Back to Journals » Research and Reports in Urology » Volume 10
Trans-perineal pumpkin seed oil phonophoresis as an adjunctive treatment for chronic nonbacterial prostatitis
Authors Tantawy SA , Elgohary HMI , Kamel DM
Received 11 March 2018
Accepted for publication 18 May 2018
Published 18 September 2018 Volume 2018:10 Pages 95—101
DOI https://doi.org/10.2147/RRU.S167896
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Sayed A Tantawy,1,2 Hany MI Elgohary,3 Dalia M Kamel2,4
1Department of Physiotherapy, Centre of Radiation, Oncology and Nuclear Medicine, Cairo University, Giza, Egypt; 2Physiotherapy Department, College of Medical and Health Sciences, Ahlia University, Manama, Kingdom of Bahrain; 3Department of Physiotherapy for Surgery, Faculty of Physical Therapy, Cairo University, Giza, Egypt; 4Department of Physiotherapy for Women’s Health, Faculty of Physical Therapy, Cairo University, Giza, Egypt
Background: A significant number of men who are younger than 50 years visit urologists for interminable prostatitis. This study aimed to thoroughly investigate the effect of pumpkin seed oil (PSO) phonophoresis on chronic nonbacterial prostatitis (CNBP).
Subjects and methods: Sixty patients with CNBP were randomly assigned to three groups: Group A, wherein patients were treated with PSO using phonophoresis; Group B, where patients underwent trans-perineal continuous low-intensity ultrasound (LIUS); and Group C, wherein patients underwent placebo LIUS. All three groups received their corresponding treatments daily for up to 3 weeks. The NIH-Chronic Prostatitis Symptom Index (NIH-CPSI), residual urine determined by urodynamic measurements, and flow rate were used to analyze study outcomes. The white blood cell (WBC) count in the prostatic secretion was determined.
Results: Comparisons of the intragroup mean values of all measurements in Groups A and B before and after the end of the treatment showed a significant improvement in residual urine, flow rate, WBC count, and NIH-CPSI (p < 0.05), whereas no significant change was found in Group C (p > 0.05). Between-group comparisons of all variables showed a significant difference was found after intervention (p < 0.05). Postintervention comparisons between Groups A and B showed a significant difference in all measurements, except for WBC, in favor of Group A. Comparing the changes between Groups A and C, a significant difference was found in all measurements (p < 0.05). Furthermore, all parameters differed significantly when comparing Groups B and C (p < 0.05).
Conclusion: The current study showed that PSO phonophoresis can produce a significant effect in the management of CNBP and can, therefore, be considered a safe, noninvasive method for the treatment of CNBP.
Keywords: pumpkin seeds oil, phonophoresis, ultrasound, physiotherapy, cucurbita pepo, chronic non-bacterial prostatitis
Introduction
Ultrasound has been observed to be most frequently used as a therapeutic modality in the field of physiotherapy and sports medicine. It is also widely used in the clinical management of traumatic and acquired conditions that affect the musculoskeletal system. The use of ultrasound has been advocated for the enhancement of the transdermal penetration of medications – this is termed phonophoresis.1
A cream or medication gel, which acts as a coupling medium, is applied over the ultrasound probe, which is then used over the target area of treatment.2 To facilitate the application of the gel or cream, determination of the properties of a good ultrasound conductor is important to achieve positive therapeutic outcomes; otherwise, the treatment may yield ineffective therapeutic results.3
Based on proper examination and testing of several examples or classification, essential oils, such as birch sweet, German chamomile, wintergreen, and camphor, are categorized as having properties that help in inflammatory conditions. Similarly, geranium, clary sage, orange, parsley, rose geranium, and some other essential oils are characterized as having significant therapeutic effects, specifically related to female hormonal and gynecologic symptoms.4
Nakhostin-Roohi et al have assessed the special effects of virgin olive oil phonophoresis for anterior knee pain among female athletes.5 Furthermore, Chinese herbal medicines, such as guizhi (cassia twig) and cinnamic aldehyde, particularly its oil derivatives, have also been reported to be used in phonophoresis.6
The present study has focused on the Cucurbita pepo variant. Cucurbita is a globally well-known and old-fashioned herbal medicinal product that has been used over the past several centuries. Furthermore, its medicinal and therapeutic use and significance have been described in many textbooks and traditional medicinal manuscripts. In Europe, C. pepo in the form of ethanolic pumpkin seeds using its soft extract has been widely used. This has primarily been registered as a remedy for several urological conditions, specifically those related to an enlarged prostate gland and micturition problems related to an overactive bladder.7,8
Pumpkin seed oil (PSO) is rich in antioxidants.9 The active components of C. pepo L. seeds are D5-, D7-, and D8-sterols. D7-sterols are largely found in a predominant proportion in C. pepo. These are significantly registered to be the vital active components of pumpkin seeds in the treatment of mild prostatic hyperplasia.10
The significant fraction that consists of D7-sterols is widely believed to be responsible for its therapeutic effects. These sterols have not been found in any other sterol-containing plant extracts used in the treatment of benign prostatic hyperplasia (BPH).11
Pumpkin seeds contain considerable amounts of D7-phytosterols, either in the free form or bound to sugar molecules. Furthermore, a lipid–steroidal extract has also been confirmed to have an inhibitory effect on 5-α-reductase in cultured human prostate fibroblasts. The pharmacological mechanism of action of PSO is well documented through the inhibition of 5-α-reductase, which is primarily responsible for the conversion of testosterone into dihydrotestosterone (DHT).12,13
A study conducted by Schilcher et al14 concluded that orally administered D7-phytosterol-rich Cucurbita pepo subspecies pepo (C. pepo subs. pepo) seeds (3–4 days before prostatectomy) resulted in a reduced volume of DHT in the prostate tissue of patients.
Another remarkable benefit is the high content of omega-6 (linoleic acid), which is a necessary fatty acid. Omega-6 has been found to relieve symptoms associated with benign expansion and decrease the risk for prostate cancer.15 The administration of PSO and saw palmetto oil are suggested to be clinically safe and may be effective as complementary and alternative medicine treatments for BPH.12
With its perceived therapeutic and other useful effects on the prostate, the possibility that PSO can be used as a coupling medium for therapeutic ultrasound in the clinics should be given proper consideration. Although it is predominantly made of both linoleic and oleic acid, the proportion of linoleic acid is comparatively low; however, its high unsaturated fatty acid property provides a high oxidative stability for use in different industrial settings.16 Therefore, the present study was conducted to investigate the trans-perineal use of PSO as a topical coupling ultrasound medium for therapeutic ultrasound application on nonbacterial prostatitis (CNBP).
Subjects and methods
Design
A randomized controlled comparative trial was conducted. The patients were screened accordingly. Bladder residual urine, urinary flow rate, white blood cells (WBCs) in prostatic-specific specimens, and NIH-Chronic Prostatitis Symptom Index (NIH-CPSI) were recorded before and after 3 weeks of treatment to ensure the benefits of the utilized modalities. After screening, 60 male patients with CNBP were randomly assigned to three different groups: Group A, wherein patients were treated with phonophoresis by using PSO; Group B, wherein patients underwent continuous low-intensity ultrasound (LIUS); and Group C, wherein patients underwent placebo LIUS, with the ultrasound machine working with no output. The three groups received their preagreed protocol five times per week for 3 weeks.
Participants
For the analysis, the participants were recruited from the registry files of patients diagnosed with CNBP from the outpatient clinic, Department of Urology and Nephrology, Kasr Elaini Hospital, Cairo University. They were examined and diagnosed accordingly by a medical practitioner/urologist. The patients who were included in the study were required to have normal laboratory findings. Patients with concomitant infection, autoimmune diseases, diabetes mellitus, cancer, heart problems/pacemaker, implants (metal, silicone, saline, etc), acute and postacute injuries, thrombophlebitis, impaired sensation, psychiatric diseases, and those with well-known contraindications of ultrasound were excluded from the study. Similarly, those with hypersensitivity to PSO and its derivatives were excluded from the study sample. All patients stopped the administration of any form of medications used for the treatment of CNBP during the treatment protocol. Complete blood count, urinalysis, urine culture, and routine biochemical tests were conducted to rule out other diseases.
Sample size
Power analysis was initially conducted to calculate the sample size. The sample size for this study was calculated using the NIH-CPSI measure collected from a previous study.17 The mean difference and SD were 2.01 and 2.03, respectively (two-sided test; a = 0.05; power desired, 80%). The present study required a sample size of 17 patients for each group. 20 patients were enrolled in each group to account for dropout rates of 20%.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The Research Ethics Committee (P.T.REC/012/001810) of the Faculty of Physical Therapy, Cairo University, approved this study, which was registered with ClinicalTrials.gov under the identifier number NCT03461263. All the participants were informed about the complete study design thoroughly, both in verbal and written form, and all patients in this study provided signed informed consent.
Measures
To accumulate desired data for the analysis, for all subjects, bladder residual urine and urinary flow rate were measured using the urodynamic examination system. Furthermore, the WBCs in prostatic-specific specimens and NIH-CPSI questionnaire were recorded both before and after 3 weeks of treatment. Symptoms were monitored and recorded on the basis of changes in terms of the NIH-CPSI, which was recorded both before and after treatment for all the three groups.
Urodynamic examination system
Cystometry was evaluated with a DANTIC UD 5000/5500 urodynamic examination system (Megamed, Germany). Each patient underwent multichannel cystometry before starting the study and, again, after 3 weeks. Some urodynamic parameters were estimated for each patient in terms of the urinary residual volume and urine flow rate.
WBC count
WBC count in prostatic-specific specimens was quantified by a well-trained hematologist by using the Hematocytometer cell-counting chamber.
NIH-CPSI
The NIH-CPSI is a 13-item index designed to evaluate symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS).18 It has shown reliability, validity, and responsiveness to change, and it has been used as the primary outcome variable in multiple large-scale studies of CP/CPPS treatments.19
Intervention
For Group A, a trans-perineal phonophoresis using 3-mL PSO as a coupling medium (obtained from the Faculty of Pharmacy, Cairo University; pharmaceutical grade, pure product, preprepared for therapeutic purposes) was applied for 8 min per session five times a week for 3 consecutive weeks. The patient was placed in a lateral recumbent position while both lower limbs were flexed toward the chest. The area to be treated was cleaned by using an alcohol-based formulation. Continuous ultrasonic waves with a frequency of 1 MHz and a power density of 1.5 W/cm2, 100% duty cycle, 8 min per session were applied while the ultrasound head was moved in a circular direction over the perineal area throughout the session. An LIUS device (Sonopuls 490 u. Manufacturer; ENRAF NONIUS B.V, Netherlands) was used in the present study.
For Group B, LIUS waves using the same duty cycle, time, and manner of application were used trans-perineally, five times a week, for three consecutive days. Regular gel was used as a coupling medium. For Group C, placebo LIUS was conducted five times a week for 3 consecutive weeks.
Statistical analyses
The SPSS version 21 was used for data analysis. For all demographic and quantitative variables of interest, summary statistics, namely, the mean ± SD, were calculated. To assess the degree of differences before and after treatment among the three groups, independent and paired t-test procedures were conducted. Furthermore, the differences were analyzed for the same group on pre- and posttreatment basis and on pre- and posttreatment difference among groups. One-way analysis of variance was used to assess inter-group differences in various measurements.
Results
The study sample for the present study was 69 patients with CNBP. Of the 69 participants, nine did not qualify in the study and were excluded consequently from the results due to various reasons: reluctance to participate (n = 4), inadequate evaluation measures (n = 2), unwillingness to remain in the treatment procedures (n = 2), and not receiving three sessions of the selected interventions (n = 1). Thus, the study included 60 participants, with 20 participants in each group.
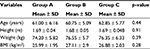
The demographic data for groups were tested preintervention to determine homogeneity, and no significant difference was found (p > 0.05) as shown in Table 1. No significant differences were also found between the three groups for the baseline parameters (p > 0.05) as presented in Table 2.
The comparisons of intragroup mean values of all measurements in Groups A and B, before and after the treatment was completed, showed a significant improvement in residual urine, flow rate, and WBC (p < 0.05), with mean differences of −31.35 and −15.55, 8.6 and 4.4, and −1.8 and −1.59, respectively, whereas no significant change was found in Group C (p > 0.05).
Statistically significant improvement in NIH-CPSI, including total scores, pain symptoms, urination symptoms, and quality of life impact, was observed among patients in Groups A and B (p < 0.05), whereas no significant change was found in Group C (p > 0.05).
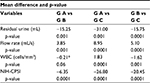
Comparing the results of all variables between the groups, significant differences were found after intervention, in favor of Group A (p < 0.05), as shown in Table 2.
Postintervention comparisons between Groups A and B showed a significant difference in all measurements, in favor of Group A (residual urine, flow rate) and NIH-CPSI, including pain symptoms, urination symptoms, and quality of life impact, except for WBC (p < 0.05), with mean differences of −15.25, 3.8, −6.35, and −6.35, respectively, as presented in Table 3.
Comparing the changes between Groups A and C showed significant differences in residual urine, flow rate, WBC, and NIH-CPSI, including pain symptoms, urination symptoms, and quality of life impact (p < 0.05), with mean differences of −31.0, 8.95, −1.83, and −26.8, respectively. Furthermore, all parameters differed significantly when comparing Groups B and C (p < 0.05), as shown in Table 3.
Discussion
The findings of the present study showed that phonophoresis using PSO can be used as an alternative for CNBP treatment. This was demonstrated through the significant reduction of WBC count, NIH-CPSI, and residual urine in the urinary bladder, whereas a significant increase was recorded in the urine flow rate. To date, studies conducted on the current area of interest have emphasized mechanisms by which trans-perineal phonophoresis exerts its effects through different pathways. Future research is recommended to clearly determine the principles concerning these influences in CNBP.
In 2013, Li et al17 appraised the clinical potency and safety of trans-perineal LIUS to treat chronic prostatitis (CP). The study reported the NIH-CPSI scores, in addition to regular prostatic testing and assessment of prostatic fluid. Findings showed that trans-perineal ultrasound is profoundly useful for CP, particularly in releasing prostatic discomfort and simultaneously holding the benefits of being safe, simple to use, easy to handle, and generally acceptable.
Furthermore, trans-rectal low-intensity pulsed ultrasound (LIPUS) has been reported to be productive in counteracting the clinical manifestations of CP and chronic pelvic pain syndrome (CP/CPPS). LIPUS has been found to produce its effects through controlling cytokine excretion.20
Furthermore, LIPUS treatment could hinder interleukin (IL)-1-beta-induced COX-2 production via the integrin beta-1 receptor served by the phosphorylation of ERK ½ because COX-2 is produced because of pain.21 Therefore, LIPUS is considered as an effective clinical method for the treatment of CP/CPPS.
The mechanisms of increasing skin permeability provoked through the clinical application of low-frequency ultrasound have been attributed to the temporary acoustic cavitation generated over the skin membrane via micro-jets affecting the skin surface, although other mechanisms may play insignificant roles. Generally, low-frequency pulsed ultrasound has revealed a much greater potential to allow high-molecular weight components to pass through the skin and affect deep structures.22
The dispersion of LIUS through the skin has two principal real values: heating phenomenon and production of cavitation, wherein both mechanisms might be combined as cavitation may result in heating.23
Herbal medicine were dispensed to males with symptomatic BPH and CNBP, where practitioners designated phytochemical products in the corresponding way as they designate medications, and then investigated the mixture of saw palmetto with PSO, with notable improvement in the maximum flow rate (MFR) value in patients with BPH after 3 months of therapy.24,25
Our study showed similar effectiveness on MFR in the ultrasound group, but the combination of PSO with ultrasound had more benefits and was more effective.
The effect of PSO is characterized by its restraint on 5-α-reductase that transforms testosterone into DHT26,13 as well as epithelial compression in the prostate transition area as explained by Marks et al.24 The use of PSO for patients with prostate problems showed that testosterone-induced prostatic hyperplasia was inhibited.26 Similarly, intake of PSO resulted in an actual decrease in prostatic weight.13
In our study, integration of treatment via PSO and ultrasound phonophoresis resulted in significant symptomatic recovery, with a statistically significant difference in flow rate, residual urine, WBC, and NIH-CPSI. Further, more significant results can be achieved if the trial is reconducted with a larger sample over a more extended measurement period. With regard to the study outcomes, it could be proposed that PSO phonophoresis is clinically supported and may be acceptable as an alternative therapy for BPH.
Moon et al27 found that the urinary flow rate of patients with BPH administered with sitosterol showed significant improvement from an initial MFR after 4 weeks of therapy and progressed up to 12 weeks.
Essential omega-3 fatty acids are primarily associated with initiating anti-inflammatory responses; PSO is rich in omega-3 fatty acids, specifically α-linolenic acid. Its main property involves anti-inflammatory, antioxidative, hypocholesterolemic, hypolipidemic, hypotensive, and vasoconstrictive effects. A plausible explanation for the reduction of symptoms of prostatitis with the application of phonophoresis may be the enhancement of properties of PSO because an omega-3 fatty acid exerts its effects on prostatic tissue.28
Ultrasound helps in increasing the production of blood vessels and fibroblasts and increases the permeability of the cell membrane.29 Moreover, this may possibly explain the enhancement observed in Group B in addition to the effects of the PSO as a coupling medium to the physiological effects exerted in the prostate tissue in Group A.
Furthermore, PSO was found to include high levels of delta-7-sterine, which inhibits the DHT receptors that cause cancer in the prostate gland. Delta-7-sterine is a mild steroid that competes with DHT. DHT is known to be a significant factor in enlarged prostate tissue and hair loss. In addition, PSO contains phytosterols with beta-sitosterol, which are particularly beneficial for men.10
Abdel-Rahman30 conducted a similar study to properly explore the impact of pumpkin seeds administered through diet supplementation on prostate hypertrophy and reported that ingestion of a high dose of pumpkin seeds inhibits prostate growth.
For patients who experience urological problems frequently, treatment using PSO may be advised with micturition manifestations correlated to BPH during the initial disease stages, particularly those associated with an overactive bladder.12
Future researchers should be directed to examine the use of other different herbal sources that can be presented to the body by using different modes of LIUS by phonophoresis. The outcome measures of the study were limited to the use of some urodynamic parameters, such as urinary flow rate and residual urine, WBCs, and NIH-CPSI, which were insufficient measures to strongly support the use of the treatment protocol; therefore, further studies should be conducted along with the measurement of prostate weight, inflammatory markers in prostatic secretions (tumor necrotic factor [TNF] and interleukin-6 [IL-6] or 1B, NF-κB), and DHT levels.
Conclusion
CNBP can be treated by many modalities to reduce the inflammatory process and diminish prostatic signs and symptoms. These modalities include trans-perineal PSO phonophoresis and LIUS, which are considered adjunctive treatments for this debilitating problem.
Acknowledgment
The authors thank all patients who participated in this study.
Author contributions
SA Tantawy and HMI Elgohary designed and conducted research. DM Kamel collected the data and undertook statistical analysis. HMI Elgohary and SA Tantawy wrote the manuscript. DM Kamel: revised and edited the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Byl NN. The use of ultrasound as an enhancer for transcutaneous drug delivery: phonophoresis. Phys Ther. 1995;75(6):539–553. | ||
Low J, Reed A. Electrotherapy Explained: Principles and Practice. 3rd ed. Oxford: Butterworth Heinemann; 2000:94–95. | ||
Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin Drug Deliv. 2010;7(12):1415–1432. | ||
Therapeutic Properties of Essential Oils. (2015). [Blog] AIRASE. Available from: http://airase.com/therapeutic-uses-of-essential-oils/. Accessed August 12, 2017. | ||
Nakhostin-Roohi B, Khoshkhahesh F, Bohlooli S. Effect of virgin olive oil versus piroxicam phonophoresis on exercise-induced anterior knee pain. Avicenna J Phytomed. 2016;6(5):535–541. | ||
Zhao J, Wang Q, Wu J, et al. Therapeutic effects of low-frequency phonophoresis with a Chinese herbal medicine versus sodium diclofenac for treatment of knee osteoarthritis: a double blind, randomized, placebo-controlled clinical trial. J Tradit Chin Med. 2016;36(5):613–617. | ||
ESCOP. Cucurbitae semen (pumpkin seed). In: European Scientific Cooperative on Phytotherapy, editor. ESCOP Monographs. 2nd ed. Stuttgart: Georg Thieme Verlag; 2009:50–56. | ||
Wisher D. Martindale. The complete drug reference. 37th ed. J Med Libr Assoc. 2012;100(1):75–76. | ||
Edwards SE, Rocha IDC, Williamson EM, Heinrich M. Phytopharmacy: an Evidence-Based Guide to Herbal Medicinal Products. Chichester: John Wiley & Sons, Ltd; 2015. | ||
Damiano R, Cai T, Fornara P, Franzese CA, Leonardi R, Mirone V. The role of Cucurbita pepo in the management of patients affected by lower urinary tract symptoms due to benign prostatic hyperplasia: a narrative review. Arch Ital Urol Androl. 2016;88(2):136–143. | ||
Vahlensieck W, Theurer C, Pfitzer E, Patz B, Banik N, Engelmann U. Effects of pumpkin seed in men with lower urinary tract symptoms due to benign prostatic hyperplasia in the one-year, randomized, placebo-controlled GRANU study. Urol Int. 2015;94(3):286–295. | ||
Hong H, Kim CS, Maeng S. Effects of pumpkin seed oil and saw palmetto oil in Korean men with symptomatic benign prostatic hyperplasia. Nutr Res Pract. 2009;3(4):323–327. | ||
Tsai YS, Tong YC, Cheng JT, Lee CH, Yang FS, Lee HY. Pumpkin seed oil and phytosterol-F can block testosterone/prazosin-induced prostate growth in rats. Urol Int. 2006;77(3):269–274. | ||
Schilcher H, Dunzendorfer U, Ascali F. D7-Sterols: the prostatotropic principle of pumpkin seeds? Urologe B. 1987;27:316–319. | ||
Chua ME, Sio MC, Sorongon MC, Dy JS. Relationship of dietary intake of omega-3 and omega-6 Fatty acids with risk of prostate cancer development: a meta-analysis of prospective studies and review of literature. Prostate Cancer. 2012;2012:826254. | ||
Stevenson DG, Eller FJ, Wang L, Jane JL, Wang T. Inglett GE. Oil and tocopherol content and composition of pumpkin seed oil in 12 cultivars. J Agric Food Chem. 2007;55(10):4005–4013. | ||
Li HS, Wang B, Han L, Wang CH, Xin ZC. [Transperineal ultrasonic therapy for chronic prostatitis]. Zhonghua Nan Ke Xue. 2013;19(1):49–53. Chinese [with English abstract]. | ||
Litwin MS, McNaughton-Collins M, Fowler FJ Jr, et al. The National Institutes of Health chronic prostatitis symptom index: development and validation of a new outcome measure. J Urol. 1999;162(2):369–375. | ||
El-Nashaar A, Fathy A, Zeedan A, Al-Ahwany A, Shamloul R. Validity and reliability of the arabic version of the National Institutes of Health Chronic Prostatitis Symptom Index. Urol Int. 2006;77(3):227–231. | ||
Karpukhin VT, Nesterov NI, Roman DL. [Ultrasonic therapy of chronic prostatitis]. Vopr Kurortol Fizioter Lech Fiz Kult. 1977;(3):75–77. Russian [with English abstract]. | ||
Doan N, Reher P, Meghji S, Harris M. In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts, and monocytes. J Oral Maxillofac Surg. 1999;57(4):409–419; discussion 420. | ||
Nakamura T, Fujihara S, Yamamoto-Nagata K, Katsura T, Inubushi T, Tanaka E. Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng. 2011;39(12):2964–2971. | ||
Holt RG, Roy RA. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol. 2001;27(10):1399–1412. | ||
Marks LS, Partin AW, Epstein JI, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol. 2000;163(5):1451–1456. | ||
Carbin BE, Larsson B, Lindahl O. Treatment of benign prostatic hyperplasia with phytosterols. Br J Urol. 1990;66(6):639–641. | ||
Gossell-Williams M, Davis A, O’Connor N. Inhibition of testosterone-induced hyperplasia of the prostate of sprague-dawley rats by pumpkin seed oil. J Med Food. 2006;9(2):284–286. | ||
Moon YT, Oh CH, Kim SC. Clinical effect of Sitosterol (PronalR) on the treatment of benign prostatic hypertrophy. Korean J Androl Soc. 1990;8(1):23–29. | ||
Johnson M, Bradford C. Omega-3, Omega-6 and Omega-9 fatty acids: implications for cardiovascular and other diseases. J Glycomics Lipidomics. 2014;4:123. | ||
Franco de Oliveira R, Pires Oliveira DA, Soares CP. Effect of low-intensity pulsed ultrasound on l929 fibroblasts. Arch Med Sci. 2011;7(2):224–229. | ||
Abdel-Rahman MK. Effect of pumpkin seed (Cucurbita pepo L) diets on benign prostatic hyperplasia (BPH): chemical and morphometric evaluation in rats. World J Chem. 2006;1(1):33–40. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.