Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Transplantation of Human Amniotic Mesenchymal Stem Cells Up-Regulates Angiogenic Factor Expression to Attenuate Diabetic Kidney Disease in Rats
Authors Ni Y, Chen Y, Jiang X, Pu T, Zhang L, Li S, Hu L, Bai B, Hu T, Yu L, Yang Y
Received 3 June 2022
Accepted for publication 10 January 2023
Published 7 February 2023 Volume 2023:16 Pages 331—343
DOI https://doi.org/10.2147/DMSO.S371752
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gian Paolo Fadini
Yu Ni,1,* Yuqin Chen,2,* Xuheng Jiang,3 Tao Pu,1 Ling Zhang,4 Shaobin Li,2 Linhong Hu,1 Bing Bai,1 Tingting Hu,1 Limei Yu,2 Yibin Yang1,2
1Department of Nephrology, Affiliated Hospital of Zunyi Medical University, Zunyi, 563003, People’s Republic of China; 2Key Laboratory of Cell Engineering of Guizhou Province, Zunyi City, Affiliated Hospital of Zunyi Medical University, Zunyi, 563003, People’s Republic of China; 3Department of Emergency, Affiliated Hospital of Zunyi Medical University, Zunyi, 563003, People’s Republic of China; 4Zhuhai Campus of Zunyi Medical University, Zhuhai, 519041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Limei Yu, Department of Nephrology, Affiliated Hospital of Zunyi Medical University, Zunyi, 563003, People’s Republic of China, Email [email protected] Yibin Yang, Key Laboratory of Cell Engineering of Guizhou Province, Zunyi City, Affiliated Hospital of Zunyi Medical University, Zunyi, 563003, People’s Republic of China, Email [email protected]
Background and Aims: Diabetic kidney disease (DKD) is a prevalent and intractable microvascular complication of diabetes mellitus (DM), the process of which is closely related to abnormal expression of angiogenesis-regulating factors (ARFs). Stem cell transplantation might be a novel strategy for treating DKD. This study aims to explore the effect of transplantation of human amniotic mesenchymal stem cells (hAMSCs) on renal microangiopathy in a type 1 DKD rat model (T1DRM).
Methods: Seventy-two rats were randomly divided into three groups, including normal control group, DKD group, and hAMSCs transplantation group. T1DRM was established using a rat tail vein injection of streptozotocin (STZ) (55 mg/kg). hAMSCs were obtained from placental amniotic membranes during cesarean delivery and transplanted at 3 and 4 weeks through penile veins. At 6, 8, and 12 weeks following transplantation, blood glucose levels, renal function, pathological kidney alterations, and the expressions of ARFs’ mRNA and protein were analyzed.
Results: In T1DRM, transplanted hAMSCs that were homed at the injured site of kidneys increased ARFs’ expression and decreased blood glucose levels. Compared to the DKD group, the levels of 24-h urinary protein, serum creatinine, urea, and kidney injury molecule-1 (KIM-1) were reduced in hAMSCs transplantation group. In terms of renal pathology such as the degree of basement membrane thickening, hAMSCs transplantation was also less severe than the DKD group, thereby alleviating kidney injury.
Conclusion: hAMSCs transplantation might ameliorate STZ-induced chronic kidney injury through increasing ARFs’ expression in kidneys and lowering blood glucose levels.
Keywords: DKD, hAMSCs, renal microangiopathy, angiogenesis-regulating factors
Introduction
Diabetic kidney disease (DKD), as a microvascular complication of diabetes mellitus (DM), has become a worldwide public health concern.1 Approximately 40% of patients are at risk for developing DKD.2 DM is the second leading cause of end-stage renal disease (ESRD) in China, with rising prevalence and mortality.3 The onset and progression of DKD are closely associated with vascular endothelial cell dysfunction, structural anomalies, and dysregulated expression of various angiogenesis-regulating factors (ARFs).4,5 Recent studies found that ameliorating the aberrant expression of ARFs, which play a crucial role in the evolution of DKD, may slow the disease’s progression.6,7 Therefore, it is essential to evaluate the potential of anti-angiogenic treatment for DKD is crucial.8 Moreover, a recent animal study suggested that angiogenesis is a potential therapeutic target for early-stage DKD.5
Stem cell therapy has recently emerged as a research hotspot in treating many chronic diseases, with promising results in diabetic microangiopathy and other aspects involving stem cell migration, direct differentiation, and paracrine and immune regulation. The high glucose environment of diabetic nephropathy makes it possible for stem cells to migrate to damaged tissues and perform their repair functions. Simultaneously, oxidative stress may affect the paracrine effects of mesenchymal stem cells (MSCs) under hypoxia.9 According to previous studies, transplanting bone marrow mesenchymal stem cells (BM-MSCs) into diabetic mice inhibited DKD inflammation and fibrosis while improving glomerular sclerosis,10 and umbilical cord mesenchymal stem cells (UC-MSCs) exert therapeutic effects on DKD animal models, manifested by diminished chronic inflammation contributing to DKD progression.11 BM-MSCs engraftment accelerates angiogenesis in wound healing by secreting vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) as discovered by An et al12 to summarize, stem cell transplantation might ameliorate DKD through secreting ARFs. However, the effect of stem cells and ARFs secreted by them on renal microangiopathy in DM is rarely reported.
Previous studies have demonstrated that MSCs could migrate, proliferate, and differentiate into mature endothelial cells, as well as generate a variety of cytokines and growth factors, inducing angiogenesis and wound healing.12,13 Specifically, Zhang et al14 found that the transplantation of uMSC-Exos could induce angiogenesis and bone healing, and its mechanism might involve upregulation of hypoxia-inducible factor-1α (HIF-1α) and VEGF. hAMSCs, which can be extracted from the human placenta, are promising stem cells due to their accessibility, high plasticity, low immunogenicity, and potent multi-directional differentiation ability. They are especially without ethical concern.15 However, hAMSCs play role in how to improve the outcome of diabetic kidney microangiopathy, which remains unclear. Therefore, this experiment was designed to explore the effect of hAMSCs transplantation on renal microangiopathy in a type 1 DKD rat model (T1DRM), and whether or not it was related to the expression of ARFs in kidneys or blood glucose levels.
Materials and Methods
Isolation, Culture, and Morphological Observation of hAMSCs
All experiments were approved by the Ethics and Research Committee of Zunyi Medical University (Approval no.: 2017–112). Informed consent was obtained from amniotic membrane donors. Full-term placentas from pregnant women were collected. They tested negative for serum hepatitis A, hepatitis B, human immunodeficiency virus (HIV), and Treponema pallidum. Amniotic membranes were stripped under sterile conditions and placed in sterile bottles containing D-Hanks. hAMSCs were isolated and cultured as described previously.16,17 Related cell surface markers, such as CD44, CD90, CD73, CD105, CD34, CD11 b, CD19, CD45, and human leukocyte antigen DR (HLA-DR)(BioLegend, USA), were detected using flow cytometry to identify the phenotype of hAMSCs. Fat, calcium nodules, and cartilage differentiation were observed using Oil Red O, alizarin red, and toluidine blue staining. Vimentin expression was detected in hAMSCs using immunohistochemical staining.
Rat Models and hAMSCs Transplantation
Healthy Sprague–Dawley (SD) male rats aged 3–4 months and weighing 200 ± 20 g (Specific pathogen-free grade, license number-SCXK, Chongqing; 2012–0005) were provided by the Daping Hospital of Third Military Medical University. All animal experiments were approved by Zunyi Medical College’s Animal Experiment Ethics Committee (No.: 81260118). Rats were housed in cages at 20–25℃ and 40–70% relative humidity. All rats were fed normally, with free access to water and food. As described, T1DRM induced by STZ (American Sigma, 55 mg/kg) was used.18,19 After 72 h, blood was collected from the caudal vein on three consecutive days to measure blood glucose levels. A blood glucose level of ≥16.65 mmol/L, urine protein excretion rate of ≥30 mg/24 h, urine output >150% of the previous one, and positive urine glucose for three consecutive days were used as modeling criteria. A total of 72 rats were randomly allocated to (1) NC group (n = 24), and the same dose of citric acid-sodium citrate was injected intraperitoneally, and the same quantity of PBS was transplanted to replace hAMSCs; (2) DKD group (n = 24): the same quantity of PBS was transplanted to replace hAMSCs after modeling; and (3) MSC group (n = 24): PBS-hAMSCs transplantation was performed at 3 and 4 weeks after modeling, and 0.2 mL cell suspension (1×107/mL) was collected using a microsyringe. PBS-hAMSCs were injected slowly through the penile vein (2.0×106 cells/each). Each group was divided into three sub-groups: 6-, 8-, and 12-week (at least six rats in each group). The Western blot analysis was supplemented, and the rats were modeled twice in the eighth week. Blood, urine samples, and kidney tissues were collected for further analysis after hAMSC treatment at each time point. All rats were anesthetized with 7% chloral hydrate and euthanized. All animal experiments were completely randomized and blinded.
Histological Analysis, Immunohistochemistry, and Immunofluorescence Staining
Kidney tissue specimens were fixed with 10% formaldehyde and 4% paraformaldehyde for 16 h and then submitted to the Pathology Department for paraffin embedding and sectioning. Hematoxylin–eosin (H&E) and periodic acid-Schiff (PAS) staining were performed to observe morphological changes in the kidney tissues under a light microscope. Following conventional dewaxing and hydration, the sections were soaked in citrate buffer repair solution (0.01 M, pH 6.0) for immunohistochemistry staining. Goat serum was added to each section for 30 min. Primary antibodies against Thrombospondin-1 (TSP-1) (Rabbit, 1:300, 18304-1-AP, Proteintech, USA), VEGF (Rabbit, 1:200, ab32152, Abcam, UK), Tyrosine kinase receptor-2 (Tie-2) (Rabbit, 1:100, bs-1300R, Beijing Bioss), and Angiopoietin-1 (Ang-1) (Rabbit, 1:200, 23302-1-AP, Proteintech, USA) were added and refrigerated overnight under 4℃. Thereafter, the goat anti-mouse/rabbit IgG secondary antibody (ZB-2301, ZSGB-Bio, Beijing) was added and incubated in a constant temperature box. After developing, dehydration, and drying, neutral balsam was used for mounting. The images were taken under a microscope, and the integrated optical density (IOD) was measured using Image-Pro Plus 6.0. After routine deparaffinization and hydration, goat serum was added to each section for immunofluorescence staining. Fluorescently labeled primary antibodies against TSP‑1 (Rabbit, 1:300, 18304-1-AP, Proteintech, USA), VEGF (Rabbit, 1:200, ab32152, Abcam, UK), Tie-2 (Rabbit, 1:100, bs-1300R, Beijing Bioss), Ang-1 (Rabbit, 1:200, 23302-1-AP, Proteintech, US), and Anti-human cell nuclei (MAB1281) (Mouse, 1:100, Merck Millipore, USA) were added and placed in the refrigerator at 4℃ overnight. A fluorescent secondary antibody (1:800, ZF-0314, ZSGB-Bio, Beijing) was added. After mounting with glycerol, the slides were immediately observed and photographed under a fluorescence microscope (Eclipse Ci-e, Nikon, Japan).
Quantitative Real-Time PCR
TRIzol reagent (Solarbio, Beijing) was used for RNA extraction from kidneys, and Nanodrop 1000 (Nanodrop, USA) was used to test RNA purity and concentration. RT-qPCR was performed according to the instructions (Takara Bio, Dalian). The following primer pairs were used: VEGF forward 5’-CGGAGAGCAACGTCACTATG-3’, reverse 5’-GGTCTGCATTCACATCTGCT-3’; Ang-1 forward 5’-CCATGCTGGAGATAGGAACC-3’, reverse 5’-TGGATTTCAAGACGGGATGT-3’; Ang-2 forward 5’-TCAACTCTGGCTCAGGA-3’, reverse 5’-GGCCTCTTCTCTTCATCATGC-3’; TSP-1 forward 5’-AACAAGAACGCCAAGTGCAA-3’, reverse 5’-CAGCCGTCAAGGTCTGTGTC-3’; Tie-2 forward 5’-GGCTGGCCGCTACCTACTAA-3’, reverse 5’-TCCGGTGGATGGTGAATATG-3’; β-actin forward 5’- GACCTGACCGACTACCTCATG-3’, and reverse 5’-TCTCCTTGATGTCCCGCAC-3’. The qPCR was performed, and 2−ΔΔCT relative quantification method was used to analyze the results.
Western Blot
After extracting protein from the kidney tissue, protein concentration was measured using a BCA protein assay kit (Thermo Fisher Scientific). Based on the concentration determination results, the samples were loaded and analyzed using protein blotting. The following primary antibodies were used: Ang-1 (rabbit, 1:1000, Proteintech, Wuhan China, 23302-1-AP), VEGF (mouse, 1:3000, Proteintech, Wuhan China, 66828-1-lg), TSP-1 (mouse, 1:3000, Proteintech, Wuhan China, 67241-1-lg), β-actin (mouse, 1:5000, ABclonal, AC004), and tubulin (mouse, 1:50,000, Proteintech, Wuhan China, 66031-1-lg). After washing, goat anti-mouse (1:5000, Thermo Fisher Scientific, 31,430) and goat anti-rabbit (1:5000, Thermo Fisher Scientific, 31,460) secondary antibodies were added. The signals were detected using a gel imaging system, and the gray values of the protein bands were analyzed using ImageJ software.
Statistical Analysis
Statistical analyses were performed using Statistics 18.0 software. All data are presented as mean ± standard deviation (x ± s). One-way analysis of variance was used to compare multiple groups. The least significant difference (LSD) was used when the variance was homogeneous. Dunnett’s T-test was used for correction when the variance was heterogeneous. A Pearson’s correlation analysis was performed. Statistical significance was set at α=0.05, ΔP<0.05, and *P<0.01 were considered statistically significant.
Results
Characteristics and Identification of hAMSCs
We observed that hAMSCs were adherent multi-shaped cells. Concurrently, hAMSCs expressed vimentin, a mesenchymal cell marker (Figure 1A). Further research revealed that hAMSCs could differentiate into osteoblasts, chondroblasts, and adipocytes (Figure 1B). The flow cytometry analysis exhibited that third-generation hAMSCs highly expressed CD44, CD73, CD90, and CD105 but not CD34, CD11b, CD19, CD45, and HLA-DR (Figure 1C).
Evaluation of the Homing Ability of hAMSCs in Kidneys
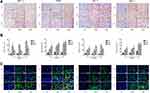
MAB1281 was used as a tracer to explore the homing ability of hAMSCs. Immunofluorescence staining revealed positive labeling at 2, 4, and 8 weeks after the hAMSCs were injected into veins (6, 8, and 12 weeks after establishing the diabetic model). In addition, positive MAB1281 expression was observed in kidney tissue. However, MAB1281 expression (Figure 2) was absent in the DKD and NC groups. This result indicates that hAMSCs could be homed in DKD rat kidneys.
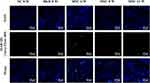 |
Figure 2 Image of hAMSCs homing in kidney tissue (×40 Green: MAB1281, blue: nucleus). |
hAMSCs Transplantation Improved the Renal Function of STZ-Induced T1DRM
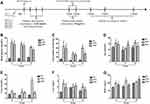
An STZ-induced T1DRM was established to explore the effect of hAMSCs transplantation on DKD. After 6, 8, and 12 weeks of transplantation, the animals were sacrificed, and specimens were collected for further analysis (Figure 3A). The results revealed that the hAMSCs transplantation group had significantly higher blood glucose levels than the NC group but lower levels than the model group (Figure 3B). In addition, the 24-h urine protein level in the transplantation group was higher than that in the NC group but lower than that in the model group (Table 1 and Figure 3C). The changes in serum creatinine (SCr) and cystatin C (Cys-c) levels in the transplantation group were insignificant compared to those in the NC and DKD groups (Figures 3D and F). However, the urea level was higher in the hAMSCs treatment group than that in the NC group but significantly lower than that in the DKD group (P<0.05) (Table 2 and Table 3, Figure 3E). Moreover, the transplantation group had a higher KIM-1 level than the NC group but a lower KIM-1 level than the DKD group (P<0.05), except for the 12-week group (Table 2 and Table 3, Figure 3G). These results indicate that transplantation of hAMSCs alleviates kidney injury in DM rats.
 |
Table 1 Comparison of Blood Glucose and 24-h Urine Protein Levels Among Three Rat Groups |
 |
Table 2 Comparison of SCr and Urea Levels Among Three Rat Groups |
 |
Table 3 Comparison of Serum Cys-c and Urine KIM-1 Levels Among Three Rat Groups |
hAMSCs Transplantation Reduced the Pathological Changes of Kidneys in Renal Tissues
The comparison between hAMSCs transplantation and DKD groups exhibited a significant reduction in kidney weight index (Figure 4A). H&E and PAS staining demonstrated no obvious abnormalities in the glomeruli, renal tubules, and interstitium in the NC group. However, pathological changes, such as tubular hypertrophy, increased tubulointerstitial inflammatory cell infiltration, tubular basement membrane thickening, and increased mesangial matrix, were observed in two diabetic model groups, whereas pathological changes were relatively reduced in the DKD + hAMSCs group (Table 4, Figures 4B and C).
 |
Table 4 Comparison of Kidney/Body Weight Among Three Rat Groups |
The Effect of hAMSCs Intervention on the Expressions of Renal ARFs mRNA and Protein in Each Group
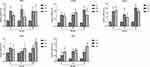
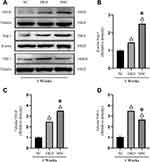
As shown in Figures 5–7, hAMSCs transplantation promoted vascular growth factor expression. TSP-1, VEGF, Ang-1, Ang-2, and Tie-2 mRNA expression levels in kidney tissues of DKD and MSC groups were upregulated to different degrees compared to the control group at each time point. Compared to the DKD group, the MSC group had a significantly upregulated Ang-1 expression level at 12 weeks but downregulated TSP-1 and VEGF expression levels at 8 and 12 weeks, respectively. However, VEGF and Ang-1 mRNA levels were increased in the MSC group, whereas Ang-2 and Tie-2 mRNA expressions were not significantly changed, compared to the DKD group (Figure 5). Immunohistochemistry and fluorescence showed that TSP-1, VEGF, Ang-1, and Tie-2 expressions were significantly elevated in kidney tissue of DKD and MSC groups compared to the NC group (P<0.05). TSP-1 expression was lower in the MSC group than in the DKD group (P<0.05). VEGF and Ang-1 protein expressions were higher in the DKD group at the same time point. A significant difference in VEGF expression was observed only at the sixth and eighth weeks (P<0.05). Ang-1 expression was statistically obvious only at 8 weeks (P<0.05). However, Tie-2 expression was higher in the DKD group at 6 and 8 weeks but lower in the DKD group at 12 weeks (P<0.05; Figure 6). VEGF, Ang-1, and TSP-1 protein expression levels were analyzed at 8 weeks by Western blot, indicating the same results (P<0.01) (Figure 7). These results show that hAMSCs transplantation can regulate angiogenic factor levels in T1DRM.
Correlation Analysis
Correlation analysis was performed based on the immunohistochemical staining findings of each index. The results revealed positive correlations were observed between TSP-1 and 24-h urine protein, 24-h urine protein and KIM-1, TSP-1 and KIM-1, KIM-1 and urea, VEGF and Ang-1, Tie-2 and Ang-1, and VEGF and Tie-2 (P<0.05). Additionally, negative correlations between Ang-1 and 24-h urine protein, Tie-2 and 24-h urine protein, VEGF and 24-h urine protein, Tie-2 and KIM-1, Tie-2 and TSP-1, VEGF and TSP-1, and Ang-1 and TSP-1 were revealed (P<0.05) (Figures 8A–H). These results suggested both positive and negative associations between diabetic kidney AFs. Furthermore, hAMSCs modulated the expressions of AFs, ultimately improving DKD.
Discussion
With the development of regenerative medicine, stem cell therapy for kidney disease has become a new research hotspot. hAMSCs will be a viable and novel therapy in the future because they have low immunogenicity and multi-lineage differentiation potential, and they are easier to obtain than other stem cells and without any social or ethical constraints in particular. However, little is known about the effect of hAMSCs on ARFs in DKD. In the present study, an STZ-induced T1DRM was constructed, in which hAMSCs transplantation was performed to explore its efficacy and potential mechanism in reducing diabetic kidney injury. This study indicated that hAMSCs could be homed to the kidneys of T1DRM, effectively reduce the levels of blood glucose, 24-h urine protein, KIM-1, and urea, ameliorating renal pathological injury and alleviating microangiopathy. Its potential mechanism might be relevant with regulating the expression of ARFs and blood glucose in DKD rats.
Numerous studies have shown that MSCs can reduce organ tissues lesions through homing to damaged organ tissues.11,20 In this study, the homing ability of hAMSCs in kidneys was proved by using a tracer named MAB1281 shown in Figure 2. It was also observed that the homing of hAMSCs could ameliorate renal injury and change the expression of ARFs in T1DRM.
Kidney angiogenesis is an adaptive response to ischemia, in which many ARFs such as angiogenin (Angs), VEGF, Tie-2, and thrombospondin-1 (TSP-1) are involved. Numerous studies also have shown that Ang-1, Ang-2, Tie-2, VEGF, HIF-1α, stromal-derived factor-1 (SDF-1), TSP-1, pigment epithelium-derived factor (PEDF), and other ARFs are abnormally expressed in the kidneys of DM rat.4–8 Previous experiments conducted by the research group indicated that administration of Ang-1 adenovirus vector and L-mimosine (HIF-1α degradation inhibitor) improved the expression of abnormal angiogenic factors in diabetic kidneys.21 In the present study, hAMSCs upregulated the expressions of Ang-1/Ang-2/Tie-2 and VEGF, while down-regulated the expression of TSP-1, suggesting that hAMSCs transplantation could regulate the abnormal expressions of angiogenic factors in DKD to a certain extent.
Ang/Tie-2 systems play an important role in regulating angiogenesis.22 Ang-1 has biological functions including stabilizing blood vessels, promoting blood vessel maturation, reducing blood vessel leakage, and resisting endothelial cell apoptosis and inflammation,23 whereas Ang-2 is a natural competitive antagonist of Ang-1 and is a factor necessary for initiating blood vessel remodeling. It is primarily expressed in active or repaired vascular endothelial remodeling.22,24 Researchers found that the decrease of Ang-1/Ang-2 ratio, as well as the activation of Ang/Tie pathway, which is closely related to the pathological changes of DKD.25–27 In our study observed that urinary protein excretion decreased obviously; furthermore, the expression of Ang-1 and Ang-2 was up-regulated at each time point, the expression of Tie-2 was up-regulated at 6 and 8 weeks, and the ratio of Ang-1/Ang-2 increased after hAMSCs transplantation in T1DRM. A study revealed that insulin-like growth factor (IGF) and Ang-2 were highly expressed after transplanting BM-MSCs into diabetic mice wound, promoting endothelial cell proliferation and angiogenesis.28 This finding is consistent with our study; hAMSCs transplantation could increase the expression of Ang-2 in the MSC group. In addition, our study also confirmed that Ang-1 was negatively correlated with 24-h urine protein, urine KIM-1, and blood urea, indicating that hAMSCs transplantation can partially correct abnormal Ang/Tie-2 expression in DKD, and Ang-1 elevation can enhance new blood vessel maturation and stability.
Previous studies found that a high level of VEGF may result in aberrant blood vessel formation, macrophages activation, and even mesangial dilation. Its highly inhibited expression can reduce glomerular hypertrophy, basement membrane thickening, and proteinuria excretion.29 However, as the condition progresses, the level of VEGF may decrease resulting in endothelial cell death and capillary thinning.30 Ang-2 was proved to promote vascular atrophy in the absence of VEGF.31 Moreover, VEGF could promote endothelial nitric oxide synthase (eNOS) phosphorylation through the PI3K/Akt signaling pathway, on the one hand, which could maintain the glomerular endothelial cell function through stimulating endothelial cells to release nitric oxide, on the other hand, which can concurrently increase vascular permeability and urinary protein excretion.32,33 Above all, the appropriate levels of VEGF might have positive results through promoting the maturation and stability of new blood vessels in a certain stage of diseases. Researchers transplanted exosomes derived from BM-MSCs into a rat model of renal ischemia-reperfusion injury and discovered that VEGF and CD31 expressions were upregulated in the exosome group, and the density of renal blood vessels increased, promoting angiogenesis.34 This finding is consistent with the finding on the upregulation of VEGF in the MSC group in this study. However, VEGF mRNA expression was lower than in the DKD group, which may be due to abnormal expression of several growth factors and cytokines, including TGF-β1 and HIF-1, as well as kidney gene and protein differential expressions.35
Thrombospondin −1 (TSP-1), first isolated from human platelets, was the first endogenous angiogenesis inhibitor to be identified. Some researchers have proposed that TSP-1 or its derivatives directly inhibit tumor angiogenesis.36 In this study, correspondingly, TSP-1 expression was lower in the MSC group than in the DKD group. Correlation analysis also revealed that a positive correlation between 24-h urine protein and TSP-1 and negative correlation with VEGF, Tie-2, and Ang-1. The negative correlation between TSP-1 and Tie-2, VEGF, and Ang-1. Positive correlations existed between Ang-1 and VEGF and Tie-2. In conclusion, these results suggested that ARFs in diabetic kidneys interact, resulting in renal dysfunction. In addition, alterations in ARFs following hAMSCs transplantation are associated with improved DKD.
Conclusions
This study demonstrated that hAMSCs transplantation therapy could ameliorate diabetic nephropathy in DM rats. The underlying mechanism might involve altered expressions of kidney-related angiogenic factors and decreased blood glucose levels. Consequently, our findings may provide new treatment strategies for delaying DKD progression.
Data Sharing Statement
The data from this study are available from the corresponding author upon reasonable request.
Ethics Approval
All animal experimental procedures were performed in accordance with the guidelines of the Regulation for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, 1988, revised in March 2017) and approved by the ethics committee of the Affiliated Hospital of Zunyi Medical University. This study was also conducted in accordance with the Guiding Principles for the Care and Use of Laboratory Animals (China).
Acknowledgments
We are grateful to Hongyang Wang from the Cell Engineering Department for supporting the culture and identification of human amniotic mesenchymal stem cells. The authors are eternally grateful to all participants in the study.
Author Contributions
All authors made substantial contributions to conception and design, data acquisition, or data analysis and interpretation; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant nos. 81260118 and 81860139), a grant from the Zunyi Science and Technology Plan Project (2021) No. 66, Zunyi Science and Technology Bureau Zunshikehe Shenshikehe No. [2014]59, a grant from the Zunyi Science and Technology Plan Project (2022) No. 281, a grant from Basic Research Program (Natural Science) of Guizhou Province (2023, No.626).
Disclosure
Dr Limei Yu reports a patent A complete culture medium and culture method of human amniotic mesenchymal stem cells licensed to Affiliated Hospital of Zunyi Medical University. The authors report no other conflicts of interest in this work.
References
1. Bikbov B, Purcell CA, Levey AS.; Collaboration GBDCKD. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. doi:10.1016/S0140-6736(20)30045-3
2. Ebaid H, Bashandy SAE, Abdel-Mageed AM, Al-Tamimi J, Hassan I, Alhazza IM. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr Metab. 2020;17:6. doi:10.1186/s12986-019-0419-7
3. Daios S, Kaiafa G, Pilalas D, et al. Endothelial dysfunction and platelet hyperaggregation in type 2 diabetes mellitus: the era of novel anti-diabetic agents. Curr Med Chem. 2021;28(20):3935–3963. doi:10.2174/0929867327666201009143816
4. Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58(7):1471–1478. doi:10.2337/db09-0119
5. Tanabe K, Maeshima Y, Sato Y, Wada J. Antiangiogenic therapy for diabetic nephropathy. Biomed Res Int. 2017;2017:5724069. doi:10.1155/2017/5724069
6. Wen D, Huang X, Zhang M, et al. Resveratrol attenuates diabetic nephropathy via modulating angiogenesis. PLoS One. 2013;8(12):e82336. doi:10.1371/journal.pone.0082336
7. Gowd V, Kang Q, Wang Q, Wang Q, Chen F, Cheng KW. Resveratrol: evidence for its nephroprotective effect in diabetic nephropathy. Adv Nutr. 2020;11(6):1555–1568. doi:10.1093/advances/nmaa075
8. Zhang A, Fang H, Chen J, He L, Chen Y. Role of VEGF-A and LRG1 in abnormal angiogenesis associated with diabetic nephropathy. Front Physiol. 2020;11:1064. doi:10.3389/fphys.2020.01064
9. Liu Y, Tang SC. Recent progress in stem cell therapy for diabetic nephropathy. Kidney Dis. 2016;2(1):20–27. doi:10.1159/000441913
10. Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:36. doi:10.1186/s13098-017-0233-1
11. Xiang E, Han B, Zhang Q, et al. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res Ther. 2020;11(1):336. doi:10.1186/s13287-020-01852-y
12. An Y, Liu WJ, Xue P, et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018;9(2):58. doi:10.1038/s41419-017-0082-8
13. Patel DB, Gray KM, Santharam Y, Lamichhane TN, Stroka KM, Jay SM. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med. 2017;2(2):170–179. doi:10.1002/btm2.10065
14. Zhang Y, Hao Z, Wang P, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1alpha-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52(2):e12570. doi:10.1111/cpr.12570
15. Liu QW, Huang QM, Wu HY, et al. Characteristics and therapeutic potential of human amnion-derived stem cells. Int J Mol Sci. 2021;22:2.
16. Lm Y. Complete medium and human amnion-derived mesenchymal stem cell culture method; 2011.
17. Rc Z. Stem Cells: Basics and Clinical Translation. Springer Science Business Media Dordrecht; 2015.
18. Brosius FC
19. Bai Y, Wang J, He Z, Yang M, Li L, Jiang H. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by targeting transforming growth factor beta (TGF-beta)/smad pathway and pro-inflammatory cytokines. Med Sci Monit. 2019;25:3069–3076. doi:10.12659/MSM.914860
20. Zhang Y, Ye C, Wang G, et al. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. Biomed Res Int. 2013;2013:526367. doi:10.1155/2013/526367
21. Li S, Zou H, Gong M, et al. Angiopoietin-1 promotes the integrity of neovascularization in the subcutaneous matrigel of type 1 diabetic rats. Biomed Res Int. 2019;2019:2016972. doi:10.1155/2019/2016972
22. Yan ZX, Luo Y, Liu NF. Blockade of angiopoietin-2/Tie2 signaling pathway specifically promotes inflammation-induced angiogenesis in mouse cornea. Int J Ophthalmol. 2017;10(8):1187–1194. doi:10.18240/ijo.2017.08.01
23. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi:10.1038/nature10144
24. An YA, Sun K, Joffin N, et al. Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis. Elife. 2017;6:1. doi:10.7554/eLife.24071
25. Karalliedde J, Gnudi L. Endothelial factors and diabetic nephropathy. Diabetes Care. 2011;34:S291–296. doi:10.2337/dc11-s241
26. Khalaf N, Helmy H, Labib H, Fahmy I, El Hamid MA, Moemen L. Role of angiopoietins and Tie-2 in diabetic retinopathy. Electron Physician. 2017;9(8):5031–5035. doi:10.19082/5031
27. Desideri S, Onions KL, Baker SL, et al. Endothelial glycocalyx restoration by growth factors in diabetic nephropathy. Biorheology. 2019;56(2–3):163–179. doi:10.3233/BIR-180199
28. de Mayo T, Conget P, Becerra-Bayona S, Sossa CL, Galvis V, Arango-Rodriguez ML. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS One. 2017;12(6):e0177533. doi:10.1371/journal.pone.0177533
29. Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12(5):993–1000. doi:10.1681/ASN.V125993
30. Nakagawa T, Sato W, Kosugi T, Johnson RJ. Uncoupling of VEGF with endothelial NO as a potential mechanism for abnormal angiogenesis in the diabetic nephropathy. J Diabetes Res. 2013;2013:184539. doi:10.1155/2013/184539
31. Zhang Y, Liu J, Zou T, et al. DPSCs treated by TGF-beta1 regulate angiogenic sprouting of three-dimensionally co-cultured HUVECs and DPSCs through VEGF-Ang-Tie2 signaling. Stem Cell Res Ther. 2021;12(1):281. doi:10.1186/s13287-021-02349-y
32. Hou N, Huang N, Han F, Zhao J, Liu X, Sun X. Protective effects of adiponectin on uncoupling of glomerular VEGF-NO axis in early streptozotocin-induced type 2 diabetic rats. Int Urol Nephrol. 2014;46(10):2045–2051. doi:10.1007/s11255-014-0807-x
33. Shukla R, Pandey N, Banerjee S, Tripathi YB. Effect of extract of Pueraria tuberosa on expression of hypoxia inducible factor-1alpha and vascular endothelial growth factor in kidney of diabetic rats. Biomed Pharmacother. 2017;93:276–285. doi:10.1016/j.biopha.2017.06.045
34. Zou X, Gu D, Xing X, et al. Human mesenchymal stromal cell-derived extracellular vesicles alleviate renal ischemic reperfusion injury and enhance angiogenesis in rats. Am J Transl Res. 2016;8(10):4289–4299.
35. Zhang D, Shao S, Shuai H, et al. SDF-1alpha reduces fibronectin expression in rat mesangial cells induced by TGF-beta1 and high glucose through PI3K/Akt pathway. Exp Cell Res. 2013;319(12):1796–1803. doi:10.1016/j.yexcr.2013.03.030
36. Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and −2. Cold Spring Harb Perspect Med. 2012;2(5):a006627. doi:10.1101/cshperspect.a006627
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.