abouassaly R, Klein EA, El-Shefai A, Stephenson A. Impact of using 29 MHz high-resolution micro-ultrasound in real-time targeting of transrectal prostate biopsies: initial experience. World J Urol. 2020;38(5):1201–1206. doi:10.1007/s00345-019-02863-y
141. Lughezzani G, Saita A, Lazzeri M, et al. Comparison of the diagnostic accuracy of micro-ultrasound and magnetic resonance imaging/ultrasound fusion targeted biopsies for the diagnosis of clinically significant prostate cancer. Eur Urol Oncol. 2019;2(3):329–332. doi:10.1016/j.euo.2018.10.001
142. Chessa F, Schiavina R, Ercolino A, et al. Diagnostic accuracy of the Novel 29 MHz micro-ultrasound “ExactVuTM” for the detection of clinically significant prostate cancer: a prospective single institutional study. A step forward in the diagnosis of prostate cancer. Arch Ital Urol Androl. 2021;93(2):132–138. doi:10.4081/aiua.2021.2.132
143. Avolio PP, Lughezzani G, Paciotti M, et al. The use of 29 MHz transrectal micro-ultrasound to stratify the prostate cancer risk in patients with PI-RADS III lesions at multiparametric MRI: a single institutional analysis. Urol Oncol. 2021;39:
144. Huber PM, Afzal N, Arya M, et al. Focal HIFU therapy for anterior compared to posterior prostate cancer lesions. World J Urol. 2021;39(4):1115–1119. doi:10.1007/s00345-020-03297-7
145. Shoji S, Hiraiwa S, Uemura K, et al. Focal therapy with high-intensity focused ultrasound for the localized prostate cancer for Asian based on the localization with MRI-TRUS fusion image-guided transperineal biopsy and 12-cores transperineal systematic biopsy: prospective analysis of oncological and functional outcomes. Int J Clin Oncol. 2020;25(10):1844–1853. doi:10.1007/s10147-020-01723-9
146. Radiology Assistant. Prostate Imaging – Reporting and Data System. 2019. Version 2.1. PIRADS. Available from: https://radiologyassistant.nl/abdomen/prostate/prostate-cancer-pi-rads-v2. Accessed March 19, 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
