Back to Journals » Drug Design, Development and Therapy » Volume 15
Toxicological Evaluation of a Probiotic-Based Delivery System for P8 Protein as an Anti-Colorectal Cancer Drug
Authors An BC , Yoon YS, Park HJ , Park S , Kim TY, Ahn JY, Kwon D, Choi O, Heo JY, Ryu Y, Kim JH, Eom H, Chung MJ
Received 6 July 2021
Accepted for publication 23 October 2021
Published 27 November 2021 Volume 2021:15 Pages 4761—4793
DOI https://doi.org/10.2147/DDDT.S319930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Byung Chull An,1 Yeo-Sang Yoon,1 Ho Jin Park,1 Sangkyun Park,1 Tai Yeub Kim,1 Jun Young Ahn,1 Daebeom Kwon,1 Oksik Choi,1 Jin Young Heo,1 Yongku Ryu,1 Joong-Hyun Kim,2 Heejong Eom,2 Myung Jun Chung1
1R&D Center, Cell Biotech, Co., Ltd., Gimpo-si, Gyeonggi-do, Korea; 2Laboratory Animal Center, Osong Medical Innovation Foundation, Chungbuk, Cheongju, 28160, Korea
Correspondence: Myung Jun Chung Tel +82-31-987-6205
Fax +82-31-987-6216
Email [email protected]
Purpose: This study aimed to toxicological evaluate a probiotics-based delivery system for p8 protein as an anti-colorectal cancer drug.
Introduction: Lactic acid bacteria (LAB) have been widely ingested for many years and are regarded as very safe. Recently, a Pediococcus pentosaceus SL4 (PP) strain that secretes the probiotic-derived anti-cancer protein P8 (PP-P8) has been developed as an anti-colorectal cancer (CRC) biologic by Cell Biotech. We initially identified a Lactobacillus rhamnosus (LR)-derived anti-cancer protein, P8, that suppresses CRC growth. We also showed that P8 penetrates specifically into CRC cells (DLD-1 cells) through endocytosis. We then confirmed the efficacy of PP-P8, showing that oral administration of this agent significantly decreased tumor mass (∼ 42%) relative to controls in a mouse CRC xenograft model. In terms of molecular mechanism, PP-P8 induces cell-cycle arrest in G2 phase through down-regulation of Cyclin B1 and Cdk1. In this study, we performed in vivo toxicology profiling to obtain evidence that PP-P8 is safe, with the goal of receiving approval for an investigational new drug application (IND).
Methods: Based on gene therapy guidelines of the Ministry of Food and Drug Safety (MFDS) of Korea, the potential undesirable effects of PP-P8 had to be investigated in intact small rodent or marmoset models prior to first-in-human (FIH) administration. The estimated doses of PP-P8 for FIH are 1.0× 1010 – 1.0× 1011 CFU/person (60 kg). Therefore, to perform toxicological investigations in non-clinical animal models, we orally administered PP-P8 at doses of 3.375 × 1011, 6.75 × 1011, and 13.5× 1011 CFU/kg/day; thus the maximum dose was 800– 8000-fold higher than the estimated dose for FIH.
Results: In our animal models, we observed no adverse effects of PP-P8 on clinicopathologic findings, relative organ weight, or tissue pathology. In addition, we observed no inflammation or ulceration during pathological necropsy.
Conclusion: These non-clinical toxicology studies could be used to furnish valuable data for the safety certification of PP-P8.
Keywords: biologics, therapeutic protein, probiotics derived anti-cancer protein P8, Pediococcus pentosaceus SL4, Lactobacillus rhamnosus, colorectal cancer, drug delivery system, protein secretion system, oral administration, non-clinical trial, toxicology finding
Introduction
CRC is a widespread fatal disease. Each year, approximately 1 million people around the world are diagnosed with CRC, of whom ~50% will be dead within 5 years of diagnosis.1 Chemotherapy for CRC be subjected to suppress or prevent the recurrence of CRC after surgical resection. However, the efficacy of chemotherapy agents is compromised by their potential toxicity.2 The molecularly targeted therapies be used to treatment for solid cancers, although safer than general chemotherapeutic agents, have significant side effects.3 Accordingly, more effective and safer treatments are urgently required.
To overcome safety limitations, bioactive components such as natural and biological substances have been proposed as substitutes for conventional chemotherapeutic agents.2,4 Bioactive components are probiotics or probiotic-derived biomolecules (postbiotics). Probiotics are functional foods constituted by non-pathogenic intestinal bacteria that confer unique health benefits on the host.5–7 Probiotics has been reported to have therapeutic potential against various diseases;8 diarrhea,9 inflammatory bowel disorder (IBD),10 ulcerative colitis (UC),11 Crohn’s disease (CD),12 lactose intolerance,13 and cancers.14,15 Furthermore, the recommended amount of probiotics in functional foods (not be less than 108 to 109 colony-forming units/g) corrects dysbiosis and maintains eubiosis of gut microbiota.16 Dysbiosis, a reduced diversity of gut microbiota, is associated with damage to gut barrier functions such as intestinal immune system modulation, inflammatory cell activation, and the structure of the intestinal barrier.17
Therefore, we recently proposed using a lactic acid bacteria (LAB)-based drug delivery system (DDS) for secretion of proteins with therapeutic effects. Because LAB are intestinal microbes in humans, they are generally regarded as safe. Moreover, secretion of a probiotic-derived anti-CRC protein by LAB would likely have very few or mild adverse side effects.18 LAB are beneficial microorganisms that confer health benefits on the host and play roles in therapy;19 recent studies have demonstrated that some LAB strains effectively inhibit the development of CRC via multiple pathways.20–22 However, despite their importance to human health, few proteins have been isolated from LAB and evaluated for therapeutic potential.23–25
Recently, we described the first LAB DDS with the ability to suppress CRC that employed the delivery of a probiotic-derived anti-cancer protein directly to the intestine. A major advantage of food-grade LAB-based DDS is that it is unlikely to be pathogenic; consistent with this idea, LAB have never been reported to pose a risk to health.18,26 Moreover, our approach could lead to the development of more cost-effective, intestine-specific, long-term therapies for human CRC.
We also isolated a novel biologic agent, the Lactobacillus rhamnosus-derived P8 protein, which exhibits anti-CRC properties in both cell culture and xenografts.27,28 To develop a new LAB-based biologic, we generated a transgenic Pediococcus pentosaceus SL4 (PP) DDS expressing P8 (PP-P8) for oral administration, and verified its anti-CRC efficacy in a xenograft model.27,29 Innovative biologics such as PP-P8 have been genetically engineered in an effort to create new therapeutics with fewer off-target activities.30,31
Furthermore, oral administration of PP-P8 can accelerate recovery from chemically induced dysbiosis. Correlation analysis showed that PP-P8 can contribute to rebiosis.32
Novel approaches for the development of anti-cancer drugs holds great promise for cancer therapy; however, new strategies also have a higher risk of failure due to issues with efficacy or safety. In the case of PP-P8, we adopted live LAB as a drug delivery vehicle. Moreover, the anti-cancer P8 protein, which is secreted when PP-P8 reach the intestine, is also derived from probiotics, further increasing the probability that it is safe. We used genetic engineering tools and a recombinant plasmid [pCBT24-2-PK-P8-PK-P8 plasmid (accession number: KCCM12181P)] to create a LAB-based DDS capable of secreting P8 protein in the intestine.
In this study, we performed toxicological analysis of PP-P8 to confirm the safety of this system. The findings reported in this article will be useful for determining a suitable dose of PP-P8 for clinical applications, before the function of PP-P8 in normal tissues and physiology is fully understood.
Materials and Methods
Bacterial Strains and Culture
Lactobacillus rhamnosus (LR) KCTC 12202BP was isolated from human feces and Pediococcus pentosaceus SL4 (PP) KCTC 10297BP was isolated from kimchi. Both strains were obtained from the culture collection maintained at Cell Biotech (Gimpo, Korea) and cultured for 18–24 h in MRS broth (Difco, Detroit, MI, USA) at 37°C.27 Escherichia coli strains were cultured for 18–24 h in Luria–Bertani broth (LB broth, Difco) or on MacConkey agar (Difco) at 37°C.
Purification of Recombinant P8 from Escherichia coli
A P8 gene with restriction enzyme sites (5ʹ-NdeI/3ʹ-SalI) was synthesized by Cosmogenetech (Seoul, Korea) and then cloned into the Escherichia coli expression vector (pET-22b; Novagen, Madison, WI, USA), which was described previously.27 The resultant pET-22b::P8 construct was transformed into Escherichia coli strain C41(DE3) (Novagen), which was cultured in M9 medium to an optical density (OD) of 0.6. The recombinant P8 protein (r-P8) contains a hexa-histidine (6×His) and a TEV protease cleavage site in its N-terminal region. R-P8 protein was purified by binding to Ni2+-NTA agarose (Qiagen, Valencia, CA, USA), and then the 6×His tag was removed using TEV protease (GenScript, Piscataway, NJ, USA). To confirm the homogeneity of the purified r-P8, r-P8 was applied to a size exclusion column (HiLoad 26/60 Superdex 200 pg; GE Healthcare, Boston, MA, USA).
Cell Proliferation Assay
DLD-1 cells (1 X 103 cells/well) were seeded into 96-well plates. After 24 h, r-P8 protein (0, 40 μM) was added to each well and incubated for a further 72 h. Next, both cells [control and r‐P8 treatment] were stained for 30 min with the Live cell marker Syto9 (Green) (LIVE/DEAD® Viability/Cytotoxicity Kit; ThermoFisher scientific, Waltham, MA, USA) after removal of the medium. Live cells were visualized and images were analyzed using ImageXpress®Micro Confocal microscope (Molecular Devices).27,28
Generation of Transgenic Pediococcus pentosaceus SL4 Harboring the pCBT24-2-PK-P8-PK-P8 Plasmid
An artificial P8 expression system [pyruvate kinase promoter (PK)-secretion signal peptide (usp45)-P8] was designed to enable the insertion of synthesized DNA fragments. A two codon-optimized artificial P8 expression system with restriction enzyme sites (5ʹ-NheI/3ʹ-SalI or 5ʹ-BamHI/NdeI-3ʹ) was synthesized by ATUM (Newark, CA, USA) and cloned into the expression vector pCBT24-2 (KCCM12182P). Two copies of the artificial P8 expression system were used to maximize the expression and secretion of P8 protein. Finally, the pCBT24-2-PK-P8-PK-P8 plasmid (accession number: KCCM12181P) was transformed into PP cells, which were described previously.27 The transformant selected using MRS agar plates containing erythromycin (10 mg/mL) was inoculated into 10 mL MRS broth containing erythromycin and cultured at 37°C for 15 h (no shaking). Next, 1 mL pre-culture was inoculated into 10 mL M9 minimal medium containing erythromycin and cultured at 37°C for 48 h (no shaking). The pure culture supernatant was collected by centrifugation and then concentrated using the TCA precipitation method to isolate the secreted protein. Finally, the secreted PP-P8 protein was detected by Western blotting.33
Detection of PP-P8 Protein in the PP-P8 Culture Supernatant
To collect PP-P8 culture media without cells, the culture was centrifuged at 7000 rpm for 15 min at 4°C, and the supernatant was subjected to microfiltration (MF) on a 0.2 μm ULTA filtration system (Cytiva, Marlborough, MA, USA). To concentrate PP-P8 in the culture medium, a 3 kDa hollow-fiber membrane (UFP-3-C-4X2MA, 0.014 m2, 60 cm) was connected to an ÄKTA flux 6 system (Cytiva), and the trans-membrane pressure (TMP) value was maintained at 1.5 bar. The culture concentrate (300 mL) was recovered by ultrafiltration (UF) and dialysis with binding buffer (20 mM Na-P, pH 7.4), and the amount of P8 protein in the concentrated solution was determined by SDS-PAGE and Western blotting. To purify the P8 protein from the concentrated solution, an anti-P8 pAb-coupled resin was prepared using CNBr-activated Sepharose 4B.34 Purification of P8 protein from the concentrated solution by immuno-affinity chromatography (IAC) was performed as described.35 The amount of purified PP-P8 protein was determined by SDS-PAGE and Western blotting. Next, purified PP-P8 protein was subjected to quadrupole time-of-flight (Q-TOF) LC/MS/MS analysis for identification (Gyeonggido Business & Science Accelerator Bio Center, Suwon-si, Gyeonggi-do, Korea).
Animal Care and Maintenance
ICR mice (8-week-old) were used for the single-dose oral toxicity study. ICR mice (6-week-old) were used for the 2 week repeated oral dose range-finding (DRF) study, the 4 week repeated oral toxicity study, and the 4 week recovery test. All test mice were obtained from Samtako Bio Korea (Osan-si, Gyeonggi-do, Korea). The toxicity tests were conducted in facilities of the Chemon Preclinical Research Center (Yongin-Si, Gyeonggi-do, Korea), which has been approved by the Korean government, and animals were maintained in accordance with the guide for the care and use of laboratory animals.36 The animal use protocol was reviewed and approved by The Chemon Institutional Animal Care and Use Committee (Chemon IACUC). These selections were accepted by Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and Use Committee (Chemon IACUC; See below).
- Single-dose oral toxicity study in ICR mice [Chemon Study No. 18-MA-0928; Approval No. 18-M597]
- Two-week repeated oral DRF study in ICR mice [Chemon Study No. 17-MR-0814; Approval No. 18-M132]
- Four-week repeated oral toxicity study and 4 week recovery test in ICR mice [Chemon Study No. 17-MR-0816; Approval No.18-M534]
The Marmoset has several advantages in toxicology studies.37 Marmosets (32 animals/sex/group; males, 1.5–3 years; females, 1.5–3 years) were obtained from Kbio Health (Cheonju-si, chungbuk-do, Korea). The animal use protocol was accepted by AAALAC International and the Osong Medical Innovation Foundation (OMIF) Institutional Animal Care and Use Committee (IACUC; KBIO-IACUC-2019-011-1), and was in accordance with OMIF animal testing center Standard Operating Procedure (SOP) recommendations for 4 week repeated dose toxicity and studies.
Justification for Selection of the Dose Level
The amount of active probiotic is generally expressed as CFU (colony-forming units), a unit of measure used in microbiology to estimate the number of bacteria capable of forming colonies in a sample of a probiotic-based biologic. Although general clinical trials have indicated that probiotic doses of 10–20 billion CFU per day are sufficient to maintain immune and digestive health, products with 50–100 billion CFU are gradually becoming more common.38,39 Therefore, it may not be necessary to use a high CFU dose to achieve greater probiotic benefits, especially given that the goal of probiotics is to balance the gut microbiota.40
In the toxicity study, we tested PP-P8 over a three-point dose escalation [3.375 × 1011 CFU/kg, 6.75×1011 CFU/kg, and 13.5×1011 CFU/kg]. The maximum dose [13.5 × 1011 CFU/kg] of PP-P8 was 800-fold higher than the optimal starting dose for a clinical trial [1 × 1011 CFU/person (60 kg) ≒ 1.7×109 CFU/kg]. Although most people do not need a huge CFU to achieve general probiotic benefits, high-dose PP-P8 regimens (>50 billion CFU) have been considered for clinical treatment of CRC patients with extreme gastrointestinal dysfunction or dysbiosis.41,42 Although a daily dose of 10–15 billion CFU is advisable for individuals seeking everyday immune and digestive support, treatment with higher doses may be considered for people who experience significant changes in their gut microbiome caused by illness, intense antibiotic therapy, or environmental toxins. However, individuals who think they require probiotics should consult with a medical professional about their health status and determine whether a high-dose probiotic regimen might improve their health.
Observation and Examination of Toxicity Studies
Clinical Signs
All animals were observed once a day, and any clinical symptoms of toxicity or mortality were recorded. Electrocardiograms (ECGs) of marmosets were conducted before treatment, after treatment, and after recovery to measure heart rate, P-R interval, QRS interval, and QT interval. These examinations were performed by a veterinarian from Konkuk University Veterinary Medical Teaching Hospital (Seoul, Korea).
Body Weight and Food Consumption
Body weights of all animals were measured once weekly after the start of treatment, and food consumption was recorded. Food consumption was calculated based on the difference between the supplied amount and the remaining amount.
Urinalysis
Urinalysis of all animals was performed during the last week of treatment using Multistix 10 SG strips (Siemens AG, München, Germany) and a urine analyzer (Clinitek Advantus, AG). After collection of fresh urine, the samples were analyzed for glucose (GLU), bilirubin (BIL), ketone body (KET), specific gravity (SG), pH, protein (PRO), urobilinogen (URO), nitrite (NIT), occult blood (BLO), leukocyte (LEU), and color.
Electrocardiographic (ECG) Examination
In the lead ECG test, bipolar lead (I, II, III), unipolar lead (aVL, aVF, aVR), S wave, Q-T interval, P-R interval, R wave, P-R interval, QRS duration, T wave, and heart rate were analyzed by Professor Kim Jung-Hyun of the Veterinary Teaching Hospital, Konkuk University, Seoul, Korea.44,45
Ophthalmological Tests
Eyes of all animals in each group were examined both before treatment and after treatment, including the recovery period. The ocular fundus examination was performed using a fundus camera (Retimera 1.0.; IIScience, Yangsan, Korea) after the pupil was dilated with a mydriatic drug (Ocu-Tropine; Samil-Pharm, Seoul, Korea).
Necropsy and Organ Weight
After an overnight fast, necropsy of all animals was performed under anesthesia with 5% isoflurane, and moribund or dead animals also underwent necropsies soon after they were found.46–48 Blood samples were collected from the posterior vena cava, after which the animal was euthanized by exsanguination. The anatomic contents of animals in all orifices, all organs, and external surfaces were examined and recorded. To perform microscopic and histopathological examinations, tissues and organs of all animals were individually collected and preserved in 10% neutral buffered formalin. In addition, absolute and relative organ weights were measured. The major organs measured were as follows: thymus, spleen, liver, lung, adrenal gland left/right, brain, kidney left/right, and heart.
Hematology
Each blood sample was transferred to CBC bottles (EDTA 3 K; BD Biosciences, San Jose, CA, USA) and assayed on a hematology analyzer (Advia 120E; Siemens Healthcare, Malvern, PA USA). Hematological measurements included eosinophil (EOS), mean corpuscular hemoglobin concentration (MCHC), hematocrit (HCT), neutrophil (NEU), hemoglobin (HGB), mean corpuscular volume (MCV), large unstained cell (LUC), lymphocyte (LYM), red blood cell (RBC), white blood cell (WBC), platelet (PLT), mean corpuscular hemoglobin (MCH), monocyte (MONO), mean platelet volume (MPV), basophil (BASO), red cell distribution width (RDW), and hemoglobin distribution width (HDW).
Blood Biochemistry
Blood samples were transferred into Vacutainer tubes (SST II Advance; BD, Hunter Boulevard, UK) and allowed to clot for 20 min at room temperature. After centrifugation at 3000 rpm for 10 min, hematological parameters in plasma were analyzed on an AU680 Chemistry system (Beckman Coulter, Brea, CA, USA). The following hematological parameters were studied: albumin (ALB), alkaline phosphatase (ALP), aspartate aminotransferase (AST), albumin/globulin (A/G ratio), blood urea nitrogen (BUN), total protein (TP), creatinine (CRE), total bilirubin (TBIL), calcium ion (Ca2+), triglyceride (TG), potassium ion (K+), chloride ion (Cl−), total cholesterol (TCHO), creatine phosphokinase (CPK), alanine aminotransferase (ALT), glucose (GLU), sodium ion (Na+), and inorganic phosphorus (IP).
Histopathology
Organs of all animals were collected and fixed in 10% neutral buffered formalin at necropsy. After fixation for 18 h, paraffin embedding was conducted and 3–4 µm sections were prepared according to routine histological methods.49 All slides for histopathological examination were prepared using sectioned-tissues or -internal organs. Representative sections of each organ or tissue on slides were stained with hematoxylin and eosin for light microscopy. Histopathological examinations and analyses were performed by KNOTUS (Incheon, Korea). The following organs were examined: liver, kidney, thymus, adrenal gland, lung, parathyroid, jejunum, esophagus, thyroid, salivary gland, heart, eye, ileum, brain, duodenum, and pituitary gland.
Statistical Analysis
Body weight, food consumption data, blood biochemistry data, hematological data, and organ weight values were analyzed for homogeneity of variance using Levene’s test.50 Statistical analyses were performed using one-way analysis of variance (ANOVA) on the Prism 4. The results are presented as mean and standard deviation (means ±). Multiple comparisons were performed using Tukey’s multiple comparison test. A value of P < 0.05 was regarded as statistically significant.
Results
Probiotic-Based DDS (PP-P8) Resulted in Anti-CRC Activity via PP-P8 Secretion
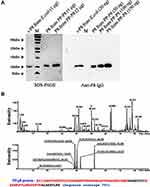
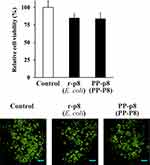
Previous studies suggested that P8 protein suppresses proliferation of CRC, and we have reported that P8 protein can be detected in intestinal juice, blood, and CRC tissue.27–29 To date, however, no study has compared the anti-CRC efficacy between PP-secreted P8 protein (PP-P8) and Escherichia coli-expressed P8 protein (r-P8), despite the importance of this comparison for drug dose determination. We successfully purified P8 protein (PP-P8) from PP-P8 culture media by IAC and then visualized P8 protein by SDS-PAGE and Western blotting (Figure 1A). Subsequently, we characterized the purified P8 using LC-MS/MS (Figure 1B). We then tested the anti-proliferative effect of P8 protein at a concentration of 20 μM; previously, we reported 40 μM as the optimal concentration of P8 protein, but in this study the purification of P8 gave a poor yield. We observed no difference in anti-proliferative activity (Figure 2).
Single-Dose Oral Toxicity Study in ICR Mice
[Chemon Study No. 18-MA-0928]
The toxicology of acute single-dose oral administration of PP-P8 in male and female ICR mice was tested at three increasing doses [3.375 × 1011 CFU/kg, 6.75×1011 CFU/kg, and 13.5×1011 CFU/kg]. The maximum dose [13.5 × 1011 CFU/kg] was 800-fold higher than the optimal starting dose for a clinical trial [1 × 1011 CFU/person (60 kg) ≒ 1.7×109 CFU/kg]. Each test article was orally administered in a single dose of 200 μL/mouse using an oral Zonde needle. In this toxicity test, PP-P8 treatment-related mortality was not detected at any dose, including vehicle control (PP-EV), and no abnormal clinical symptoms were detected during the clinical observation period. We detected no meaningful differences in body weight relative to the PP-EV control at any dose tested. Moreover, necropsy revealed no significant changes in 12 principal organs in treated vs non-treated groups. These toxicological results showed that lethal dose (ALD) of PP-P8 mice was higher than the maximum dose tested in this study [13.5 × 1011 CFU/kg].
Two-Week Repeated Oral DRF Study in ICR Mice
[Chemon Study No. 17-MR-0814]
To investigate the appropriate dose of PP-P8 for the 4 week repeated oral toxicity study and 4 week recovery test, we conducted a 2 week repeated oral DRF study in male and female ICR mice.
As with the previous study, we tested PP-P8 over a three-point dose escalation [3.375 × 1011 CFU/kg, 6.75×1011 CFU/kg, and 13.5×1011 CFU/kg]. Each test article was orally administered in a single dose at a volume of 200 μL/mouse using an oral Zonde needle. During the experimental period, we measured and recorded clinical data on food consumption, body weight, mortality, serum biochemistry, urine biochemistry, ophthalmic features, hematology, and organ masses. No deaths or clinical symptoms were associated with PP-P8 oral administration, and we observed no significant changes in food intake or body mass between the PP-P8-treated and PP-EV-treated groups. Ophthalmic examination, urinalysis, hematological tests, clinical biochemistry tests, and necropsy findings revealed no significant abnormalities in either group.
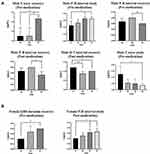
Males exhibited a slight increase in spleen mass as PP-P8 dose increased, although spleen mass was unaffected in females (Table 1). Therefore, the increase in spleen mass symptom may be dose–response related and should be confirmed in a 4 week repeat administration trial. However, we conclude that a maximum dose of 13.5×1011 CFU/kg is appropriate for a 4 week repeat administration trial, because abnormal spleen hyperplasia was only observed in males and the symptoms were not severe.
 |
Table 1 Absolute (g) and Relative (%) Organ Weights (DRF) |
Four-Week Repeated Oral Toxicity Study and 4 Week Recovery Test in ICR Mice
[Chemon Study No. 17-MR-0816]
To assess the long-term toxicity of repeated oral dosing with PP-P8, we tested PP-P8 at increasing doses [3.375 × 1011, 6.75 × 1011, and 13.5×1011 CFU/kg]. During the experimental period, we evaluated the same clinical features as before the study.
No clinical symptoms or death were associated with PP-P8 oral administration, and we observed no significant differences in food intake or body mass between the PP-P8-treated and PP-EV-treated groups. Ophthalmic examination, clinical biochemistry tests, urinalysis, hematological test, and necropsy findings revealed no significant abnormalities in any group.
Males exhibited a slight increase in spleen mass as PP-P8 dose increased, although spleen mass was unaffected in females (Table 1). The dose–response relationship implied that this change was a consequence of PP-P8 administration. Therefore, we concluded that this symptom should be followed in the 4 week repeated oral toxicity study in the mouse model and the 4 week repeated oral toxicity study in the marmoset model, and that the effect of PP-P8 administration on absolute and relative organ weights should also be followed. The abnormal spleen symptoms in males were not observed in the PP-P8 4 week repeated oral toxicity study in mouse (Table 2, Figure 3A). Moreover, because the abnormal spleen hyperplasia was only observed in males, and the symptoms were not severe, the maximum dosage of 13.5×1011 CFU/kg was considered to be appropriate for the 4 week repeated administration trial (Table 2, Figure 3B).
 |
Table 2 Absolute and Organ Weights (Mouse 4 Weeks Repeated) |
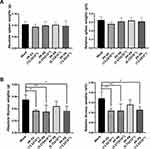 |
Figure 3 Follow-up study of PP-P8 oral administration in spleen and thymus of male mice. In a 2 week repeated oral dose range-finding (DRF) study of PP-P8, absolute and relative organ weights were monitored. A dose–response relationship was observed for spleen mass in male mice (Table 1), indicating that the influence of PP-P8 administration should be checked in a 4 week repeated oral toxicity study. (A) PP-P8 was not associated with abnormal spleen symptoms. (B) Abnormal thymus hyperplasia was observed in some animals; however, the symptoms were not severe, and no dose–response relationship was observed. * and **Represent significant differences (p<0.05 and p<0.01, respectively). |
Four-Week Repeated Oral Toxicity Study and 4 Week Recovery Test in Marmosets
[KBIOHEALTH 2019–011-CS]
To confirm the safety of PP-P8 using marmoset, which is closer to human, we performed a 4 week repeated oral toxicity test and a 4 week recovery test using the same doses as in the ICR mouse model described above. Each test article was orally administered as a single dose at a volume of 3 mL/kg using an oral catheter. During the experimental period, we evaluated clinical signs, mortality, food consumption, body weight, organ masses, electrocardiography, serum biochemistry, urine biochemistry, hematology, histopathological examination, prothrombin time examination, and ophthalmic features.
Clinical Signs, Mortality, Food Intake, and Body Mass
No deaths were associated with any dose of orally administered PP-P8, and examination of clinical contents showed no abnormalities during the experimental period. No changes in weight were observed: average initial body mass was 340 ± 26 g; in the PP-P8–treated and recovery groups, average body mass was 334 ± 28 g and 339 ± 24 g, respectively (Table 3). PP-P8 caused no significant difference in any symptom monitored (Data not shown).
 |
Table 3 Body Weight of Marmoset |
ECG
ECG evaluation revealed no significant difference among test groups (Table 4, Figure 4). When we compared ECG before and after administration of 0.9% saline (G1), used as a negative control, we observed reductions in heart rate (42.9% and 44.2%, respectively) and mild conduction disturbances in two of eight animals. In addition, we observed mild left ventricular hypertrophy (38.4%) in one animal.
 |
Table 4 ECG Evaluation |
Next, when we compared ECG before and after administration of the maximum dose of PP-EV [13.5 × 1011 CFU/kg; G2], used as a positive control, we observed mild left ventricular hypertrophy (20%) in one of eight animals. Comparison of ECG before and after administration of low-dose PP-P8 [3.375 × 1011 CFU/kg; G3] revealed a slight right ventricular hypertrophy (33.3% and 10%) in two of eight animals and mild left ventricular hypertrophy (6.25%, 25.9%, and 11.5%) in three animals. Comparison of ECG before and after administration of maximum-dose PP-P8 (G4) revealed mild or significant left ventricular hypertrophy (26.3%, 57.8%, and 9.75%) in three of eight animals. After recovery, ECG examination revealed no significant difference among test groups (Table 4). We observed no difference in ECG before administration vs after recovery. In the positive-control group, we observed mild left ventricular hypertrophy (5.26%) in one of six animals. When we compared before administration of low-dose PP-P8 vs after recovery, we observed slight left ventricular hypertrophy or reduction (37.5% and 20%) in two of six animals, and a slight right ventricular hypertrophy (33.3%) in one animal. In the group receiving maximum-dose PP-P8, we observed mild left ventricular hypertrophy (15.3% and 12%) in two of six animals.
Ophthalmic Examination
Ophthalmic examination showed no pathological effects of PP-P8 oral administration in any of the animals tested (data not shown).
Hematology
We performed hematological tests using whole blood collected before or after 4 week PP-P8 administration or 4 week recovery. Comparison of the hematological test results before vs after PP-P8 administration revealed a significant decrease in the MCHC level in both sexes in the low-dose PP-P8 group. Other statistically significant changes in the PP-P8 administration group were not correlated with dose and were within the normal physiological range (Table 5).
 |
Table 5 Hematological Values |
Blood Coagulation Test
We observed no statistically significant changes in blood coagulation. However, the recovery group of 0.9% saline administration (G1) exhibited a mild decrease (0.76-fold) in prothrombin time (PT) and a slight increase (1.15-fold) in activated partial thromboplastin time (APTT) (Table 6).
 |
Table 6 Coagulation Time |
Blood Biochemistry
Blood biochemical tests were performed using serum samples of all marmosets. We observed no statistically significant changes in blood biochemistry after PP-P8 oral administration.
Comparison of hematological test results between pre-treatment and post-treatment revealed a significant increase in Ca level in males of the maximum-dose PP-P8 of recovery group. By contrast, the recovery group of 0.9% saline administration exhibited a significant decrease in IP. Other statistically significant changes observed in the PP-P8 administration group were not correlated with dose and were within the normal physiological range (Table 7).
 |
Table 7 Serum Biochemical |
Urinalysis
Urinalysis by a semi-quantitative method revealed proteinuria exceeding the normal range in one male in the maximum-dose PP-P8 recovery group, one female in the PP-EV group (positive control), and one female in the 0.9% saline (negative control) recovery group, both pre- and post-treatment. Two females in the low-dose PP-P8 recovery group exhibited proteinuria exceeding the normal range after treatment. We did not observe any statistically significant changes in other urinalysis results between the pre- and post-treatment groups (Table 8).
 |
Table 8 Urinalysis Values |
Necropsy Findings
During necropsy, we observed hepatic fibrosis, adrenal lesions, and lung lesions in one female in the low-dose PP-P8 group. However, these abnormal findings were observed only in one sex and not in other experimental groups. A mild inflammation of the small intestine was observed in one female and one male in the low-dose PP-P8 administration group (G3). In addition, intussusception was observed in one male in the low-dose PP-P8 administration group, and one male each in the 0.9% saline recovery group (G5) and the low-dose PP-P8 administration group (G6). However, this abnormal finding was observed only in one sex, and was not detected in the maximum-dose group (G4) or in the corresponding recovery group (G7) (Table 9).
 |
Table 9 Gross Organ Observation |
Organ Weight
Weights of the heart and left adrenal gland were elevated in one male in the 0.9% saline group (G1). Weights of liver and salivary glands were elevated in one animal, and the weight of the heart exceeded the normal physiological range in one animal. In addition, in one animal in the low-dose PP-P8 recovery group (G6), the liver exceeded the normal physiological weight range. However, increases in organ weights in other subjects were not accompanied by gross findings (Tables 10 and 11).
 |  |  |
Table 10 Absolute Organ Weights |
 |  |  |
Table 11 Relative Organ Weights |
Histopathology
Bilateral extramedullary hematopoiesis and vacuolation lesions in the cytoplasm were observed in the adrenal cortex and medullary tissues of most individual cases (Table 12). Fatty changes, extramedullary hematopoiesis, and glycogen accumulation were also observed in the liver. Hepatocyte necrosis and fibrosis were also detected in some individuals. In the kidney, we observed infiltration of inflammatory cells in the interstitium, extramedullary hematopoiesis in the medulla, infarction/dilatation of the tubules, and a dark brown granular material in the tubules.
 |  |  |
Table 12 Histopathological Examination |
Proliferation of C cells, infiltration of inflammatory cells, and atrophy/involution of ectopic thymus were observed in the thyroid gland.
In the lung, the alveolar cavity contained macrophages that engaged in phagocytosis of exogenous substances. In addition, infiltration of acute inflammatory cells and inflammatory cells were observed around the bronchioles in some individual cases.
In the digestive system, we observed infiltration of inflammatory cells in the stomach, duodenum, jejunum, and ileum, as well as enlargement and proliferation of Brunner's gland.
In the brain, we observed pigmentation of intrameningeal and cyst formation or fibrosis in pituitary gland tissue in a small number of cases.
Discussion
To evaluate the in vivo toxicity of PP-P8, developed as an anti-CRC drug,27,28 we performed several studies of PP-P8 using mouse and small primate models:
- Single-dose oral toxicity study in ICR mice [Chemon Study No. 18-MA-0928]
- Two-week repeated oral DRF study in ICR mice [Chemon Study No. 17-MR-0814]
- Four-week repeated oral toxicity study and 4 week recovery test in ICR mice [Chemon Study No. 17-MR-0816]
- Four-week repeated oral toxicity study and 4 week recovery test in marmosets [KBIOHEALTH 2019-011-CS]
In the single-dose oral toxicity study using ICR mice, we observed no significant changes. However, the 2 week repeated oral DRF study in ICR mice revealed that relative and absolute spleen mass increased slightly in males, dependent upon PP-P8 dose, but spleen mass was unaffected in females (Table 1). This symptom may be dose–response related; therefore, it is judged by the influence of PP-P8 administration. However, this symptom was not observed in the 4 week repeated oral toxicity study and 4 week recovery test using ICR mice, and we observed no other significant changes in these animals. To verify the safety of PP-P8 using marmoset, which is close to human, we performed a 4 week repeated oral toxicity test and a 4 week recovery test [KBIOHEALTH 2019–011-CS] in this species. This toxicity study was performed using 32 marmosets, of which 18 marmosets were examined after 4 weeks of recovery following the 4 week repeated oral administration test. During the experimental period, we evaluated food consumption, body weight, electrocardiography, PT, clinical signs, ophthalmic features, hematology, urine biochemistry, serum biochemistry, organ masses, mortality, and histopathological features to assess toxicity. During the test period, we observed no deaths or abnormal changes associated with PP-P8 administration.
ECG revealed significant left ventricular hypertrophy in one of eight animals in the maximum-dose PP-P8 group (G4) and a significant decrease in heart rate in two of eight animals in the 0.9% saline group (G1). However, we concluded that the decrease in heart rate was due to the ECG characteristics of small primates under anesthesia. Moreover, the ECG levels in other marmosets changed by <40%, and we observed no significant change before vs after PP-P8 administration.
The blood coagulation test revealed a significant decrease (0.76-fold) in PT and a mild increase (1.15-fold) in APTT in the 0.9% saline recovery group (G1). However, we concluded that this change was not due to PP-P8 administration, as it was observed only in one sex and the values were within the normal physiological range.51
Semi-quantitative urinalysis revealed proteinuria exceeding the normal range in several marmosets throughout the entire experimental period. However, comparison between pre- and post-treated animals indicated that this symptom was not related to PP-P8 administration. Monkeys have a wider normal range of proteinuria (0–179 mg/dl) than humans (<16 mg/dl);52 in addition, gross lesions were observed only in one sex, and we observed no dose–response relationship. Therefore, based on individual weight, frequency, and degree of incidence, we concluded that this symptom was unrelated to PP-P8 administration. A weight increase in the right adrenal gland and gross lesions were observed in one animal of the low-dose PP-P8 group, and histopathological examination revealed bilateral mild extramedullary hematopoiesis and vacuolation of the cytoplasm in the adrenal cortex and medulla. However, these mild lesions occur naturally in marmoset monkeys, and it shows a mild degree even compared to other experimental groups. Therefore, we concluded that these findings were unrelated to PP-P8 administration.
Statistically significant changes in other organ weights did not exhibit a dose–response relationship, but rather appeared sporadically throughout the study. Consequently, there was judged not to be pathological findings by combining individual weight and pathological findings.
Histopathological observation revealed intussusception in one male in the low-dose PP-P8 group (G3) and one animal each in the 0.9% saline (G1) and low-dose PP-P8 recovery groups (G6). In addition, we observed inflammation in the small intestine in one male and one female in the low-dose PP-P8 group (G3), and one female in the maximum-dose PP-P8 group (G4). Spontaneous gastroenteritis can be observed in marmosets,44 implying that these lesions may not lead pathological changes. Because this change was observed in only one sex and was not present in the high-concentration group, we concluded that it was not related to PP-P8 administration. The symptoms frequently observed by histopathology, including extramedullary hematopoiesis, infiltration of inflammatory cells, and fatty degeneration of the liver, are naturally occurring lesions in marmosets.46–48 Other lesions were also sporadic, irrespective of PP-P8 dosage.
In conclusion, we determined that these findings were unrelated to PP-P8 administration. Because we observed no severe symptoms or observed them in only one sex group of the mouse model. Furthermore, to investigate the toxicity of PP-P8 oral administration in a nonclinical model close to that of human, we performed repeated oral administration of PP-P8 for 4 weeks at doses of 3.375×1011 and 13.5×1011 CFU/kg in the marmoset, a small primate. The results of the study revealed no toxic changes related to PP-P8 administration in any experimental group. The No Observed Adverse Effect Level (NOAEL) for PP-P8 in this study was 13.5×1011 CFU/kg for both male and female marmosets. As a future study, we intend to submit a new drug (IND) application to the Korea Ministry of Food and Drug Safety (MFDS) with the aim o performing a clinical PP-P8 trial. The toxicity data reported in his study will provide valuable data for the safety certification of probiotics based on a drug delivery system, because PP-P8 is the first agent to be used for anti-cancer drug development.
Abbreviations
LAB, Lactic acid bacteria; PP, Pediococcus pentosaceus SL4; LR, Lactobacillus rhamnosus; PP-P8, Pediococcus pentosaceus SL4 (PP) secretes the probiotic-derived anti-cancer protein P8; CRC, Colorectal cancer; IND, Investigational new drug; MFDS, The Ministry of Food and Drug Safety; FIH, First-in-human; IBD, Inflammatory bowel disorder; UC, Ulcerative colitis; CD, Crohn’s disease; DDS, Drug delivery system; r-P8, Recombinant P8 protein; 6×His, Hexa-histidine; OD, Optical density; PK, Pyruvate kinase promoter; MF, Microfiltration; TMP, Trans-membrane pressure; UF, Ultrafiltration; IAC, Immuno-affinity chromatography; Q-TOF, Quadrupole time-of-flight; DRF, Dose range-finding; Chemon IACUC, Chemon Institutional animal care and use committee; AAALAC International, Assessment and Accreditation of Laboratory Animal Care International; OMIF, Osong Medical Innovation Foundation; SOP, Standard Operating Procedure; CFU, Colony-forming units; ECGs, Electrocardiograms; GLU, Glucose; BIL, Bilirubin; KET, Ketone body; SG, Specific gravity; PRO, Protein; URO, Urobilinogen; NIT, Nitrite; BLO, Occult blood; LEU, Leukocyte; EOS, Eosinophil; MCHC, Mean corpuscular hemoglobin concentration; HCT, Hematocrit; NEU, Neutrophil; HGB, Hemoglobin; MCV, Mean corpuscular volume; LUC, Large unstained cell; LYM, Lymphocyte; RBC, Red blood cell; WBC, White blood cell; PLT, Platelet; MCH, Mean corpuscular hemoglobin; MONO, Monocyte; MPV, Mean platelet volume; BASO, Basophil; RDW, Red cell distribution width; HDW, Hemoglobin distribution width; ALB, Albumin; ALP, Alkaline phosphatase; AST, Aspartate aminotransferase; A/G ratio, Albumin/globulin; BUN, Blood urea nitrogen; TP, Total protein; CRE, Creatinine; TBIL, Total bilirubin; Ca2+, Calcium ion; TG, Triglyceride; K+, Potassium ion; Cl−, Chloride ion; TCHO, Total cholesterol; CPK, Creatine phosphokinase; ALT, Alanine aminotransferase; GLU, Glucose; Na+, Sodium ion; IP, Inorganic phosphorus; PT, Prothrombin time; APTT, Thromboplastin time.
Consent for Publication
All authors agree with publication of this study.
Acknowledgments
This study was supported by the World Class 300 Project, funded by the Small and Medium Business Administration [SMBA, S2367890 (S2416714)], Korea.
Disclosure
All authors have no conflicts of interest to report.
References
1. Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet. 2005;365(9454):153–165. doi:10.1016/S0140-6736(05)17706-X
2. Zhang L, Ren X, Alt E, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464(7291):1058–1061. doi:10.1038/nature08871
3. Widakowich C, de Castro G
4. Widakowich C, de Castro GJ, de Azambuja E, et al. Review: side effects of approved molecular targeted therapies in solid cancers. Oncologist. 2010;12(12):1443–1455.
5. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, et al. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–543. doi:10.1016/j.nut.2011.08.013
6. Perez M, Asto E, Huedo P, et al. Derived postbiotics of a multi-strain probiotic formula clinically validated for the treatment of irritable bowel syndrome. FASEB J. 2020;34(S1):1.
7. Tomasik P, Tomasik P. Probiotics, non-dairy prebiotics and postbiotics in nutrition. Appl Sci. 2020;10(4):1470. doi:10.3390/app10041470
8. Harish K, Varghese T. Probiotics in humans–evidence based review. Calicut Med J. 2006;4(4):e3.
9. Narayan SS, Jalgaonkar S, Shahani S, et al. Probiotics: current trends in the treatment of diarrhoea. Hong Kong Med J. 2010;16(3):213–218.
10. Moeinian M, FarnazGhasemi-Niri S, Mozaffari S, et al. Synergistic effect of probiotics, butyrate and l-Carnitine in treatment of IBD. J Med Hypotheses Ideas. 2013;7(2):50–53. doi:10.1016/j.jmhi.2013.02.003
11. Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5(2):111–125. doi:10.1177/1756283X11428502
12. Jonkers D, Penders J, Masclee A, et al. Probiotics in the management of inflammatory bowel disease. Drugs. 2012;72(6):803–823. doi:10.2165/11632710-000000000-00000
13. Oak SJ, Jha R. The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr. 2018;59(11):1675–1683. doi:10.1080/10408398.2018.1425977
14. Mario U, Giulia M, Francesco B, et al. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012;12(Suppl 1):S1–S35.
15. Ranjbar S, Seyednejad SA, Azimi H, et al. Emerging roles of probiotics in prevention and treatment of breast cancer: a comprehensive review of their therapeutic potential. Nutr Cancer. 2019;71(1):1–12. doi:10.1080/01635581.2018.1557221
16. Belizário EJ, Faintuch J. Microbiome and gut dysbiosis. Exp Suppl. 2018;109:459–476.
17. Backhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611–622. doi:10.1016/j.chom.2012.10.012
18. Steidler L, Vandenbroucke K. Genetically modified Lactococcus lactis: novel tools for drug delivery. Int J Dairy Technol. 2006;59(2):140–146. doi:10.1111/j.1471-0307.2006.00255.x
19. Quigley EM. Gut microbiota and the role of probiotics in therapy. Curr Opin Pharmacol. 2011;11(6):593–603. doi:10.1016/j.coph.2011.09.010
20. Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol. 2014;20(24):7878–7886. doi:10.3748/wjg.v20.i24.7878
21. Sadeghi-Aliabadi H, Mohammadi F, Fazeli H, et al. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran J Basic Med Sci. 2014;17:815–819.
22. An BC, Ryu Y, Hong S, et al. Probiotics as potential therapeutics for colorectal cancer. Am J Biomed Sci Res. 2020;9(2). DOI:10.34297/AJBSR.2020.09.001362
23. Hörmannsperger G, von Schillde MA, Haller D. Lactocepin as a protective microbial structure in the context of IBD. Gut Microbes. 2013;4(2):152–157. doi:10.4161/gmic.23444
24. von Schillde MA, Hörmannsperger G, Weiher M, et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe. 2012;11(4):387–396. doi:10.1016/j.chom.2012.02.006
25. Flambard B, Juillard V. The autoproteolysis of Lactococcus lactis lactocepin III affects its specificity towards β-casein. Appl Environ Microbiol. 2000;66(12):5134–5140. doi:10.1128/AEM.66.12.5134-5140.2000
26. An BC, Ryu Y, Choi O, et al. Genetic engineering of a probiotic-based drug delivery system for colorectal cancer therapy. Cancer Rep Rev. 2020;4:2–3.
27. An BC, Ryu Y, Yoon YS, et al. Colorectal cancer therapy using a Pediococcus pentosaceus SL4 drug delivery system secreting lactic acid bacteria derived protein P8. Mol Cells. 2019;30:755–762.
28. An BC, Hong S, Park HJ, et al. Anti-colorectal cancer effects of probiotic-derived P8 protein. Genes. 2019;10(8):624. doi:10.3390/genes10080624
29. Kim B-K, Yoon Y-S, Ryu R, et al. Probiotic-derived P8 protein induce apoptosis via regulation of RNF152 in colorectal cancer cells. Am J Cancer Res. 2021;11(3):746–759.
30. Giezen TJ, Mantel-Teeuwisse AK, Straus SM, et al. Safety-related regulatory actions for biological approved in the United States and the European Union. JAMA. 2008;300:1887–1896.
31. Amouzadeh HR, Vargas HM. Safety pharmacology assessment of biopharmaceuticals. In: Vogel HG, editor. Drug Discovery and Evaluation: Safety and Pharmacokinetic Assays. Berlin: Springer; 2013:555–560.
32. Chung Y, Ryu Y, An BC, et al. A synthetic probiotic engineered for colorectal cancer therapy modulates gut microbiota. Microbiome. 2021;9(1):122. doi:10.1186/s40168-021-01071-4
33. Jabir SM, Saleh MY, Sulaiman MJ, et al. Green synthesis of silver nanoparticles using annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials. 2021;11(2):384. doi:10.3390/nano11020384
34. Kavran MJ, Leahy JD. Coupling antibody to cyanogen bromide-activated sepharose. Method Enzymol. 2014;541:27–34.
35. Moser CA, Hage SD. Immunoaffinity chromatography: an introduction to applications and recent developments. Bioanalysis. 2010;2(4):769–790. doi:10.4155/bio.10.31
36. OECD. OECD principles of good laboratory practice; 1997. ENV/MC/CHEM(98)17.
37. Smith D, Trennery P, Farningham D, et al. The selection of marmoset monkeys (Callithrix jacchus) in pharmaceutical toxicology. Lab Anim. 2001;35(2):117–130. doi:10.1258/0023677011911444
38. Williams TN. Probiotics. Am J Health Syst Pharm. 2010;67(6):449–458. doi:10.2146/ajhp090168
39. Kliger B, Cohrssen A. Probiotics. Am Fam Physician. 2008;78(9):1073–1078.
40. Senok AC, Ismaeel AY, Bottaet GA. Probiotics: facts and myths. Clin Microbiol Infect. 2005;11(12):956–958. doi:10.1111/j.1469-0691.2005.01228.x
41. Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53(1):108–114. doi:10.1136/gut.53.1.108
42. Bibiloni R, Fedorak NR, Tannock WG, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100(7):1539–1546. doi:10.1111/j.1572-0241.2005.41794.x
43. Korte S, Everitt J. Chapter 27 - The use of the marmoset in toxicity testing and nonclinical safety assessment studies. In: The Common Marmoset in Captivity and Biomedical Research. Academic Press. 2019:493–513.
44. Hanton G, Sobry C, Daguès N, et al. Cardiovascular toxicity of minoxidil in the marmoset. Toxicol Lett. 2008;180(3):157–165. doi:10.1016/j.toxlet.2008.05.018
45. Tucker MJ. A survey of the pathology of marmosets (Callithrix jacchus) under experiment. Lab Anim. 1984;18(4):351–358. doi:10.1258/002367784780865397
46. Kaspareit J, Friderichs-Gromoll S, Buse E, et al. Background pathology of the common marmoset (Callithrix jacchus) in toxicological studies. Exp Toxicol Pathol. 2006;57(5–6):405–410. doi:10.1016/j.etp.2006.02.013
47. David MJ, Dick JE
48. Ali IH, Jabir MS, Al-Shmgani HS, et al. Pathological and immunological study on infection with Escherichia coli in ale balb/c mice. In
49. Kareem SH, Naji AM, Taqi ZJ, et al. Polyvinylpyrrolidone loaded-MnZnFe2O4 magnetic nanocomposites induce apoptosis in cancer cells through mitochondrial damage and P53 pathway. J Inorg Organomet Polym Mater. 2020;30(12):5009–5023. doi:10.1007/s10904-020-01651-1
50. Smither JS, Nelson M, Eastaugh L, et al. Experimental respiratory Marburg virus haemorrhagic fever infection in the common marmoset (Callithrix jacchus). Int J Exp Pathol. 2013;94(2):156–168. doi:10.1111/iep.12018
51. Collins MG, Rogers NM, Jesudason S, et al. Spontaneous glomerular mesangial lesions in common marmoset monkeys (Callithrix jacchus): a benign non-progressive glomerulopathy. J Med Primatol. 2014;43(6):477–487. doi:10.1111/jmp.12134
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.