Back to Journals » Clinical Ophthalmology » Volume 18
Transepithelial Accelerated Crosslinking for Progressive Keratoconus: A Critical Analysis of Medium-Term Treatment Outcomes
Authors Vilares-Morgado R , Ferreira AM , Cunha AM , Moreira R, Torrão L , Neves-Cardoso P , Pinheiro-Costa J
Received 2 December 2023
Accepted for publication 24 January 2024
Published 8 February 2024 Volume 2024:18 Pages 393—407
DOI https://doi.org/10.2147/OPTH.S450916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Rodrigo Vilares-Morgado,1,2 Ana Margarida Ferreira,1,2 Ana Maria Cunha,1,2 Raúl Moreira,1,2 Luís Torrão,1,2 Pedro Neves-Cardoso,1,2 João Pinheiro-Costa1– 3
1Department of Ophthalmology, Centro Hospitalar Universitário de São João, Porto, Portugal; 2Department of Surgery and Physiology, Faculty of Medicine of Porto University, Porto, Portugal; 3Department of Biomedicine, Faculty of Medicine of Porto University, Porto, Portugal
Correspondence: Rodrigo Vilares-Morgado, Department of Ophthalmology, Centro Hospitalar Universitário de São João, Alameda Prof. Hernâni Monteiro, Porto, 4202-451, Portugal, Tel +351 914 471 067, Fax +351 225 512 100, Email [email protected]
Purpose: To report the 4-year outcomes of transepithelial accelerated corneal collagen crosslinking (TE-ACXL) in the treatment of eyes with progressive keratoconus (KC).
Methods: Eyes of patients who underwent TE-ACXL (6mW/cm2 for 15 minutes) for progressive KC and presented 48 months of follow-up were included. Corrected distance visual acuity (CDVA), keratometry measurements (Kmax, maximum keratometry, Kmean, mean keratometry and Astg, corneal astigmatism), thinnest corneal thickness (PachyMin), and topographic, and tomographic indices (specifically the posterior radius of curvature from the 3.0 mm centered on the thinnest point of the cornea (PRC), and the D-index) were analysed preoperatively and every 12 months after TE-ACXL, up to 48 months. Progression after TE-ACXL was considered when eyes presented ≥ 1 criteria: (1) increase of ≥ 1D in Kmax or increase of ≥ 0.75D in Kmean or increase of ≥ 1D in Astg; (2) reduction of ≥ 0.085 mm in PRC; (3) decrease ≥ 5% in PachyMin.
Results: 41 eyes from 30 patients were included, with a mean age at crosslinking of 20.90± 4.69 years. There was a significant increase in Kmean (+0.64± 1.04 D, p< 0.001; +0.98 ± 1.49 D, p< 0.001; +1.27± 2.01 D, p< 0.001; +1.13± 2.00 D, p=0.006) and a significant decrease in PRC throughout follow-up (− 0.12± 0.22, p=0.002; − 0.15± 0.24, p< 0.001; − 0.17± 0.43, p=0.021; − 0.16± 0.43, p=0.027). PachyMin decreased significantly at 36 and 48 months (− 8.50± 15.93 μm, p=0.004; − 7.82± 18.37, p=0.033). According to our progression criteria, there was a major progression rate throughout follow-up (57.1%, 61.1%, 58.8%, and 67.9%, respectively). Surgery and follow-up were uneventful in all subjects. Eleven eyes (26.8%) required further procedures, ≥ 36 months after the initial TE-ACXL, due to persistent progressive disease.
Conclusion: TE-ACXL proved to be a safe therapeutic option for progressive KC. However, its efficacy is deemed unsatisfactory, as a notable proportion of affected eyes may continue to advance within a 4-year timeframe, necessitating additional procedures to halt the disease’s course.
Keywords: progressive keratoconus, trans-epithelial corneal crosslinking, epi-on crosslinking, keratoconus progression
Introduction
Keratoconus (KC) is a progressive, bilateral, and typically asymmetric corneal disease associated with both genetic factors (family history) and environmental factors (eye rubbing and nocturnal ocular compression).1–5 Nowadays, it is no longer considered non-inflammatory, as many pro-inflammatory factors have been linked to its etiopathogenesis.6–9 Typically, it presents with paracentral corneal thinning and steepening, leading to progressive irregular astigmatism.4,5 Epidemiological indices of KC conspicuously vary around the world, with prevalence rates ranging from as low as 0.2 to as high as 4790 per 100000 persons. Incidence figures, though available in a limited number of studies, also exhibit wide dispersion, ranging from as low as 1.5 to as high as 25 per 100000 persons per year. Prevalence rates have typically been higher in India, Iran, and the Middle East, while incidence rates are elevated among individuals of Asian ancestry.4,10,11 Currently, corneal tomography and biomechanics are the most useful tools for early diagnosis,12 while topographic, tomographic, and pachymetric parameters are usually combined to monitor disease progression.13,14 The “Global Consensus on Keratoconus and Ectatic Diseases” defined ectasia progression as a consistent change in ≥2 of the following parameters: (1) steepening of the anterior corneal surface; (2) steepening of the posterior corneal surface; (3) thinning and/or an increase in the rate of corneal thickness change from the periphery to the thinnest point. Furthermore, the consensus panel agreed that a change in both uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) is not required to document progression, and that while specific quantitative data are lacking to further define progression, these data would likely be machine/technology specific.15 Although not validated, some parameters are frequently used as KC progression criteria in the literature, in studies that use the Pentacam® tomography system (OCULUS Optikgeräte GmbH, Wetzlar, Germany).13,16–21 However, KC progression after corneal collagen crosslinking (CXL) is usually determined through longitudinal evaluation of specific topographic or tomographic parameters, such as maximum keratometry (Kmax), mean keratometry (Kmean), corneal astigmatism (Astg), posterior radius of curvature from the 3.0 mm centered on the thinnest point (PRC), or minimum pachymetry (PachyMin).22,23 Some studies also use longitudinal CDVA evaluation to determine KC progression after CXL.24,25
Keratoconus treatment has improved significantly in recent years, with the development of new treatment modalities, for earlier stages of the disease, decreasing the disease progression and the need of keratoplasty, improving patients’ vision-related quality of life.26 CXL is one of these effective treatment modalities. It is a safe and minimally invasive intervention. Its primary objective is to improve the corneal biomechanical properties and decrease disease progression.27,28 The Dresden Protocol, first described by Wollensak et al29 is considered the standard (or conventional, C-CXL) procedure, as it was the first established CXL protocol. It involves epithelium removal to facilitate stromal riboflavin impregnation, followed by application of a 0.10% riboflavin 5-phosphate solution for 30 minutes, and exposure to UVA radiation (365 nm, 3 mW/cm2) for 30 minutes, resulting in a total energy dose of 5.4 J/cm2.29 Nevertheless, numerous studies on this technique reported low rates of significant complications, including infectious keratitis, sub-epithelial haze, sterile corneal infiltrates, corneal scarring, postoperative pain, and delayed visual rehabilitation. The primary risk factor appeared to be the 8 to 9 mm of corneal de-epithelialization required for the Dresden protocol (epi-off).28,30–32 In response to these reported complications, alternative options such as partial de-epithelialization,33–35 mechanical disruption without debridement,36,37 and direct application of trans-epithelial techniques (TE-CXL or epi-on) were developed.38,39 Subsequently, accelerated protocols (A-CXL) emerged, grounded in the Bunsen-Roscoe law of photochemical reciprocity, thereby maintaining the total dose of radiation energy. Finally, a combination of the trans-epithelial approach and the accelerated protocol gave rise to transepithelial accelerated crosslinking (TE-ACXL).28,32,40,41 Nonetheless, the efficacy of TE-ACXL may be constrained by several factors. The primary challenge involves the penetration of riboflavin, a large water-soluble molecule, through the corneal epithelium. As the corneal epithelium is inherently lipophilic, it forms a barrier that impedes the easy passage of such molecules. To enhance riboflavin diffusion, alternative approaches include adding substances that can loosen tight junctions of corneal epithelial cells, such as ethylenediaminetetraacetic acid (EDTA). A buffering agent, trometamol (Tris), is commonly incorporated to maintain a stable pH in the riboflavin solution, thereby improving the efficacy of EDTA. Additionally, benzalkonium chloride (BAC) may be utilized due to its surfactant properties, which affect the integrity of tight junctions between corneal epithelial cells, leading to increased permeability. Furthermore, TE-ACXL faces the challenge of potential partial absorption of UVA by the corneal epithelium, limiting oxygen diffusion to the corneal stroma. Another consideration is the assumption underlying TE-ACXL, suggesting that increasing irradiation intensity over a reduced period has an equivalent biological effect in the cornea compared to longer exposure periods with lower energy. However, this premise may not necessarily hold true.38,42 Hence, comprehensive, long-term studies on the efficacy and safety of TE-ACXL are necessary to fully evaluate the effectiveness of this technique in managing progressive KC and to identify the specific patient populations that will derive the greatest benefits from its application. The purpose of the present study is to evaluate the visual, topographic, tomographic and pachymetric 4-year outcomes, as well as associated complications, in patients with progressive keratoconus who underwent a single treatment of TE-ACXL.
Methods
Study Design
Retrospective, single-center, observational, cohort study of eyes with progressive keratoconus who underwent TE-ACXL (6 mW/cm² for 15 minutes), from January 2016 to July 2019, and were followed for at least 48 months afterwards in the Department of Ophthalmology of Centro Hospitalar Universitário de São João (Porto, Portugal). The study was approved by the Institutional Ethics Review Board of the institution. The protocol conformed with the canons of the Declaration of Helsinki for research involving human participants, as well as the European Union’s General Data Protection Regulation. Informed consent was waived in view of the retrospective nature of the study.
The study’s inclusion criteria were: age between 18 and 32, pachymetry at its thinnest point (PachyMin) ≥ 380 µm, and documented progression of keratoconus in at least two Pentacam HR® (OCULUS Optikgeräte GmbH, Wetzlar, Germany) exams, with a minimum interval of 12 months between the two exams before the procedure. Progression after TE-ACXL was considered when eyes presented ≥1 criteria: (1) increase of ≥1D in Kmax or increase of ≥0.75D in Kmean or increase of ≥1D in Astg; (2) decrease of ≥0.085 mm in PRC; (3) decrease ≥5% in PachyMin. These criteria were similar to previous published criteria for assessing KC progression after CXL.22–25 Exclusion criteria were previous history of cornea surgery, presence of corneal leucoma, severe dry eye, compromised corneal epithelial healing, active infectious keratitis, connective tissue disease, pregnancy or lactation.
Clinical, visual, corneal topographic, tomographic, and pachymetric parameters were evaluated preoperatively and every 12 months postoperatively up to 48 months. CDVA was recorded via a Snellen chart and converted to logarithm of minimal angle of resolution (logMAR) units. Kmax, Kmean, Astg, PRC, PachyMin, index of height decentration (IHD), index of vertical asymmetry (IVA), index of surface variance (ISV), keratoconus index (KI), Pentacam® topographical keratoconus classification (KC; Pentacam®-derived Amsler-Krumeich stages), and the Belin/Ambrósio D-index were determined using the Pentacam HR® (OCULUS Optikgeräte GmbH, Wetzlar, Germany). Disease progression was assessed every 12 months after TE-ACXL. Evidence of postoperative KC progression, according to the previous criteria, or need of other corneal procedures were interpreted as treatment failures.
Surgical Technique and Postoperative Care
All surgeries were performed in the operative room. Topical local anesthesia was used, with oxybuprocaine hydrochloride 4mg/L eyedrops. A special TE riboflavin magistral preparation, prepared by the hospital pharmacy, containing 0.25% riboflavin, EDTA, Tris, BAC, and 0.45% phosphate-buffered saline, was instilled in the cornea every 3 minutes for 30 minutes before UV-A irradiation. Corneal stromal saturation was confirmed through slit-lamp assessment of the anterior chamber flare. Afterwards, patients were exposed for 15 minutes to an UV-A light beam, with an intensity of 6mW/cm2, to achieve a total energy dose of 5,4J/cm2. During this period, to avoid corneal dehydration, both riboflavin magistral preparation and sterile balance sodium solution (BSS® Sterile Irrigating Solution, Alcon®) eyedrops were administered alternatively every 3 minutes. In the postoperative period, topical antibiotic eye drops (ofloxacin 0.30%, Floxedol®, EDOL®) were prescribed for a week, steroids eye drops (fluorometholone 0.10%, FML®, Liquifilm®) were prescribed for 2 weeks and nonpreserved sodium hyaluronate 0.15% eyedrops (HYABAK®, Théa®) were prescribed as needed. Patients were reviewed the following day, at 3 months, and every 6 months thereafter.
Statistical Analysis
Categorical variables were summarized as counts and proportions. Continuous variables were described as mean and standard deviation (or median and interquartile range, when they did not present a normal distribution). The postoperative variation in CDVA, topographic, tomographic, and pachymetric parameters was obtained by subtracting their preoperative values from the subsequent measurements at each follow-up visit (positive delta values represented an increase in that parameter, whilst negative delta values depicted a decrease). Preoperative and postoperative outcomes were compared with paired t-tests or the Wilcoxon test, while multiple related samples were compared via within-subjects ANOVA test. Comparisons between the group of eyes that had progression throughout our study and those that did not present progression were conducted with independent samples t-tests, Mann–Whitney U, Chi-square, and Fisher’s exact tests, as suitable The same methodology was used to compare eyes that required further corneal procedures and eyes that did not require further corneal procedures. A p-value lower than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics software (version 29.0 for Mac OS; SPSS Inc., Chicago, IL, USA).
Results
The present study included 41 eyes from 30 patients, 22 male and 8 female patients. Bilateral TE-ACXL was performed in 11 patients (9 male and 2 female patients). Mean baseline age was 20.90 ± 4.69 years (ranging from 18 to 32). Table 1 summarizes baseline parameters. KC preoperative distribution is depicted in Figure 1. 18 out of 41 eyes presented grade 3 KC, while 6 eyes presented grade 2, 6 eyes presented grade 2.5, and 6 eyes presented grade 3.5 KC. All 41 eyes completed the 48-month follow-up, with no intraoperative or postoperative complications. However, 11 eyes from the original 41 eyes were excluded from the comparison between 48 months and baseline values because they were submitted to either an epithelial-off crosslinking (n=6), an intracorneal ring segment (n=4), or a corneal penetrating transplant (n=1), after 36 months of follow-up. These eyes presented either significant KC progression despite TE-ACXL, with anterior corneal steepening and corresponding visual deterioration, or significant deterioration in CDVA, which could not be corrected through contact lenses or glasses. There were no cases of corneal scar development or endothelial toxicity due to the TE-ACXL procedure. Table 2 summarizes the variation in CDVA, topographic, tomographic and pachymetric parameters, throughout follow-up.
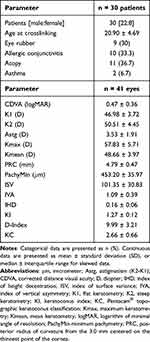 |
Table 1 Baseline Demographic, Clinical, Visual, Corneal Topographic, Tomographic and Pachymetric Characteristics of Patients Undergoing Trans-Epithelial Accelerated Crosslinking |
 |
Table 2 Mean Changes in Visual, Corneal Tomographic, Topographic and Pachymetric Parameters Between 12, 24, 36, and 48 Months and Baseline Values |
 |
Figure 1 Distribution of baseline Pentacam® topographical keratoconus classification (KC; Pentacam®-derived Amsler-Krumeich stages). |
Visual Acuity
Figure 2 illustrates CDVA evolution over time. The mean baseline CDVA was 0.47 ± 0.36 logMAR units (which corresponds approximately to a Snellen visual acuity of 20/63). Mean variation was −0.10 ± 0.20 logMAR units at 12 months (p=0.045), −0.10 ± 0.33 logMAR units at 24 months (p=0.099), −0.07 ± 0.34 logMAR units at 36 months (p=0.226), and −0.17 ± 0.33 logMAR units at 48 months postoperatively (p=0.013). BCVA improved significantly at 12 and at 48 months. Final BCVA was 0.29 ± 0.30 logMAR units (which corresponds approximately to a Snellen visual acuity of 20/40).
 |
Figure 2 Corrected distance visual acuity (CDVA) in logMAR notation at baseline, 12, 24, 36, and 48 months after Trans-Epithelial Accelerated Crosslinking (TE-ACX). |
Topography and Topographic Indices
The mean preoperative Kmax was 57.83 ± 5.71 D. There were no significant variations in this parameter, except at 36 months (+1.08 ± 2.40D; p=0.013). At 48 months, Kmax increased by less than 1D (+0.61 ± 3.03; p=0.299). Average baseline Kmean was 48.66 ± 3.97 D. There was a significant increase in Kmean throughout follow-up [+0.64 ± 1.04 D at 12 months (p<0.001); +0.98 ± 1.49 D at 24 months (p<0.001); +1.27 ± 2.01 D at 36 months (p<0.001), and +1.13 ± 2.00 D at 48 months (p=0.006)]. There were no significant variations in corneal astigmatism throughout follow-up (Table 2). Figures 3 and 4 depict the evolution of Kmax and Kmean, respectively, throughout follow-up. There were no significant variations in ISV, IVA, IHD, or KI throughout follow-up.
 |
Figure 3 Maximum keratometry (Kmax) in diopters (D) at baseline, 12, 24, 36, and 48 months after Trans-Epithelial Accelerated Crosslinking (TE-ACX). |
 |
Figure 4 Mean keratometry (Kmean) in diopters (D) at baseline, 12, 24, 36, and 48 months after Trans-Epithelial Accelerated Crosslinking (TE-ACX). |
Tomography, Tomographic Indices, and Pachymetry
The mean baseline PachyMin and D-index values were 453.20 ± 35.97 µm and 9.99 ± 3.21 units, respectively. There was a significant decrease of PachyMin at 36 months (−8.50 ± 15.93 µm; p=0.004), and at 48 months (−7.82 ± 18.37; p=0.033). Figure 5 depicts the evolution of PachyMin throughout follow-up. Additionally, there was a significant increase in the D-index throughout follow-up [+0.51 ± 1.03 units at 12 months (p=0.007); +0.69 ± 1.25 units at 24 months (p=0.002); +1.02 ± 1.72 units at 36 months (p=0.002); +1.15 ± 1.64 units at 48 months (p<0.001)]. Regarding PRC, there was a significant decrease in this parameter throughout follow-up (−0.12 ± 0.22 at 12 months; p=0.002; −0.15 ± 0.24 at 24 months; p<0.001; −0.17 ± 0.43 at 36 months; p=0.021; −0.16 ± 0.43 at 48 months; p=0.027).
 |
Figure 5 Minimum pachymetry (PachyMin) in micrometres (μm) at baseline, 12, 24, 36, and 48 months after Trans-Epithelial Accelerated Crosslinking (TE-ACX). |
Progression and Need of Further Procedures
Table 3 discriminates the number of patients that presented at least one progression criteria at 12, 24, 36, and 48-month follow-up. There was an increase ≥ 1.0 D in Kmax in 25.7% (9/35) of the studied eyes at 12 months, 33.3% (12/36) at 24 months, 35.3% (12/34) at 36 months and 35.7% (10/28) at 48 months. Regarding Kmean, 42.9% (15/35) of the study eyes registered an increase ≥ 0.75 D at 12 months, 47.2% (17/36) at 24 months, 55.9% (19/34) at 36 months, and 42.9% (12/28) at 48 months. There was a reduction of at least 0.085 mm in PRC in 54.2% (19/35) of the study eyes at 12 months, 52.8% (19/36) at 24 months, 52.9% (18/34) at 36 months, and 53.6% (15/28) at 48 months. There was a decrease of ≥ 5% in PachyMin in 2.9% (1/35) of the study eyes at 12 months, 11.1% (4/36) at 24 months, 17.6% (6/34) at 36 months, and 13.8% (4/29) at 48 months.
 |
Table 3 Number and Proportion of Eyes Classified as Progressors According to the Criteria Defined in the Methods Section |
Twenty out of 35 eyes (57.1%) presented progression at 12 months, 22 out of 36 eyes (61.1%) presented progression at 24 months, 20 out of 34 eyes (58.8%) presented progression at 36 months, and 19 out of 28 eyes (67.9%) presented progression at 48 months. All 11 eyes that required further procedures after 36 months of follow-up presented progression at 12 months, at 24 months, and at 36 months.
The baseline parameters of the group that presented progression and the group that did not progress throughout follow-up were compared in Table 4. There were no significant differences in baseline age, CDVA, corneal Astg, Kmax, Kmean, PRC, PachyMin, and D-index. Additionally, the baseline characteristics of the group that required further corneal procedures and the group that did not require further procedures throughout follow-up were compared in Table 5. There were also no significant differences in baseline age, CDVA, corneal Astg, Kmax, Kmean, PRC, PachyMin, and D-index.
 |
Table 4 Baseline Parameters: Progressive Vs Non-Progressive Post TE-ACXL Keratoconus Cases |
 |
Table 5 Baseline Parameters: Eyes Requiring versus Eyes Not Requiring Additional Procedures |
Discussion
In our retrospective study, 41 eyes from 30 patients underwent TE-ACXL (with chemical enhancers for trans-epithelial riboflavin diffusion, and an UV-A light beam intensity of 6mW/h for 15 minutes to achieve a total dose intensity of 5,4J/cm2) due to progressive KC. These patients were followed for 48 months. Eleven eyes required additional procedures, 36 months or later after the initial TE-ACXL. There were no significant differences in baseline age, CDVA, corneal Astg, Kmax, Kmean, PRC, PachyMin, and D-index between eyes that required further corneal procedures and eyes that did not require further interventions. Our primary outcome was the detection of progression of the KC, despite TE-ACXL. After undergoing TE-ACXL, there was a significant improvement in CDVA at 12 and at 48 months. However, there was also a significant increase in Kmean and D-index throughout follow-up, and a significant decrease in PRC and PachyMin (only at 36 and 48 months for the latter). Furthermore, 20/35 eyes (57.1%), 22/36 eyes (61.1%), 20/34 eyes (58.8%), and 19/28 eyes (67.9%) presented progression at 12, 24, 36, and 48 months. All 11 eyes that required further procedures after 36 months of follow-up presented progression at 12 months, at 24 months, and at 36 months. There were no intraoperative or postoperative complications regarding the procedure. Therefore, even though there was a significant improvement in CDVA, most eyes still progressed after undergoing TE-ACXL, which raises questions about its effectiveness in halting progression in KC, especially considering that there are newer techniques to improve CXL’s efficacy and effectiveness.
Corneal CXL has been widely used for several years, with multiple prospective and relevant studies demonstrating its safety and effectiveness in progressive keratoconus.29,43 This treatment modality specifically targets the pathophysiology of the disease, decreasing or even ceasing its progression.2,28,44,45 In order to reduce associated complications, several modifications to the conventional Dresden protocol have been proposed, with overall favourable results.46 One of this modified protocols, the TE-ACXL protocol, has been thoroughly investigated since its initial development, reflecting the significant interest in this method, which naturally has a lower rate of associated complications. Most studies acknowledge its safety and short-term efficacy, albeit most of them agree that additional long-term studies are required.47–57
A significant number of non-randomized comparative trials (NRCTs), randomized clinical trials (RCTs), systematic reviews and meta-analyses have been published on the comparison between transepithelial and epithelium-off crosslinking.58–60 Wen et al performed a systematic review and meta-analysis in 2018 which included 8 comparative studies (5 randomized controlled trials and 3 non-randomized comparative trials), with a follow-up of 12 months.59 In their review, there were no significant differences in the 12-month variation of UDVA and CDVA after epi-off or TE-CXL. More recently, in 2021, D’Oria et al also published a systematic review and meta-analysis on this comparison, which included a total of 15 studies (9 RCTs and 6 were NRCTs), with a follow-up of 12 to 36 months after CXL.58 In their study, the CDVA improved significantly more in eyes that underwent epi-off CXL, but there were no significant differences in postoperative UDVA variation. Buzzonetti et al also reported a significant improvement in CDVA from baseline to 3 years in pediatric patients with progressive KC who underwent epi-off CXL, while patients who underwent TE-CXL presented no significant differences in CDVA throughout follow-up.61 Regarding topographic postoperative differences between epi-on and epi-off CXL, Wen et al reported that the reduction in Kmean was significantly higher with epi-off CXL when compared to the TE-CXL. When the TE-CXL groups were separated into subgroups of those using chemical additives and those using iontophoresis, the differences were even more apparent. TE-CXL using chemical additives to enhance riboflavin absorption and epi-off CXL presented a similar decrease in Kmean, while TE-CXL using iontophoresis presented a significantly lower reduction in Kmean when compared to epi-off CXL.59 Henriquez et al also reported a significantly lower flattening in the Kmean in patients who undergo TE-ACXL (−0.09 D; p=0.33 vs −3.18 D; p<0.001 in the epi-off CXL). Their study presented a 5-year follow-up.22 D’oria et al reported a significantly higher flattening of Kmax with epi-off CXL.58 As for pachymetry changes, D’Oria et al reported no significant differences in central corneal thickness (CCT) changes.58 As for adverse events, the postoperative discomfort and pain is significantly lower in patients who undergo TE-CXL, as expected.23 Furthermore, in the review by Shalchi et al, which included 51 NRCTs of epi-off (45) and TE-CXL (6), haze, scarring and microbial keratitis were only reported in epi-off CXL studies, with none were reported in TE-CXL studies.62 D’Oria et al reported that TE-CXL protocols were significantly less prompt to have risks of delay in epithelial healing and persistent stromal haze, while there were no significant differences for endothelial cell density (ECD) variation, development of sterile infiltrates, or development of microbial keratitis.58 Finally, Wen et al did not compare re-treatment rates or progression rates in both protocols, while D’Oria et al did not find significant differences in the progression rates in both CXL protocols.58,59
There is an extremely relevant issue with TE-CXL, which is the wide variety of TE-CXL protocols and approaches currently used. There are many methodologies to increase corneal epithelial permeability to riboflavin (different chemical enhancers, iontophoresis), and multiple UVA irradiation protocols (18 mW/cm2 irradiation for 5 minutes, 10 mW/cm2 irradiation for 9 minutes, 6 mW/cm2 for 15 minutes, 3 mW/cm2 irradiation for 30 minutes, or even used pulsed protocols of irradiation of 45 mW/cm2 for 5:20 min), though the total irradiation dose is generally similar (5.4 J/cm2).58 Naturally, with this lack of currently agreed upon TE-CXL protocol, drawing conclusions about the non-inferiority of TE-CXL when compared to epi-off CXL is difficult. Furthermore, there are still no RCTs that compare the long-term outcomes (at least 5 years) of TE-CXL and epi-off CXL.
We compared our results to other large prospective studies with a long follow-up (≥ 36 months) that used TE-ACXL: Al Fayez et al, who evaluated 36 patients with a baseline age of 24.8 ± 4.2 years, Kmean of 48.2 ± 3.6 D, and CDVA of 0.2 ± 0.2 logMAR units;23 and, Henriquez et al, who evaluated 32 patients with a baseline age of 13.2 ± 2.6 years, Kmean 47.32 ± 2.78 D, and CDA of 0.19 ± 0.17 logMAR units).22 In our study, we found a significant improvement in CDVA of 0.10 ± 0.29 logMAR units and 0.17 ± 0.33 logMAR units in BCVA at 12 and 48 months, respectively. Henriquez et al (who used a transepithelial riboflavin solution of 0.25% riboflavin, 1.0% phosphate hydroxypropyl methylcellulose, and 0.007% BAC, and irradiated the eyes for 5 minutes with an intensity of 18 mW/cm2) reported a significant improvement in CDVA of 0.09 ± 0.17 logMAR units and 0.06 ± 0.19 logMAR units at 12 and 60 months postoperatively.22 Al Fayez et al (who used a solution of tetracaine 1% with benzalkonium chloride 0.02% every 2 minutes, for 30 minutes, before starting no-dextran riboflavin drops, and irradiated the eyes for 30 minutes with an intensity of 3 mW/cm2) reported a decrease in CDVA of 0.02 and 0.06 logMAR units at 12 and 36 months, respectively.23 In our study, there were no significant differences in Kmax throughout follow-up, while Kmean increased significantly throughout follow-up (+0.64 ± 1.04 and +1.13 ± 2.00 at 12 and 48 months, respectively). Al Fayez et al reported a significant increase in Kmax of around +0.75 and +1.00D at 24 and 36 months, respectively, while Henriquez et al did not find significant differences in Kmax or Kmean at 12 or 60 months.22,23 In our study, PachyMin decreased significantly at 36 and 48 months (−8.50 ±15.93 and −7.83 ± 18.37 μm), while Henriquez et al reported no significant changes at 12 months and a significant increase of 7.33 ± 11.78 μm at 60 months.22 In our study, there was a significant decrease in the PRC and a significant increase in the D-index throughout follow-up (from 12 to 48 months). None of the two aforementioned studies evaluated these parameters. Finally, regarding progression, we report a progression rate of 57.1%, 61.1%, 58.8%, and 67.9%, at 12, 24, 36 and 48 months respectively, while Henriquez et al reported a progression rate of 9.37% (3/32 eyes) at 60 months (progression was defined as an increase >1 D in steep keratometry assessed between 2 tests with at least 2 months apart), and Al Fayez et al reported a progression rate of 55% at 36 months (progression was defined as an increase in Kmax >1.00 D compared with the preoperative baseline examination).22,23 Our progression rate if we only considered progression as an increase >1D in Kmax would be 25.7% (9/35), 33.3% (12/36), 35.3% (12/34), and 35.7% (10/28), at 12, 24, 36, and 48 months, respectively. Comparing our progression rate to the other 2 prospective RCTs, we had a lower progression rate than Al Fayez et al and a higher progression rate than Hernandez et al. Our study sample (baseline age of 20.90 ± 4.69 years, Kmean of 48.66 ± 3.97 D, and CDVA of 0.47 ± 0.36 logMAR units) was more similar to the one from Al Fayez et al than to the one from Henriquez et al.22,23
Age at diagnosis is an important risk factor for progression. In fact, the eyes of younger patients tend to progress more rapidly than their adult counterparts.20,63,64 In fact, progression in pediatric patients with KC occurs in approximately 80% of the cases.65 Nonetheless, in our study, isolated TE-ACXL halted KC progression in around 40% of the eyes at 36 months and 30% of the eyes at 48 months. Therefore, this procedure is still relevant in progressive KC management, especially considering its excellent safety profile and low risk of adverse events.58,59 It seems that epi-off CXL might be more aggressive in improving CDVA and flattening keratometry readings when compared with TE-ACXL.58,59 This fact should be taken into account when deciding between epi-off CXL and TE-ACXL for an individual patient. For instance, cases with higher keratometry readings and worse CDVA will probably benefit more from an epi-off CXL, while cases with mild KC, lower keratometry values, and reasonable CDVA can probably be safely managed with TE-ACXL.
Our study limitations include its retrospective and non-randomized nature, the lack of a comparison epi-off CXL group, and the lack of corneal endothelial cell density longitudinal evaluation. However, we believe that we have a relevant sample size of eyes that underwent TE-ACXL (when compared to other relevant studies that were published on this technique) and that our follow-up of 48 months allows for a reasonable evaluation of long-term outcomes of TE-ACXL. Nevertheless, there is still a significant need for RCTs that define the best TE-CXL protocol and that compare long-term outcomes of epi-off and TE-CXL. Only then will we be able to clearly define which cases should be treated with epi-off, and which cases should be treated with TE-CXL.
In conclusion, our results demonstrate that TE-ACXL was a safe treatment for eyes with progressive KC, increasing CDVA 4 years after the initial procedure. However, a significant proportion of eyes with progressive KC will still likely progress after being treated with TE-ACXL, requiring further procedures to halt disease progression.
Abbreviations
A-CXL, accelerated corneal collagen crosslinking; CDVA, Corrected distance visual acuity; CXL, Crosslinking; C-CXL, Conventional crosslinking; LogMAR, logarithm of minimal angle of resolution; IHD, Index of height decentration; ISV, Index of surface variance; IVA, Index of vertical asymmetry; K1, Flat Keratometry; K2, Steep Keratometry; KC, Keratoconus classification; Kmax, Maximum keratometry; Km, Mean keratometry; SD, Standard-deviation; TE-ACXL, Transepithelial accelerated corneal collagen crosslinking; TE-CXL, transepithelial corneal collagen crosslinking; UVA, Ultraviolet A.
Data Sharing Statement
Access to any supplemental information such as the study protocol or anonymized data can be available from the Corresponding Author upon reasonable request.
Ethics/Ethical Approval
The study was approved by the Institutional Ethics Review Board of Centro Hospitalar Universitário de São João, Porto, Portugal (certificate approval number CES284/2021). The protocol conformed with the canons of the Declaration of Helsinki for research involving human participants, as well as the European Union’s General Data Protection Regulation. Informed consent was waived in view of the retrospective nature of the study. This article was redacted according to the recommendations of The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement.
Acknowledgments
Only the named authors have collaborated in the writing of this paper.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that they have no financial ties to this study. No funding or sponsors were undertaken in the preparation of the manuscript.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
1. Mas Tur V, MacGregor C, Jayaswal R, et al. A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62(6):770–783. doi:10.1016/j.survophthal.2017.06.009
2. Davidson AE, Hayes S, Hardcastle AJ, et al. The pathogenesis of keratoconus. Eye. 2014;28(2):189–195. doi:10.1038/eye.2013.278
3. Nakagawa T, Maeda N, Kosaki R, et al. Higher-order aberrations due to the posterior corneal surface in patients with keratoconus. Invest Ophthalmol Vis Sci. 2009;50(6):2660–2665. doi:10.1167/iovs.08-2754
4. Rabinowitz YS, Galvis V, Tello A, et al. Genetics vs chronic corneal mechanical trauma in the etiology of keratoconus. Exp Eye Res. 2021;202:108328. doi:10.1016/j.exer.2020.108328
5. Bui AD, Truong A, Pasricha ND, et al. Keratoconus Diagnosis and Treatment: recent Advances and Future Directions. Clin Ophthalmol. 2023;17:2705–2718. doi:10.2147/OPTH.S392665
6. Galvis V, Tello A, Barrera R, et al. Inflammation in Keratoconus. Cornea. 2015;34(8):e22–3. doi:10.1097/ICO.0000000000000499
7. Elbeyli A, Kurtul BE. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, and platelet-to-lymphocyte ratio levels are associated with keratoconus. Indian J Ophthalmol. 2021;69(7):1725–1729. doi:10.4103/ijo.IJO_3011_20
8. Loh IP, Sherwin T. Is Keratoconus an Inflammatory Disease? The Implication of Inflammatory Pathways. Ocul Immunol Inflamm. 2022;30(1):246–255. doi:10.1080/09273948.2020.1780271
9. Pinheiro-Costa J, Lima Fontes M, Luís C, et al. Serum inflammatory biomarkers are associated with increased choroidal thickness in keratoconus. Sci Rep. 2023;13(1):10862. doi:10.1038/s41598-023-37472-8
10. Santodomingo-Rubido J, Carracedo G, Suzaki A, et al. Keratoconus: an updated review. Cont Lens Anterior Eye. 2022;45(3):101559. doi:10.1016/j.clae.2021.101559
11. Salman A, Darwish T, Ghabra M, et al. Prevalence of Keratoconus in a Population-Based Study in Syria. J Ophthalmol. 2022;2022:6064533. doi:10.1155/2022/6064533
12. Ambrósio R, Lopes BT, Faria-Correia F, et al. Integration of Scheimpflug-Based Corneal Tomography and Biomechanical Assessments for Enhancing Ectasia Detection. J Refract Surg. 2017;33(7):434–443. doi:10.3928/1081597X-20170426-02
13. Duncan JK, Belin MW, Borgstrom M. Assessing progression of keratoconus: novel tomographic determinants. Eye Vis (Lond). 2016;3(1):6. doi:10.1186/s40662-016-0038-6
14. Jay JM, Akilesh G, Hans RV, et al. Progression of keratoconus in children and adolescents. Br J Ophthalmol. 2023;107(2):176. doi:10.1136/bjophthalmol-2020-316481
15. Gomes JA, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi:10.1097/ICO.0000000000000408
16. Ribeiro M, Barbosa C, Correia P, et al. Best Fit Sphere Back and Adjusted Maximum Elevation of Corneal Back Surface as Novel Predictors of Keratoconus Progression. Clin Ophthalmol. 2022;16:4239–4248. doi:10.2147/OPTH.S388614
17. Cunha AM, Correia PJ, Alves H, et al. Keratoconus enlargement as a predictor of keratoconus progression. Sci Rep. 2021;11(1):21079. doi:10.1038/s41598-021-00649-0
18. Jiménez-García M, Kreps EO, Nd S, et al. Determining the Most Suitable Tomography-Based Parameters to Describe Progression in Keratoconus. The Retrospective Digital Computer Analysis of Keratoconus Evolution Project. Eye Contact Lens. 2021;47(9):486–493. doi:10.1097/ICL.0000000000000800
19. Gustafsson I, Bergström A, Cardiakides A, et al. The Interday Repeatability of Parameters for the Assessment of Progressive Disease in Subjects With Less Advanced Keratoconus. Am J Ophthalmol. 2021;225:38–46. doi:10.1016/j.ajo.2020.12.028
20. Martínez-Abad A, Piñero DP. New perspectives on the detection and progression of keratoconus. J Cataract Refract Surg. 2017;43(9):1213–1227. doi:10.1016/j.jcrs.2017.07.021
21. Shajari M, Steinwender G, Herrmann K, et al. Evaluation of keratoconus progression. Br J Ophthalmol. 2019;103(4):551–557. doi:10.1136/bjophthalmol-2017-311651
22. Henriquez MA, Hernandez-Sahagun G, Camargo J, et al. Accelerated Epi-On Versus Standard Epi-Off Corneal Collagen Cross-Linking for Progressive Keratoconus in Pediatric Patients: five Years of Follow-Up. Cornea. 2020;39(12):1493–1498. doi:10.1097/ICO.0000000000002463
23. Al Fayez MF, Alfayez S, Alfayez Y. Transepithelial Versus Epithelium-Off Corneal Collagen Cross-Linking for Progressive Keratoconus: a Prospective Randomized Controlled Trial. Cornea. 2015;34(Suppl 10):S53–6. doi:10.1097/ICO.0000000000000547
24. Salman A, Ali A, Rafea S, et al. Long-term visual, anterior and posterior corneal changes after crosslinking for progressive keratoconus. Eur J Ophthalmol. 2022;32(1):50–58. doi:10.1177/11206721211052878
25. Salman AM, Darwish TR, Haddad YH, et al. Accelerated versus Standard Corneal Cross-linking for Progressive Keratoconus in Syria. J Ophthalmic Vis Res. 2021;16(3):338–348. doi:10.18502/jovr.v16i3.9430
26. Deshmukh R, Ong ZZ, Rampat R, et al. Management of keratoconus: an updated review. Front Med Lausanne. 2023;10:1212314. doi:10.3389/fmed.2023.1212314
27. Valera-Cornejo DA, Vega-Estrada A, Alio JL. Invasive Pharmacology Outcomes with Different Corneal Cross-Linking Protocols: a Review. J Ocul Pharmacol Ther. 2019;35(9):475–490. doi:10.1089/jop.2018.0144
28. Subasinghe SK, Ogbuehi KC, Dias GJ. Current perspectives on corneal collagen crosslinking (CXL). Graefes Arch Clin Exp Ophthalmol. 2018;256(8):1363–1384. doi:10.1007/s00417-018-3966-0
29. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi:10.1016/S0002-9394(02)02220-1
30. Heikal MA, Soliman TT, Fayed A, et al. Efficacy of transepithelial corneal collagen crosslinking for keratoconus: 12-month follow-up. Clin Ophthalmol. 2017;11:767–771. doi:10.2147/OPTH.S129037
31. Beckman KA, Gupta PK, Farid M, et al. Corneal crosslinking: current protocols and clinical approach. J Cataract Refract Surg. 2019;45(11):1670–1679. doi:10.1016/j.jcrs.2019.06.027
32. O’Brart DPS. Corneal collagen crosslinking for corneal ectasias: a review. Eur J Ophthalmol. 2017;27(3):253–269. doi:10.5301/ejo.5000916
33. Razmjoo H, Rahimi B, Kharraji M, et al. Corneal haze and visual outcome after collagen crosslinking for keratoconus: a comparison between total epithelium off and partial epithelial removal methods. Adv Biomed Res. 2014;3(1):221. doi:10.4103/2277-9175.145677
34. Hashemi H, Miraftab M, Hafezi F, et al. Matched comparison study of total and partial epithelium removal in corneal cross-linking. J Refract Surg. 2015;31(2):110–115. doi:10.3928/1081597X-20150122-06
35. Galvis V, Tello A, Carreño NI, et al. Corneal Cross-Linking (with a Partial Deepithelization) in Keratoconus with Five Years of Follow-Up. Ophthalmol Eye Dis. 2016;8:17–21. doi:10.4137/OED.S38364
36. Henriquez MA, Rodríguez AM, Izquierdo L. Accelerated Epi-On Versus Standard Epi-Off Corneal Collagen Cross-Linking for Progressive Keratoconus in Pediatric Patients. Cornea. 2017;36(12):1503–1508. doi:10.1097/ICO.0000000000001366
37. Zaheryani SMS, Movahedan H, Salouti R, et al. Corneal Collagen Cross-Linking Using Epithelium Disruptor Instrument in Progressive Keratoconus. J Curr Ophthalmol. 2020;32(3):256–262. doi:10.4103/JOCO.JOCO_59_20
38. Li W, Wang B. Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus standard corneal collagen crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2017;17(1):262. doi:10.1186/s12886-017-0657-2
39. Napolitano P, Tranfa F, D’Andrea L, et al. Topographic Outcomes in Keratoconus Surgery: epi-on versus Epi-off Iontophoresis Corneal Collagen Cross-Linking. J Clin Med. 2022;11(7):1785. doi:10.3390/jcm11071785
40. Medeiros CS, Giacomin NT, Bueno RL, et al. Accelerated corneal collagen crosslinking: technique, efficacy, safety, and applications. J Cataract Refract Surg. 2016;42(12):1826–1835. doi:10.1016/j.jcrs.2016.11.028
41. Cunha AM, Sardinha T, Torrão L, et al. Transepithelial Accelerated Corneal Collagen Cross-Linking: two-Year Results. Clin Ophthalmol. 2020;14:2329–2337. doi:10.2147/OPTH.S252940
42. Ng AL, Chan TC, Lai JS, et al. Comparison of the Central and Peripheral Corneal Stromal Demarcation Line Depth in Conventional Versus Accelerated Collagen Cross-Linking. Cornea. 2015;34(11):1432–1436. doi:10.1097/ICO.0000000000000626
43. Hersh PS, Stulting RD, Muller D, et al. United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment. Ophthalmology. 2017;124(9):1259–1270. doi:10.1016/j.ophtha.2017.03.052
44. Gordon MO, Steger-May K, Szczotka-Flynn L, et al. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2006;142(6):923–930. doi:10.1016/j.ajo.2006.07.026
45. Matthaei M, Sandhaeger H, Hermel M, et al. Changing Indications in Penetrating Keratoplasty: a Systematic Review of 34 Years of Global Reporting. Int J Med. 2017;101(6):1387–1399. doi:10.1097/TP.0000000000001281
46. Cifariello F, Minicucci M, Di Renzo F, et al. Epi-Off versus Epi-On Corneal Collagen Cross-Linking in Keratoconus Patients: a Comparative Study through 2-Year Follow-Up. J Ophthalmol. 2018;2018:4947983. doi:10.1155/2018/4947983
47. Sun L, Li M, Zhang X, et al. Transepithelial accelerated corneal collagen cross-linking with higher oxygen availability for keratoconus: 1-year results. Int Ophthalmol. 2018;38(6):2509–2517. doi:10.1007/s10792-017-0762-5
48. Tian M, Jian W, Sun L, et al. One-year follow-up of accelerated transepithelial corneal collagen cross-linking for progressive pediatric keratoconus. BMC Ophthalmol. 2018;18(1):75. doi:10.1186/s12886-018-0739-9
49. Akbar B, Intisar-Ul-Haq R, Ishaq M, et al. Transepithelial corneal crosslinking in treatment of progressive keratoconus: 12 months’ clinical results. Pak J Med Sci. 2017;33(3):570–575. doi:10.12669/pjms.333.11907
50. Huang J, Shen Y, Jian W, et al. Two-year topographic and densitometric outcomes of accelerated (45 mW/cm(2)) transepithelial corneal cross-linking for keratoconus: a case-control study. BMC Ophthalmol. 2018;18(1):337. doi:10.1186/s12886-018-0999-4
51. Zhang X, Sun L, Chen Y, et al. One-year Outcomes of Pachymetry and Epithelium Thicknesses after Accelerated (45 mW/cm(2)) Transepithelial Corneal Collagen Cross-linking for Keratoconus Patients. Sci Rep. 2016;6:32692. doi:10.1038/srep32692
52. Kır MB, Türkyılmaz K, Öner V. Transepithelial High-Intensity Cross-Linking for the Treatment of Progressive Keratoconus: 2-year Outcomes. Curr Eye Res. 2017;42(1):28–31. doi:10.3109/02713683.2016.1148742
53. Zhang X, Sun L, Tian M, et al. Accelerated (45 mW/cm(2)) Transepithelial Corneal Cross-Linking for Progressive Keratoconus Patients: long-Term Topographical and Clinical Outcomes. Front Med Lausanne. 2020;7:283. doi:10.3389/fmed.2020.00283
54. Tian M, Jian W, Zhang X, et al. Three-year follow-up of accelerated transepithelial corneal cross-linking for progressive paediatric keratoconus. Br J Ophthalmol. 2020;104(11):1608–1612. doi:10.1136/bjophthalmol-2019-315260
55. Ziaei M, Vellara H, Gokul A, et al. Prospective 2-year study of accelerated pulsed transepithelial corneal crosslinking outcomes for Keratoconus. Eye. 2019;33(12):1897–1903. doi:10.1038/s41433-019-0502-3
56. Aixinjueluo W, Usui T, Miyai T, et al. Accelerated transepithelial corneal cross-linking for progressive keratoconus: a prospective study of 12 months. Br J Ophthalmol. 2017;101(9):1244–1249. doi:10.1136/bjophthalmol-2016-309775
57. Madeira C, Vasques A, Beato J, et al. Transepithelial accelerated versus conventional corneal collagen crosslinking in patients with keratoconus: a comparative study. Clin Ophthalmol. 2019;13:445–452. doi:10.2147/OPTH.S189183
58. D’Oria F, Palazón A, Alio JL. Corneal collagen cross-linking epithelium-on vs. epithelium-off: a systematic review and meta-analysis. Eye Vis (Lond). 2021;8(1):34. doi:10.1186/s40662-021-00256-0
59. Wen D, Song B, Li Q, et al. Comparison of Epithelium-Off Versus Transepithelial Corneal Collagen Cross-Linking for Keratoconus: a Systematic Review and Meta-Analysis. Cornea. 2018;37(8):1018–1024. doi:10.1097/ICO.0000000000001632
60. Meiri Z, Keren S, Rosenblatt A, et al. Efficacy of Corneal Collagen Cross-Linking for the Treatment of Keratoconus: a Systematic Review and Meta-Analysis. Cornea. 2016;35(3):417–428. doi:10.1097/ICO.0000000000000723
61. Buzzonetti L, Petrocelli G, Valente P, et al. Iontophoretic Transepithelial Collagen Cross-Linking Versus Epithelium-Off Collagen Cross-Linking in Pediatric Patients: 3-Year Follow-Up. Cornea. 2019;38(7):859–863. doi:10.1097/ICO.0000000000001965
62. Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus. Eye. 2015;29(1):15–29. doi:10.1038/eye.2014.230
63. Barbara R, Barbara A, Castillo J, et al. Keratoconus Expert Meeting, London, 2014. Int J Keratoconus Ectatic Corneal Dis. 2014;3:141–158. doi:10.5005/ijkecd-3-3-141
64. Choi JA, Kim MS. Progression of keratoconus by longitudinal assessment with corneal topography. Invest Ophthalmol Vis Sci. 2012;53(2):927–935. doi:10.1167/iovs.11-8118
65. Perez-Straziota C, Gaster RN, Rabinowitz YS. Corneal Cross-Linking for Pediatric Keratcoconus Review. Cornea. 2018;37(6):802–809. doi:10.1097/ICO.0000000000001579
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
