Back to Journals » Cancer Management and Research » Volume 14
Transformation from Small Cell to Squamous Cell Carcinoma in a Thymic Carcinoma Patient with a Durable Response to Anlotinib: A Case Report
Authors Qin W, Zou B, Fan X, Fan B, Wang S, Wang L
Received 17 February 2022
Accepted for publication 22 April 2022
Published 29 April 2022 Volume 2022:14 Pages 1595—1602
DOI https://doi.org/10.2147/CMAR.S362858
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Wenru Qin,1,2,* Bing Zou,2,* Xinyu Fan,2,3 Bingjie Fan,2 Shijiang Wang,2 Linlin Wang2
1Department of Oncology, Weifang Medical University, Weifang, Shandong, People’s Republic of China; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 3Department of Oncology, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Linlin Wang, Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jiyan Road 440, Jinan, Shandong, 250117, People’s Republic of China, Tel +86-531-67626142, Email [email protected]
Abstract: The most common pathologic type of thymic carcinoma (TC) is squamous cell carcinoma (SCC). Small cell carcinoma is relatively rare, accounting for approximately 2% to 5% of all thymic tumors. Histologic transformation of TC has not yet been reported. Available treatments for TC patients who progress after first-line therapy are limited, which contributes to their poor prognosis. We reported an extraordinary case of a 66-year-old man who was diagnosed with thymic small cell carcinoma that transformed into SCC after third-line treatment. Surprisingly, the patient had a progression-free survival (PFS) of 25 months and an overall survival (OS) of 10 years on anlotinib as fourth-line therapy. The tolerance was well. Thus, anlotinib may be a safe and promising treatment for TC patients, especially those who undergo histologic transformation.
Keywords: thymic carcinoma, squamous cell carcinoma, small cell carcinoma, histologic transformation, anlotinib
Introduction
Thymic carcinoma (TC) is a relatively rare but highly aggressive disease with a 5-year survival rate of only 36%.1,2 The histopathologic subtypes of TC vary in their degree of differentiation and aggressiveness, and squamous cell carcinoma (SCC) is by far the most common subtype. Small-cell neuroendocrine carcinoma is relatively rare, accounting for approximately 2% to 5% of all thymic tumors.3 The histologic transformation of TC has not yet been reported, and the selection of salvage therapies for them is elusive. About 46.5% of TC patients have advanced disease at diagnosis and are administered platinum-based chemotherapy as standard first-line treatment.4,5 However, there are few treatment options for patients who progress after first-line therapy, and the efficacy of further-line treatments is poor.
Anlotinib is an oral small molecule receptor tyrosine kinase inhibitor (TKI) that exhibits significant anti-angiogenesis and broad-spectrum antitumor activity via highly potent and selective suppression of vascular endothelial growth factor receptor-2 (VEGFR-2).6,7 It was recommended for use in the treatment of multiple cancers, including non-small-cell lung cancer (NSCLC), small cell lung cancer (SCLC) and soft tissue sarcoma.6 However, the effect of anlotinib in patients with TC, especially those who undergo histologic transformation, remains unclear. We reported an uncommon case of thymic small cell carcinoma that underwent histologic transformation to SCC and discussed the patient’s long-term response to anlotinib.
Case Presentation
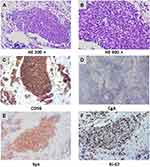
A 66-year-old man was admitted to the hospital with sternal pain in December 2011. His chest computerized tomography (CT) scan showed anterior mediastinal space occupying lesions with mediastinal lymph node metastases. A biopsy of the mediastinal lesions showed small cell carcinoma (Figure 1). The patient was diagnosed with thymic small cell carcinoma cT2N1M0 stage IVa. From December 2011 to May 2012, six cycles of first-line chemotherapy with irinotecan (65 mg/m2 d1, 8) and cisplatin (60 mg/m2 d1) were administered. During chemotherapy the patient also received 60 Gy/30f local radiotherapy for his mediastinal lesions. Partial response (PR) was achieved as assessed with Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Radiation-induced pneumonia occurred, but improved after active treatment.
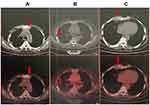
The patient felt sternal pain again in May 2017, and a positron emission tomography/computed tomography (PET-CT) scan showed progressive disease (PD) with right parasternal tissue enlargement and chest wall metastases (Figure 2). A puncture biopsy of the right parasternal tissue revealed metastatic thymic small cell carcinoma. Second-line chemotherapy with five cycles of irinotecan (65 mg/m2 d1, 8) and cisplatin (60 mg/m2 d1) was completed in September 2017. Efficacy was reported as stable disease (SD) after chemotherapy, and the patient’s pain improved.
The patient’s sternal pain worsened again in March 2018, and a CT scan showed an abnormal density in his right anterior chest wall and destruction of his ribs and sternum. Four cycles of third-line paclitaxel (175mg/m2 d1) and cisplatin (60 mg/m2 d1) chemotherapy were completed in June 2018. During the follow-up period, the patient’s response to chemotherapy was rated as SD (Figure 3A).
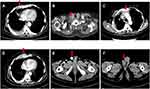
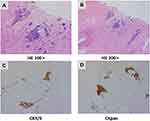
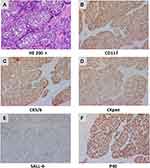
The patient reported a progressively enlarging right testicular mass and worsening sternal pain again in March 2019. An enhanced CT was performed which showed right clavicle and neck lymph node metastases, multiple new bone metastases with surrounding soft tissue masses, and right testicle metastases (Figure 3B–F). A biopsy was performed again on account of the patient’s extensive metastases. Interestingly, the pathology of the right parasternal mass biopsy showed SCC rather than small cell carcinoma (Figure 4). Due to the size of the right testicular mass, a right orchiectomy was performed, and postoperative pathology also confirmed SCC from the thymus gland (Figure 5).
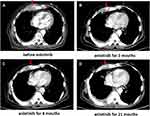
The patient’s Eastern Cooperative Oncology Group performance status (ECOG PS) score was 3. He had a history of hypertension and heart disease. His pro-B-type natriuretic peptide (pro-BNP) reached 2055 pg/mL and his left ventricular ejection fraction was 53%. Expectant and supportive treatment was administered to improve the patient’s condition. His ECOG PS score improved to 2, his pro-BNP fell to 421.5 pg/mL and his left ventricular ejection fraction improved to 61%. Even so, the patient was not likely to tolerate further chemotherapy. We therefore chose to pursue palliative radiotherapy and anti-angiogenic therapy for optimal safety and efficacy. Palliative radiotherapy of 54 Gy/27f was administered to the parasternal mass due to sternal pain, with treatments ending May 2019. A CT after the completion radiotherapy showed that the size of the right chest wall metastasis was smaller, and the patient’s chest wall pain was significantly improved. The patient started to take anlotinib orally in June 2019 at a dose of 8 mg/day, once daily on days 1–14 of a 21-day cycle (2-weeks on, 1-week off) while taking medicine to lower his blood pressure and improve the condition of his heart. The patient developed grade II hand-foot skin reactions while taking anlotinib, which improved to grade I after supportive treatment. Over a routine observation period the patient’s response to anlotinib was evaluated as SD. In July 2021, the patient died of respiratory failure because he caught a cold that developed into a pulmonary infection. The patient therefore achieved a PFS of 25 months during anlotinib treatment, and an OS of up to 10 years from diagnosis (Figure 6). The patient’s treatment timeline is shown in Figure 7.
 |
Figure 7 Timeline of disease status and the treatment. |
Discussion
Here we discuss a patient with a rare case of a thymic small cell carcinoma that transformed into SCC, who had a long-term response to anlotinib as fourth-line treatment.
The initial pathologic type of this TC was small cell carcinoma, as determined at the time of diagnosis via needle biopsy. Transformation into SCC after third-line therapy was confirmed via needle and excisional biopsy. It is possible that the patient’s primary cancer was mixed small cell and SCC because of intratumoral heterogeneity or sampling artifacts. Guo et al8 reported a case of a recurrent breast cancer whose pathological type changed from invasive ductal carcinoma to metaplastic SCC. The authors’ hypothesis was that the tumor’s transformation into breast SCC was likely to be the result of the malignant transformation of breast cancer stem/progenitor cells. Histologic transformation from adenocarcinoma to small cell carcinoma9,10 and SCC11–14 in epidermal growth factor receptor (EGFR)-mutant and anaplastic lymphoma kinase (ALK)-rearranged lung cancer patients has been identified as a potential mechanism of resistance to EGFR/ALK inhibition. Although the related mechanism by which small cell carcinoma transforms into SCC has not yet been reported, possible mechanisms for such a histologic transformation include pluripotent cancer stem cell differentiation, intratumor heterogeneity15,16 and early clonal evolution.17 Further studies are therefore crucial to fully identify the mechanism of tumor pathologic transformation.
The patient had completed third-line treatment when his pathologic transformation occurred. A recent study reported that the median PFS of patients with metastatic or unresectable TC who were treated with first-line chemotherapy was 8.4 months.18 Significantly, in our case, the patient still achieved a PFS of 2 years after fourth-line anlotinib treatment despite the tumor’s pathologic transformation. Anlotinib not only effectively inhibited the migration and proliferation of capillary-like tubes adjusted by VEGFR in endothelial cells, but also inhibited tumor cell proliferation.19 In clinical trials, anlotinib was more effective than a placebo as salvage therapy for patients with advanced NSCLC, SCLC and soft tissue sarcoma.20,21 It has been reported that VEGFR-1 and VEGFR-2 are overexpressed in TC, which suggests that angiogenesis inhibitors might be a valid treatment strategy.22 However, no study of anlotinib in TC has been published. Clinical trials are necessary to confirm the efficacy of anlotinib in the treatment of TC.
Conclusion
In summary, this is the first report of a histologic transformation of TC from small cell carcinoma to SCC. The patient achieved exceptional PFS and OS with anlotinib as fourth-line treatment, which suggests that anlotinib may be a promising strategy for treating advanced TC with a transformed pathologic type. This potential benefit should be evaluated in future prospective studies.
Abbreviations
TC, Thymic carcinoma; SCC, squamous cell carcinoma; PFS, progression-free survival; OS, overall survival; TKI, tyrosine kinase inhibitor; NSCLC, non-small-cell lung cancer; SCLC, small cell lung cancer; VEGFR, vascular endothelial growth factor receptor; SD, stable disease; CT, computerized tomography; PR, partial response; PD, progress disease; ECOG PS, Eastern Cooperative Oncology Group performance status; pro-BNP, pro-B-type natriuretic peptide.
Ethics Statement
Written informed consent was obtained from the son of patient for publication of this case report and any accompanying images. The study is exempt from ethics committee approval since treatment decisions were based on clinical reasoning.
Data Sharing Statement
The data are available from the corresponding authors upon reasonable request.
Acknowledgments
Wenru Qin and Bing Zou are co-first authors for this study.
Funding
This study was supported by the grants from the Natural Science Foundation of Shandong Province (Grant No. ZR2019LZL012).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol. 2016;99:332–350. doi:10.1016/j.critrevonc.2016.01.012
2. Radovich M, Pickering CR, Felau I, et al. The integrated genomic landscape of thymic epithelial tumors. Cancer Cell. 2018;33(2):244–258.e210. doi:10.1016/j.ccell.2018.01.003
3. Lausi PO, Refai M, Filosso PL, et al. Thymic neuroendocrine tumors. Thorac Surg Clin. 2014;24(3):327–332. doi:10.1016/j.thorsurg.2014.05.007
4. Okuma Y, Saito M, Hosomi Y, Sakuyama T, Okamura T. Key components of chemotherapy for thymic malignancies: a systematic review and pooled analysis for anthracycline-, carboplatin- or cisplatin-based chemotherapy. J Cancer Res Clin Oncol. 2015;141(2):323–331. doi:10.1007/s00432-014-1800-6
5. Wei ML, Kang D, Gu L, Qiu M, Zhengyin L, Mu Y, Chemotherapy for thymic carcinoma and advanced thymoma in adults. Cochrane Database Syst Rev. 2013;8:CD008588. doi:10.1002/14651858.CD008588.pub2
6. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi:10.1186/s13045-018-0664-7
7. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised Phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661. doi:10.1038/bjc.2017.478
8. Guo T, Chen Z, Xu J, Zhang Y. Change of pathological type to metaplastic squamous cell carcinoma of the breast during disease recurrence: case report and literature review. Front Oncol. 2020;10:32. doi:10.3389/fonc.2020.00032
9. Liu Y. Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: a case report and literatures review. Cancer Biol Ther. 2018;19(6):445–449. doi:10.1080/15384047.2018.1435222
10. Dorantes-Heredia R, Ruiz-Morales JM, Cano-García F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl Lung Cancer Res. 2016;5(4):401–412. doi:10.21037/tlcr.2016.07.10
11. Izumi H, Yamasaki A, Ueda Y, et al. Squamous cell carcinoma transformation from EGFR-mutated lung adenocarcinoma: a case report and literature review. Clin Lung Cancer. 2018;19(1):e63–e66. doi:10.1016/j.cllc.2017.10.005
12. Kuiper JL, Ronden MI, Becker A, et al. Transformation to a squamous cell carcinoma phenotype of an EGFR-mutated NSCLC patient after treatment with an EGFR-tyrosine kinase inhibitor. J Clin Pathol. 2015;68(4):320–321. doi:10.1136/jclinpath-2015-202866
13. Kaiho T, Nakajima T, Iwasawa S, Yonemori Y, Yoshino I. ALK rearrangement adenocarcinoma with histological transformation to squamous cell carcinoma resistant to alectinib and ceritinib. Onco Targets Ther. 2020;13:1557–1560. doi:10.2147/ott.S236706
14. Gong J, Gregg JP, Ma W, et al. Squamous cell transformation of primary lung adenocarcinoma in a patient with EML4-ALK fusion variant 5 refractory to ALK inhibitors. J Natl Compr Canc Netw. 2019;17(4):297–301. doi:10.6004/jnccn.2019.7291
15. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. doi:10.1056/NEJMoa1113205
16. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. doi:10.1038/nrc2499
17. Hakozaki T, Kitazono M, Takamori M, Kiriu T. Combined small and squamous transformation in EGFR-mutated lung adenocarcinoma. Intern Med. 2020;59(10):1291–1294. doi:10.2169/internalmedicine.3542-19
18. Fukuda A, Okuma Y, Hakozaki T, et al. Cisplatin and irinotecan as first-line chemotherapy for previously untreated metastatic thymic carcinoma: updated analysis. Front Oncol. 2021;11:779700. doi:10.3389/fonc.2021.779700
19. Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi:10.1111/cas.13536
20. Cheng Y, Han B, Li K, et al. Effect of anlotinib as a third- or further-line therapy in advanced non-small cell lung cancer patients with different histologic types: subgroup analysis in the ALTER0303 trial. Cancer Med. 2020;9(8):2621–2630. doi:10.1002/cam4.2913
21. Cheng Y, Wang Q, Li K, et al. Anlotinib vs placebo as third- or further-line treatment for patients with small cell lung cancer: a randomised, double-blind, placebo-controlled Phase 2 study. Br J Cancer. 2021;125(3):366–371. doi:10.1038/s41416-021-01356-3
22. Cimpean AM, Raica M, Encica S, Cornea R, Bocan V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat. 2008;190(3):238–245. doi:10.1016/j.aanat.2007.05.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.