Back to Journals » Drug Design, Development and Therapy » Volume 12
Traditional Chinese herbal formula relieves snoring by modulating activities of upper airway related nerves in aged rats
Authors Chung KT, Hsu CH , Lin CL , Wang SE, Wu CH
Received 3 November 2017
Accepted for publication 15 March 2018
Published 8 May 2018 Volume 2018:12 Pages 1165—1171
DOI https://doi.org/10.2147/DDDT.S155996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Kou-Toung Chung,* Chih-Hsiang Hsu,* Ching-Lung Lin, Sheue-Er Wang, Chung-Hsin Wu
Department of Life Science, National Taiwan Normal University, Taipei, Taiwan
*These authors contributed equally to this work
Aim: The present study investigated whether intraperitoneal treatment with the herbal formula B210 ([B210]; a herbal composition of Gastrodia elata and Cinnamomum cassia) can reduce snoring in aged rats. Also, we studied possible neural mechanisms involved in B210 treatment and subsequent reduced snoring in rats.
Methods and result: We compared pressure and frequency of snoring, activities of phrenic nerve (PNA), activities of recurrent laryngeal nerve (RLNA) and activities of hypoglossal nerve (HNA), inspiratory time (TI) and expiratory time (TE) of PNA, and pre-inspiratory time (Pre-TI) of HNA in aged rats between sham and B210 treatment groups (30 mg/mL dissolved in DMSO). We found that aged rats that received B210 treatment had significantly reduced pressure and frequency of snoring than rats who received sham treatment. Also, we observed that aged rats that received B210 treatment had significantly increased PNA, RLNA, and HNA, extended TI and TE of PNA, and prolonged Pre-TI of HNA compared to rats that received sham treatment. In other words, B210 treatment may relieve snoring through modulating activities and breathing time of upper airway related nerves in aged rats.
Conclusion: We suggested that the B210 might be a potential herbal formula for snoring remission.
Keywords: Chinese herbal medicine, snoring remission, upper airway, phrenic nerve, recurrent laryngeal nerve, hypoglossal nerve
Introduction
Sleep disordered breathing such as serious snoring and sleep apnea is common in aging.1 Snoring is a sign of resistance to the passage of air into the lungs and also a sign of breathing problems.2 Many studies have reported that sleep disordered breathing, such as snoring, was connected with many causes of death such as hypertension, myocardial infarction, and stroke.3–5 Electrical stimulation of the upper airway dilator muscles to reduce snoring has been used for many years, however, most people do not want intrusive surgery.6 Allover the world, including Taiwan, many patients with serious snoring are unwilling to use continuous positive airway pressure and to undergo surgery. Thus, seeking a potential traditional Chinese medicine as an alternative treatment may be another option for the treatment of serious snoring.7 In Taiwan, the herbal formula B210 ([B210]; a herbal composition of Gastrodia elata and Cinnamomum cassia) is a well-known, patented Chinese herbal formula (patent no 361075 issued by Intellectual Property Office of Taiwan; patent owner: Brion Research Institute of Taiwan) for patients with sleep disorders to reduce snoring. However, the possible neural mechanism concerning pharmacological effects of B210 in snoring remission is still unclear. The causes of snoring are multi-factorial, involving the complex interplay between the central and peripheral nervous system, respiratory related nerves such as phrenic nerve, and upper airway related nerves such as recurrent laryngeal and hypoglossal nerves, and their neurotransmitters, all of which play a role in snoring.8
In this study, we aimed to investigate whether a traditional Chinese herbal formula such as B210, may relieve snoring through modulating activities of the respiratory related phrenic nerve, and upper airway related nerves such as recurrent laryngeal and hypoglossal nerves in aged rats. To achieve our research goals, we designed a snoring rat model, as described in previous studies.9–12 First, we confirmed whether intraperitoneal B210 treatment could reduce snoring in these rats. Then, the activities of phrenic nerve (PNA), activities of recurrent laryngeal nerve (RLNA), and activities of hypoglossal nerve (HNA) of the snoring rat model were recorded and compared before and after intraperitoneal B210 treatment. Also, the PNA, RLNA, and HNA of the snoring rat model were compared between sham and B210 treatment groups. This is the first study to utilize neurophysiological methods to clarify the therapeutic mechanism of B210 for snoring remission in a snoring rat model. Our study reveals that B210 might be a potential herbal formula for snoring remission.
Materials and methods
Chromatographic fingerprint analysis of B210
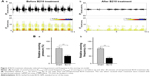
B210 (Taiwan patent no 361075) was provided free of charge by Brion Research Institute of Taiwan. High performance liquid chromatography (HPLC) fingerprints of individual herbal extracts and the combination of individual herbal extracts were generated to monitor the presence or absence of individual herbs of B210 (Figure 1). HPLC grade acetonitrile was purchased from Burdick & Jackson (Gyeonggi-do, Korea), along with methanol from Avantor (Center Valley, PA, USA). All the chemicals used were of analytical grade and solubilized in distilled H2O/MeOH. The combined extracts were dried and re-dissolved in 2 mL of 50% methanol. The prescription of the herbal formula B210 mainly contains two kinds of medicinal herbs, G. elata and C. cassia, in specific ratios. A total of 10 mL of B210 was injected into the HPLC system. For the analysis of every single herbal extract, an amount of extract of that herb equivalent to the amount of 50 mg of extract was prepared based on its yield, and analyzed using the method described previously. All samples were analyzed in triplicate.
The IC50 of B210
The half maximal inhibitory concentration (IC50) of B210 was evaluated by MTT assay. SH-SY5Y cells were plated at 37°C with 5% CO2 for 24 hours. Then, SH-SY5Y cells were treated with B210 (10–160 mg/mL dissolved in DMSO) for 24 hours to determine the IC50 of cytotoxicity. After incubation with 100 μL of MTT solution (0.5 mg/mL) at 37°C for 3 hours, 100 μL of 10% sodium dodecyl sulfate-0.01N HCl solution was added to each well and incubated at 37°C overnight to dissolve the formazan. The absorbency was measured at 570 nm with an ELISA reader (uQuant, BioTek Instruments Inc., Winooski, VT, USA), and the results were expressed as the relative cell viability of treated cells against those of the controls.
Animal preparation
Twenty-two aged male Wistar rats of 30–36 months old (486 ± 19 g) were used in this study. All rats were housed in a room kept at 25°C with access to water and food ad libitum. Experiments were performed with the consent of the Animal Care and Use Committee of National Taiwan Normal University (NTNU-ACUC-101017) under the guidelines of Taiwan Animal Protection Law for the welfare of animals. The research involves animal experiments and has considered the 3R rules of Replace, Reduce, and Refine to optimize the experimental design.
Before experiment recording, aged rats were weighed and treated with atropine (0.5 mg/Kg, intramuscular injection) to reduce bronchial secretion. After 30 minutes, the animals were anesthetized with urethane (1.2 g/Kg, intraperitoneal injection). Tracheal and vascular intubations were applied for spontaneous breathing. Then, the blood pressure was measured and the saline was administered by way of these intubations. The end-tidal fractional concentration of CO2 was continuously monitored with a CO2 analyzer (Model CD-3A carbon dioxide analyzer, AEI Technologies, Inc., Pittsburgh, PA, USA). The body temperature was maintained at 37°C–38°C with a heat blanket or lamp. In this study, six rats were used for snoring assessment, eight rats were used for neural activity recording of PNA and RLNA, and seven rats were used for recording of PNA and HNA. Data of the experimental groups were obtained from aged rats that received intraperitoneal B210 treatment. Preparation method for B210 was 30 mg/mL dissolved in DMSO. The dosage of B210 was 150 mg/Kg for each intraperitoneal treatment. Preparation method for sham treatment was used with the DMSO injection volume of 5 mL/Kg.
Snoring assessment
Snoring pressure and frequency of anesthetized rats was assessed before and after intraperitoneal B210 treatment (30 mg/mL dissolved in DMSO; the pH value was close to 7.0). As described in the Introduction section, we designed a snoring rat model as described in previous studies.9–12 Snoring pressure and frequency in the anesthetized rats was measured in a supine position. Partial upper airway obstruction and snoring were produced via external compression of the pharynx with a miniature sandbag. Sound pressure (PSOUND) and sound frequency (FSOUND) were measured by using a cardioid polar pattern studio condenser microphone (CAD M37, CAD Professional Microphones, Menton, OH, USA) positioned at 30° to the vertical and at a distance of 1.5 cm from the rat’s mouth. The microphone has a frequency response of 20 Hz–20 kHz and operates linearly between 50 Hz and 3 kHz with a manufacturer-specified sensitivity. The microphone was interfaced with a preamplifier (Symetrix SX202, Symetrix, Mountlake Terrace, WA, USA), providing the signal with a gain of 55 dB. In this study, snoring was induced via compression of the pharyngeal tissues by using a miniature sandbag (weighing 10.0 g) placed on the skin of the neck just above the larynx, forcing partial obstruction of the pharynx. In this study, we kept a constant pressure on the neck of rats before and after intraperitoneal B210 treatment.
Recording of PNA, RLNA, and HNA
The phrenic nerve of the anesthetized rat was isolated via a dorsolateral approach at the spinal level of C4–C5 and cut peripherally. The recurrent laryngeal nerve of the anesthetized rat was dissected along the trachea on the right side and was cut distally. The hypoglossal nerve of the anesthetized rat on the left side was separated via a ventral approach and cut distally. Then the PNA, RLNA, and HNA of rats were continuously recorded before and after intraperitoneal sham or B210 treatment for at least 30 minutes. The PNA, RLNA, and HNA were monitored by a bipolar electrode, amplified by a differential amplifier (Grass P511 AC amplifier, Grass Instruments Co., West Warwick, RI, USA), filtered (0.3–3 kHz), and integrated by a homemade integrator (time constant = 0.02 s). Neural signals were displayed on a digital oscilloscope (Tektronix TDS420A Oscilloscope, Tektronix, Inc., Beaverton, OR, USA) and stored simultaneously on the hard disc via the PowerLab system (ADI Instrument, Colorado Springs, CO, USA).
Statistical analysis of data
Data were retrieved and analyzed by software written in the Visual C++ language. Neural discharges of 20 respiratory cycles before and after 30 minutes of sham treatment were computed and averaged as the negative control group and positive control group, respectively. The rats that received sham treatment were treated with intraperitoneal solvent. Neural discharges of 20 respiratory cycles before and after 30 minutes of intraperitoneal B210 treatment were computed and averaged as the no-treatment control group and the experimental group, respectively. For the purpose of uniform comparison, the pressure (PSOUND) and frequency (FSOUND) of snoring, and the PNA, RLNA, and HNA after 30 minutes of intraperitoneal B210 treatment were taken as experimental data, which were further transformed into percentage of pre-treatment data, while real inspiratory time (TI), expiratory time (TE), and pre-inspiratory time (Pre-TI) of aged rats were obtained from the neurograms of PNA and HNA individually. We quantified and then compared the relative PNA, RLNA, HNA, and real TI, TE, and Pre-TI in rats that received sham and B210 treatments by Student’s t-test. All values were expressed as mean ± standard error of the mean (SEM). P-values of at least <0.05 were considered significant.
Results
Characterization of B210
HPLC fingerprints showed that the combination of individual herbal extracts had a pattern consistent with that of the standard product. By comparing the on-line ultraviolet spectra and retention times, peaks in the standard product with those of the individual herbal extracts, this showed that active ingredients of B210 such as gastrodin (formula: C13H18O7; molecular weight [MW]: 286.27 g/mol) and parishin (formula: C45H56O25; MW: 996.92 g/mol) from G. elata, cinnamic (formula: C9H8O2; MW: 148.16 g/mol), cinnamaldehyde (formula: C9H8O; MW: 132.16 g/mol), and 2-methoxycinnamaldehyde (formula: C10H10O2; MW: 162.19 g/mol) from C. cassia were identified and marked at the corresponding peaks in the fingerprint (Figure 1A). As detected by MTT assay, the IC50 of B210 is 75 mg/mL, which represents the concentration of B210 that is required for 50% inhibition in vitro (Figure 1B).
Effects of the B210 treatment on snoring of aged rats
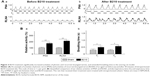
An example of the PSOUND and FSOUND of snoring of a rat before and after 30 minutes of intraperitoneal B210 treatment is demonstrated in Figure 2A. We observed that the maximal PSOUND and the highest FSOUND of snoring of a rat before B210 treatment was approximately 50 dB SPL and 16 kHz individually (Figure 2Aa). After 30 minutes of B210 treatment, we observed that the maximal PSOUND and the highest FSOUND of snoring of a rat was approximately 20 dB SPL and 9 kHz individually (Figure 2Ab). In Figure 2B, we quantified the relative snoring pressure and frequency of rats that received sham and B210 treatments. We found that both the relative snoring pressure and frequency in rats that received B210 treatment reduced significantly compared to those rats that received sham treatment (Figure 2Ba, b, N=6, P<0.01 by Student’s t-test).
Effects of the B210 treatment on PNA and RLNA of aged rats
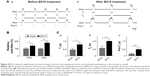
An example of PNA and RLNA of a rat before and after 30 minutes of intraperitoneal B210 treatment is demonstrated in Figure 3A. PNA and RLNA of a rat obviously increased after 30 minutes of intraperitoneal B210 treatment. Also, the TI and TE of a rat was extended after 30 minutes of intraperitoneal B210 treatment. In Figure 3B, we quantified and then compared the relative PNA and RLNA, and we found that both the relative PNA and RLNA of rats with B210 treatment significantly increased compared to those of rats with sham treatment (N=8, P<0.01 by Student’s t-test). Moreover, in Figure 3C, we quantified and then compared real TI and TE of PNA in aged rats between sham and B210 treatments, and we found that both the real TI and TE of rats with B210 treatment were significantly extended compared to those of rats with sham treatment (N=8, P<0.05 by Student’s t-test).
Effects of the B210 treatment on PNA and HNA of aged rats
An example of PNA and HNA of a rat before and after 30 minutes of intraperitoneal B210 treatment was demonstrated in Figure 4A. PNA and HNA of a rat were obviously enhanced after 30 minutes of intraperitoneal B210 treatment. Also, TI, TE, and Pre-TI of a rat were extended after 30 minutes of intraperitoneal B210 treatment. In Figure 4B, we quantified and then compared the relative PNA and HNA, and we found that both the relative PNA and HNA of rats with the B210 treatment were significantly increased compared to those of rats with sham treatment (N=8, P<0.01–0.05 by Student’s t-test). Moreover, in Figure 4C, we quantified and then compared real TI and TE of PNA, and Pre-TI of HNA in rats between sham and B210 treatments, and we found that real TI, TE, and Pre-TI of rats with the B210 treatment were significantly extended compared to those of rats with sham treatment (Figure 4C, N=8, P<0.01–0.05 by Student’s t-test).
Discussion
B210 mainly contains two kinds of medicinal herbs; G. elata and C. cassia in specific ratios. When compared to the traditional Chinese medicine, we know that none of these herbs have much therapeutic value, and to use any of them alone may prove problematic. This is the reason why we used a combined herbal formula to treat snoring in aged rats. An ingredient of B210, shown in Figure 1A, the extract of G. elata has been reported to be used for the treatment of headaches, dizziness, tetanus, and epilepsy.13 Novel pharmacological effects such as anti-angiogenic, anti-inflammatory, and analgesic activities have also been demonstrated in G. elata.14 Cinnamaldehyde, a major constituent of C. cassia, might exert anti-inflammatory and anti-depressant-like effects in aged rats when targeting the hippocampus and the frontal cortex.15 Taken together, B210 might have protective effects via reducing oxidative stress, inflammation, and depression-like effects. Furthermore, the clinical cases of more severe snoring showed great improvement after treatment with B210.
In general, snoring is caused by vibration of the soft tissues in the throat such as vocal cords and tongue. Contraction of the posterior cricoarytenoid muscles, which are controlled by the intralaryngeal abducent branch of the RLNA, dilates the vocal cords during inspiration and reduces airflow resistance.16 In this study, we observed that aged rats had enhanced inspiratory PNA and RLNA after intraperitoneal B210 treatment (Figure 3). It has been suggested that promoting RLNA may expand vocal folds in the upper airway. This is a very reasonable explanation as to why the B210 treatment may relieve snoring through promoting RLNA and expanding vocal folds in aged rats. Also, we observed that these aged rats had extended TI and TE after intraperitoneal B210 treatment (Figure 3). Breathing time is the time taken for a single breath which consists of TI and TE. These inspiration and expiration times of rats may be modified and adapted for various physical conditions and human needs. The relationships of TI and TE to end-inspiratory volume were approximately linear over the entire tidal volume range. In other words, extended TI and TE may enhance tidal volume of the lung. It is reasonable to postulate that the B210 treatment may enhance ventilation and relieve snoring in aged rats.
In addition to the recurrent laryngeal nerve, the hypoglossal nerve, and the intrinsic and extrinsic genioglossus also play an important role in snoring remission. The genioglossus muscle participates in a variety of motor functions such as phonation, swallowing, licking, mastication, and respiration.17–20 Studies in humans suggest that exercise can enhance HNA and then reduce upper airway airflow resistance during breathing.21 Respiratory-related discharge in the hypoglossal nerve is composed of pre-inspiratory and inspiratory activity.22 Pre-inspiration phase, only present in the HNA recording, corresponded to burst activity prior to the onset of PNA. By dilating the pharyngeal airway before inspiration, HNA may be particularly important in reducing upper airway airflow resistance during breathing.23,24 In this study, we observed that aged rats, after intraperitoneal B210 treatment, immediately had enhanced PNA and HNA, and prolonged Pre-TI (Figure 4). These results provide a reasonable explanation as to why B210 treatment in aged rats may reduce snoring, by enhancing contraction of the genioglossus.
In conclusion, we found that B210 treatment can cause upper airway patency and relieve snoring in aged rats through enhancing the upper airway related PNA, RLNA, and HNA, extending TI and TE of PNA, and prolonging Pre-TI of HNA. Thus, we suggest that B210 might be a potential herbal formula for snoring remission.
Acknowledgments
We thank the Brion Research Institute of Taiwan which provided our experimental materials and chromatographic fingerprint analysis of B210. This study of B210 was funded by the Brion Research Institute of Taiwan, which also owns the patent to the drug.
Disclosure
The authors report no conflicts of interest in this work.
References
Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. | ||
Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. | ||
Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. | ||
Koskenvuo M, Kaprio J, Partinen M, et al. Snoring as a risk factor for hypertension and angina pectoris. Lancet. 1985;1(8434):893–896. | ||
Koskenvuo M, Kaprio J, Telakivi T, et al. Snoring as a risk factor for ischaemic heart disease and stroke in men. Br Med J (Clin Res Ed). 1987;294(6563):16–19. | ||
Pengo MF, Steier J. Emerging technology: electrical stimulation in obstructive sleep apnoea. J Thorac Dis. 2015;7(8):1286–1297. | ||
Chen FP, Chen TJ, Kung YY, et al. Use frequency of traditional Chinese medicine in Taiwan. BMC Health Serv Res. 2007;7:26. | ||
Montserrat JM, Badia JR. Upper airway resistance syndrome. Sleep Med Rev. 1999;3(1):5–21. | ||
Amatoury J, Howitt L, Wheatley JR, Avolio AP, Amis TC. Snoring-related energy transmission to the carotid artery in rabbits. J Appl Physiol (1985). 2006;100(5):1547–1553. | ||
Nam H, Yang HJ, Kim YA, Kim HC. Impact of chronic simulated snoring on carotid atherosclerosis in rabbits. J Clin Neurol. 2013;9(4):269–273. | ||
Narayan J, Amatoury J, Cho JG, et al. Snoring effects on the baroreflex: an animal model. Respir Physiol Neurobiol. 2012;180(2–3):342–351. | ||
Narayan J, Amatoury J, Verma M, et al. Resetting the baroreflex during snoring: role of resistive loading and intra-thoracic pressure. Respir Physiol Neurobiol. 2013;185(3):489–496. | ||
Tsai CF, Huang CL, Lin YL, et al. The neuroprotective effects of an extract of Gastrodia elata. J Ethnopharmacol. 2011;138(1):119–125. | ||
Ahn EK, Jeon HJ, Lim EJ, Jung HJ, Park EH. Anti-inflammatory and anti-angiogenic activities of Gastrodia elata Blume. J Ethnopharmacol. 2007;110(3):476–482. | ||
Yao Y, Huang HY, Yang YX, Guo JY. Cinnamic aldehyde treatment alleviates chronic unexpected stress-induced depressive-like behaviors via targeting cyclooxygenase-2 in mid-aged rats. J Ethnopharmacol. 2015;162:97–103. | ||
Bartlett D Jr. Respiratory functions of the larynx. Physiol Rev. 1989;69(1):33–57. | ||
Kezirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14(5):299–305. | ||
Lowe AA. The neural regulation of tongue movement. Prog Neurobiol. 1980;15(4):295–344. | ||
Hwang JC, St John WM, Bartlett D Jr. Respiratory-related hypoglossal nerve activity: influence of anesthetics. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(3):785–792. | ||
Hwang JC, St John WM. Alterations of hypoglossal motoneuronal activities during pulmonary inflations. Exp Neurol. 1987;97(3):615–625. | ||
Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol (1985). 2000;89(2):590–598. | ||
Lee KZ, Fuller DD. Preinspiratory and inspiratory hypoglossal motor output during hypoxia-induced plasticity in the rat. J Appl Physiol (1985). 2010;108(5):1187–1198. | ||
Fukuda Y, Honda Y. Roles of vagal afferents on discharge pattern and CO2-responsiveness of efferent superior laryngeal, hypoglossal, and phrenic respiratory activities in anesthetized rats. Jpn J Physiol. 1982;32(5):689–698. | ||
Sériès F, Marc I. Influence of genioglossus tonic activity on upper airway dynamics assessed by phrenic nerve stimulation. J Appl Physiol (1985). 2002;92(1):418–423. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.