Back to Journals » International Journal of Nanomedicine » Volume 18
Toxicity and Mechanisms of Engineered Nanoparticles in Animals with Established Allergic Asthma
Authors Deng R , Zhu Y, Wu X , Wang M
Received 9 March 2023
Accepted for publication 19 June 2023
Published 29 June 2023 Volume 2023:18 Pages 3489—3508
DOI https://doi.org/10.2147/IJN.S411804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Rui Deng,1 Ya Zhu,2 Xinyue Wu,3 Mingpu Wang1
1Joint International Research Laboratory of Green Buildings and Built Environments (Ministry of Education), School of Civil Engineering, Chongqing University, Chongqing, 400045, People’s Republic of China; 2The Affiliated Kangning Hospital, Wenzhou Medical University, Wenzhou, 325035, People’s Republic of China; 3Zhejiang Provincial Key Laboratory of Organic Pollution Process and Control, Department of Environmental Science, Zhejiang University, Hangzhou, 310058, People’s Republic of China
Correspondence: Rui Deng, School of Civil Engineering, Chongqing University, Chongqing, 400045, People’s Republic of China, Email [email protected]
Abstract: Asthma is a chronic respiratory disease that is highly sensitive to environmental pollutants, including engineered nanoparticles (NPs). Exposure to NPs has become a growing concern for human health, especially for susceptible populations. Toxicological studies have demonstrated strong associations between ubiquitous NPs and allergic asthma. In this review, we analyze articles that focus on adverse health effects induced by NPs in animal models of allergic asthma to highlight their critical role in asthma. We also integrate potential mechanisms that could stimulate and aggravate asthma by NPs. The toxic effects of NPs are influenced by their physicochemical properties, exposure dose, duration, route, as well as the exposure order between NPs and allergens. The toxic mechanisms involve oxidative stress, various inflammasomes, antigen presenting cells, immune cells, and signaling pathways. We suggest that future research should concentrate on establishing standardized models, exploring mechanistic insights at the molecular level, assessing the combined effects of binary exposures, and determining safe exposure levels of NPs. This work provides concrete evidence of the hazards posed by NPs in animals with compromised respiratory health and supports the modifying role of NPs exposure in allergic asthma.
Keywords: engineered nanoparticles, allergic asthma, mouse model, toxicity, mechanisms, adjuvant effect
Graphical Abstract:
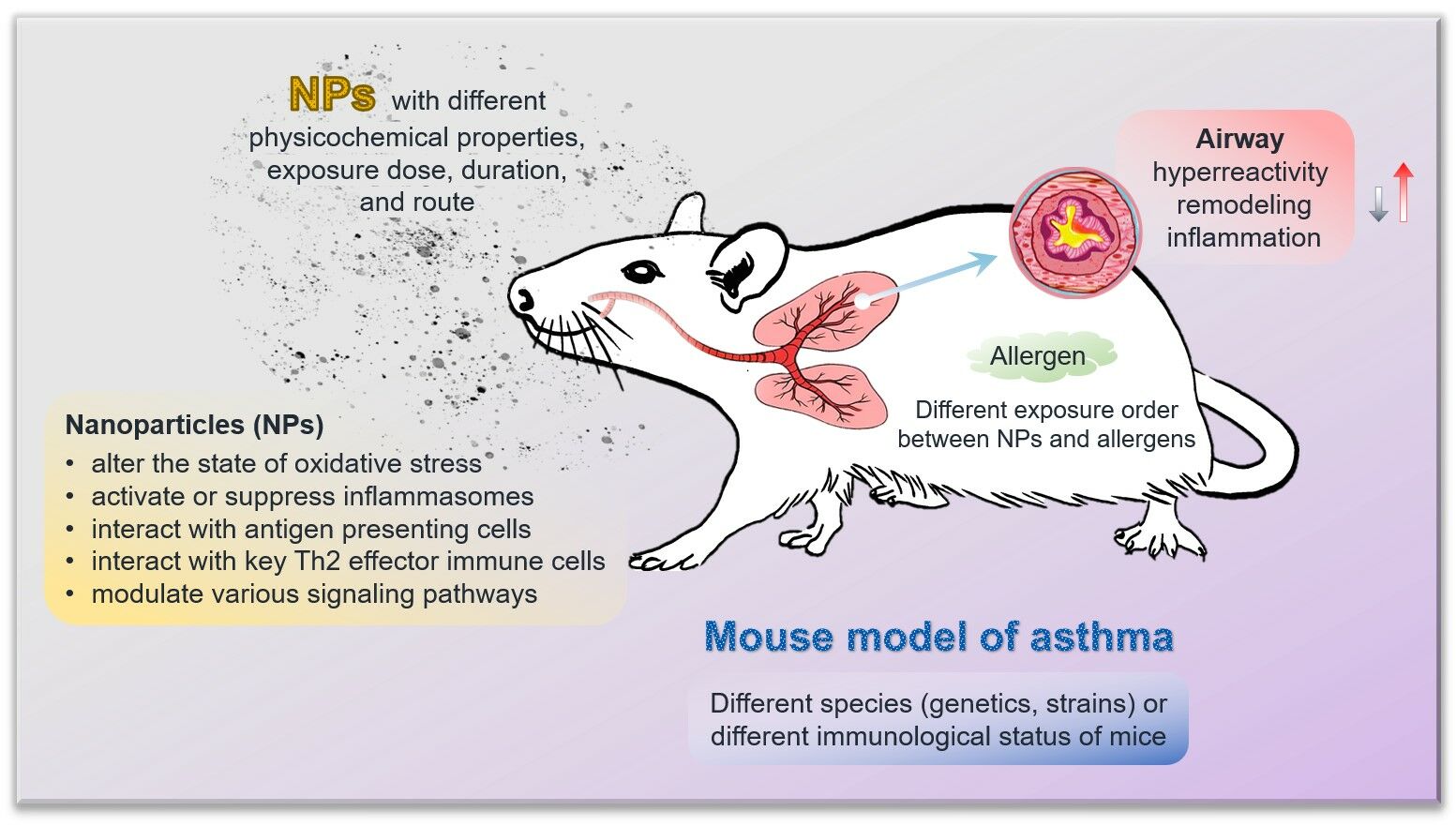
Introduction
Asthma is a type of inflammatory lung disease resulting from repeated hypersensitivity and allergic reactions.1 The clinical features of asthma include reversible airway obstruction, chronic bronchial inflammation, airway hyperresponsiveness (AHR), and bronchial smooth muscle cell hypertrophy, often presenting with wheezing, coughing, and shortness of breath.2 The generally accepted process of asthma is that chronic inflammation involving numerous resident and immune cells leads to airway remodeling with the features of smooth muscle thickening, epithelial fibrosis, and barrier alteration.3 The remodeling, coupled with intensified smooth muscle contraction and mucus secretion, gives rise to obstructive events and clinical symptoms.4 Airway remodeling and hyperreactivity underlying airway obstruction are the pathological hallmarks of asthma with a major impact on patient morbidity and mortality.3 Repeated asthma attacks can lead to irreversible airway obstruction and are associated with avoidable death and economic burden.5 More than 330 million people worldwide suffer from asthma, and the prevalence rate of asthma is rising by 50% every decade, accounting for 250,000 deaths per year.3,6
Engineered nanoparticles (NPs, at least one dimension < 100 nm) are manufactured via controlled engineering processes to provide low impurity and regular morphology, eg, sphere, tube, and plate.7 The increasing production and utilization of nanomaterials due to the development of nanotechnology has resulted in the inevitable emission of NPs, raising concerns about potential biological exposure and health effects. In addition, despite the benefits of nanomedicine, which includes the use of NPs in cancer therapy and drug delivery,8 it is crucial to evaluate the possible detrimental effects of NPs. The unique physicochemical properties of NPs (size, shape, charge, solubility, etc.) may allow them to enter tissues and cells, interact with proteins and DNA, and directly or indirectly modulate the immune system.9
Numerous studies have confirmed that different NPs tend to have different toxic effects on organisms and different mechanisms of toxicity.10,11 The toxicity of NPs has mainly been measured at the genetic, cellular, and individual levels in the laboratory.12 Mechanisms of toxicological damage have been identified and include the production of reactive oxygen species (ROS), protein misfolding, membrane disruption, and direct physical damage. As research continues, it is becoming clear that the environmental toxicology of NPs is not only a toxic risk to individual organisms, but also a threat to ecological and human health.11,12 Their ecotoxicity and toxicity to humans have rarely been reported. Opinions on the effects of NPs on human health are mainly derived from laboratory studies on specific organs or tissues of model animals exposed to NPs in vivo, or from machine learning studies based on in vitro model predictions. Previous evidence has pointed to the potential of NPs to induce and exacerbate type I hypersensitivity responses.2 Additionally, possible health hazards of NPs have been manifested by effects on immune defense, T-helper Th1/Th2 balance, and non-immunological aspects such as oxidative stress, apoptosis, autophagy, genotoxicity, and carcinogenesis.9,13 The skin, respiratory and gastrointestinal tracts of the body are connected to the external environment and are therefore the main exposure sites for NPs.5 Furthermore, NPs have the potential to translocate to different distal organs. The lung is the imperative target organ for NPs exposure, which is associated with impaired lung function, increased lung inflammation and fibrosis.14,15 NPs can deposit in the alveolar space, penetrate inside cells, and enter the circulatory system.16 Acting as allergic adjuvants, NPs can facilitate the body’s allergic immune responses to allergens and are considered to have the potential to induce and/or aggravate asthma, which is possibly associated with early mortality and reduced life expectancy.16,17 Although much progress has been made in understanding the toxicological effects of NPs, there are still many knowledge gaps regarding their effects on allergic asthma and the mechanisms involved.
This work explores the knowledge gained from different mouse models of allergic asthma and the influences of various NPs exposures on the development and exacerbation of asthma. We discuss the current understanding of the mechanisms underlying such effects and describe important findings regarding the modification of asthma upon co-exposures of NPs and other risk factors. Furthermore, we identify priority research areas for future investigation. This review will provide valuable information regarding the impact of NPs on allergic asthma and serve as a useful reference for the development of preventive strategies and regulatory guidelines to safeguard human health.
Profile of the Existing Research Articles
Relevant articles in this research field were searched using the Web of Science engine, where the search topics were “allergic asthma” and “nanoparticle* OR nanomaterial* OR nano-particle* OR nano-material*”. The asterisk “*” stands for any single or multiple characters, and there were 326 matching articles published before the year 2023. Among them, 194 articles were from the Web of Science Core Collection and we analyzed their keywords based on the CiteSpace software.18–20 The first article in this Core Collection was published in 2003, and over the past 20 years of research on allergic asthma associated with NPs, various concerns have been raised. In the keyword co-occurrence analysis for the 194 articles (Figure 1 and Table 1), the most frequent keywords with occurrence counts not less than 20 are asthma, airway inflammation, nanoparticle, and oxidative stress. The keywords with high centrality scores greater than 0.1, including dendritic cell, response, asthma, and airway inflammation, play an important role in linking different studies in the network. Keywords with higher centrality scores are more important and influential in this study field. In the keyword burst analysis (Table 2), the strength of the burst indicates the increase of the keyword over a certain period of time. The higher the strength, the more representative of the research hotspot in the field. The keywords with the earliest beginning years of burst are oxidative stress, carbon nanotube, diesel exhaust particle, antigen presenting cell, and cytokine expression, which means that they were the focus of early research in this field. The keywords with the most recent beginning years of burst are delivery, inflammation, antigen, regulatory T cell, and nanoparticle. The burst years of nanoparticle, regulatory T cell, inflammation, and delivery end in 2022, suggesting that they may represent the frontiers of research in this area.
 |
Table 2 Top 25 Keywords with the Strongest Occurrence Bursts in the Keyword Burst Analysis for the 194 Articles Related to Allergic Asthma and Nanoparticles in the Web of Science Core Collection |
After evaluating the content of the searched articles, 54 relevant articles on animal models of asthma were selected for detailed discussion in this review. They were from the Web of Science Core Collection and we analyzed their keywords based on the CiteSpace software.18–20 Articles on drug therapy, epidemiological investigation, or pure in vitro cell experiments for asthma were not concerned in our work. In the keyword co-occurrence analysis for the 54 articles (Figure 2 and Table 3), the most frequent keywords with occurrence counts not less than 10 are airway inflammation, asthma, exposure, and expression. The keywords with the highest centrality scores are mouse model, lung, particle, carbon nanotube, and asthma, which play an important and influential role in this research area. In addition, a statistical analysis of these 54 relevant articles is shown in Figure 3. Chronologically, the first article21 on the adjuvant activity of carbon black NPs (CB NPs) in allergic asthma was published in 2005. The number of published articles has increased steadily since 2009. Most studies were focused on single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), followed by nano-sized titanium dioxide (TiO2), silicon dioxide (SiO2), silver (Ag), and gold (Au). Other carbon-based NPs, including CB, graphene oxide (GO), and fullerene (C60 and C70), as well as metal oxide NPs, including zinc oxide (ZnO), iron oxide (Fe2O3), cerium dioxide (CeO2), nickel oxide (NiO), alumina (Al2O3), and copper oxide (CuO), have also been investigated in this research area.
 |
Figure 3 The number of articles closely related to the effects of NPs on the development and exacerbation of allergic asthma. An article investigating both Au and TiO2 NPs is counted twice. |
Toxicology of Various Nanoparticles in Asthma
The research on the toxicology of various NPs in asthma covered in this review is summarized in Table 4 and Table 5 and reviewed according to the types of NPs. Balb/c mice are the most widely tested species, followed by C57BL/6 mice, and females are more commonly used than males. The most extensively used allergen in the induction of allergic asthma is ovalbumin (OVA), followed by house dust mite (HDM). Inhalation and intranasal instillation are the most commonly used routes of NPs exposure, followed by oropharyngeal aspiration, intratracheal instillation, and intraperitoneal injection. The exposure order (or sequence) of NPs can be defined by the phases of allergen sensitization and challenge, but it is not uniform across different studies.
 |
Table 4 Studies on the Effects of NPs on Established Allergic Asthma |
 |
Table 5 The Role of Different Types of NPs in Allergic Asthma |
Single-Walled and Multi-Walled Carbon Nanotubes
Carbon nanotubes (CNTs) have been widely reported to facilitate the development and exacerbation of allergic asthma. SWCNTs were found to aggravate OVA-induced lung inflammation and mucus hypersecretion in mice.33 Compared to OVA alone, treatment with SWCNTs plus OVA improved the lung levels of T-helper (Th) cytokines and chemokines involved in allergy and biomarkers related to oxidative stress. Moreover, in vitro, SWCNTs partially promoted the function of bone marrow-derived dendritic cells. Therefore, the aggravating effect of SWCNTs on allergic inflammation could be attributed to increased activation of Th immunity and oxidative stress, as well as inappropriate activation of antigen presenting cells. It was also reported that SWCNTs exacerbated OVA-sensitized rat asthma, whereas this effect was antagonized by the co-administration of vitamin E.31 The application of vitamin E can lead to the reduction of oxidative stress, Th2 responses, and serum immunoglobulin (Ig) concentrations, thereby alleviating asthma symptoms.
For MWCNTs, airway fibrosis was observed in mice co-treated with OVA and MWCNTs, but not in those treated with either OVA or MWCNTs alone.30 This suggests that pre-existing allergic inflammation caused by OVA may be susceptible to airway fibrosis under MWCNTs exposure. The increase in airway resistance was observed in the MWCNTs-OVA co-exposure group rather than in the MWCNTs or OVA exposure group.28 The sensitization of MWCNTs combined with OVA accelerated airway inflammation and goblet cell hyperplasia compared to MWCNTs or OVA alone. Moreover, increased levels of OVA-specific antibodies in serum and cytokines in the lung were observed. In other words, the combination of MWCNTs and OVA enhanced allergic asthmatic responses. MWCNTs were also found to aggravate the OVA-induced airway inflammation, as evidenced by eosinophils, neutrophils, and mononuclear cell infiltration in the lung and an increase in goblet cells in the bronchial epithelium.29 MWCNTs combined with OVA elevated the levels of Th cytokines and chemokines in the lung compared to OVA alone, and MWCNTs showed adjuvant activity for OVA-specific IgG1 and IgE. In vitro, MWCNTs also improved the proliferation of OVA-specific syngeneic T cells. Therefore, similar to SWCNTs, MWCNTs could aggravate allergic airway inflammation probably via inappropriate activation of antigen presenting cells such as dendritic cells. High nickel MWCNTs were found to promote rapid eosinophilic inflammation in the lung and caused intense exacerbation of pre-existing allergic airway inflammation by facilitating the production of leukotrienes.23 This finding suggests that the potential therapeutic effects of pharmacological agents that block leukotriene biosynthesis merit further investigation. On the other hand, a study22 highlighted the potential of low-dose MWCNTs inhalation to reduce atopic immune responses to allergens. Nonetheless, MWCNTs have also been shown to promote a pro-inflammatory and pro-fibrotic environment in the lung, which may render exposed individuals chronically susceptible to allergens or pathogens.
Specially, MWCNTs aggravated the OVA-induced lung inflammation and mucus-cell metaplasia in cyclooxygenase-2-deficient (COX-2−/−) mice compared to wild-type (WT) mice.27 Levels of certain prostanoids were increased in COX-2−/− mice by exposure to OVA or MWCNTs. Moreover, MWCNTs increased OVA-induced cytokines involved in Th2, Th1, and Th17 inflammatory responses in COX-2−/− mice more than in WT mice. The aggravating effects of MWCNTs on OVA-induced airway inflammation and mucus cell metaplasia were enhanced by the COX-2 deficiency, which was associated with the activation of a mixed immune response. OVA and MWCNTs increased eosinophils and neutrophils, respectively, in bronchoalveolar lavage fluid (BALF) of mice with whole-body knockout of the Stat1 gene (Stat1−/−) and WT mice.25 OVA sensitization also inhibited MWCNTs-induced neutrophilia. Treatment with OVA or OVA plus MWCNTs increased interleukin (IL)-13 in the BALF of Stat1−/− mice more than that of WT mice. The co-exposure of MWCNTs aggravated the OVA-induced goblet cell hyperplasia, airway fibrosis, and subepithelial apoptosis, as well as increased the levels of profibrogenic mediators, but decreased IL-10 in Stat1−/− mice. The possible mechanism behind the susceptibility of Stat1−/− mice to the exacerbating effect of MWCNTs on allergic airway remodeling was an increase in pulmonary profibrogenic cytokines and collagens from fibroblasts. The study suggests a protective role for the transcription factor STAT1 in allergen-induced airway inflammation and the exacerbation of airway remodeling by MWCNTs.
For another common allergen besides OVA, HDM exposure in mice accelerated serum HDM-specific IgG1 and inflammatory cell infiltration, and increased Th2 cytokine production, collagen deposition, and mucus hyperproduction in the airways.26 Compared with HDM alone, total and specific IgG1, eosinophils, neutrophils, macrophages, collagen, mucus, and some cytokines were dose-dependently increased by HDM-MWCNTs co-exposure in mice. The study showed that MWCNTs dose-dependently enhanced the systemic immune response as well as airway inflammation and remodeling in allergic mice induced by HDM. In another study,24 MWCNTs caused neutrophilic infiltration in the lungs and increased IL-1β in BALF and pro-caspase-1 in airway epithelium and macrophages. HDM induced eosinophilic inflammation and increased IL-13. The MWCNTs exposure after HDM sensitization increased the total cell number in BALF, but decreased neutrophils and IL-1β in BALF and pro-caspase-1 in the lung. This study suggested that MWCNTs exacerbated the allergen-induced airway fibrosis by suppressing inflammasome activation and IL-1β in the lung. It was observed that not only CNTs but also carbon nanofibers elevated the OVA-IgE levels in both the injection and the airway exposure mouse models of allergy.32 CNTs induced a stronger IgE response than carbon nanofibers, and promoted eosinophil lung inflammation rather than carbon nanofibers. This suggests that the properties associated with nanotubes (eg, thin and hollow tube structure, potentially high biopersistence) appear to be important in promoting allergic responses.
Other Carbon-Based Nanoparticles
In addition to CNTs, CB NPs, GO, and fullerenes were also investigated in NPs-induced toxicity in asthma. There was a correlation between early airway toxicity and adjuvant effects of CB NPs.21 It was shown that local cytokine production early after exposure to CB NPs was predictive of allergic airway inflammation. CB NPs enhanced the allergic airway inflammation in mice and the specific Th2 response in lymph nodes induced by the antigen OVA.35 The increased neutrophilic lung inflammation was relative to the adjuvant effect of CB on allergic sensitization, which was suggested by the application of ectoin to the airways. A relatively low dose of CB NPs, with or without surface polycyclic aromatic hydrocarbons, did not aggravate the established allergic airway inflammation in asthmatic mice.34 Nevertheless, the coating of polycyclic aromatic hydrocarbons altered the biological effects of CB NPs on immune responses and airway epithelial cells.
GO administered during the sensitization phase in a murine model of asthma magnified AHR and airway remodeling with goblet cell hyperplasia and smooth muscle hypertrophy.37 GO decreased the levels of IL-4, IL-5, and IL-13 and the accumulation of eosinophils, but increased the accumulation of macrophages in BALF. GO also decreased the levels of OVA-specific IgE and IgG1, but increased IgG2a in serum. Besides, GO stimulated the production of mammalian chitinases (AMCase and CHI3L1) by macrophages, which are involved in the pathogenesis of asthma. Molecular modeling suggested that GO might directly interact with AMCase and affect its activity. In all, GO attenuated the Th2 immune response in asthmatic mice but enhanced AHR and airway remodeling along with chitinase induction. Moreover, GO exacerbated the OVA-sensitized allergic asthma, as evidenced by increased ROS and total IgE, enhanced Th2 response, and aggravated asthma symptoms, involving airway remodeling, collagen deposition, and AHR.36 However, the administration of Vitamin E as an antioxidant significantly attenuated the above-mentioned GO effects.
Two types of fullerenes, C60 and C70, were shown to have an alleviating effect on asthma. C60 inhibited the release of histamine and the decrease in core body temperature in a mast cells-dependent murine model of anaphylaxis, demonstrating the potential function of fullerenes in controlling allergic diseases such as asthma.39 C70-tetraglycolate was administered intranasally to mice either concurrently with OVA treatment or after the induction of asthma; bronchoconstriction, airway inflammation, and eosinophilia in asthmatic mice were significantly alleviated by the fullerene.38 The reason was that fullerene stimulated the production of anti-inflammatory epoxyeicosatrienoic acids in the lung, and the inhibitors of these products, such as 6-(2-propargyloxyphenyl) hexanoic acid, reversed the effects of fullerene.
Silicon Dioxide (or Silica) Nanoparticles
The influence of SiO2 NPs on allergic asthma is largely shown to be aggravating. SiO2 NPs have been found to exhibit both developmental and exacerbating effects on allergic asthma.46 With or without OVA immunization, SiO2 NPs could lead to AHR and airway remodeling due to the IL-4 increase and the Th1/Th2 cytokine imbalance. Co-exposure of OVA and 30 nm SiO2 NPs induced higher levels of OVA-specific antibodies (including IgE, IgG and IgG1) than co-exposure of OVA and 70 nm SiO2 NPs.45 Moreover, mouse splenocytes exposed to OVA plus 30 nm SiO2 NPs secreted more Th2 type cytokines than those exposed to OVA alone. Therefore, SiO2 NPs could induce Th2 type allergic immune responses depending on the particle size.
Exposure of mice to SiO2 NPs during OVA sensitization brought about a dose-dependent exacerbation of allergic airway disease.44 SiO2 NPs resulted in significantly higher OVA-specific IgE, eosinophil infiltration, and Th2 and Th17 cytokine gene and protein expression in OVA-sensitized mice. While, in healthy mice, SiO2 NPs exposure caused an increase in airway neutrophils. This suggests that SiO2 NPs could facilitate allergen sensitization and the manifestation of airway disease in allergic mice and cause innate immune responses in healthy mice. Three types of SiO2 NPs with different shapes (mesoporous, spherical, and polyethylene glycol-conjugated) could induce airway inflammation and AHR in healthy mice.43 In asthmatic mice, the co-exposure of OVA and SiO2 NPs caused more severe airway inflammation than the exposure of OVA alone. Therefore, SiO2 NPs could give rise to airway inflammation which was not affected by the immunological status of the mice. Moreover, among these three SiO2 NPs, mesoporous SiO2 NPs caused the most severe airway inflammation, followed by spherical SiO2 NPs, and polyethylene glycol-conjugated SiO2 NPs caused the least inflammation.42
SiO2 NPs significantly elevated the characteristic markers of asthma, including AHR, levels of inflammatory mediators and IgE, inflammatory cell infiltration, and mucus production.41 It was indicated that SiO2 NPs had the potential to intensify asthmatic inflammation via the activation of the NOD-like receptor pyrin domain-containing 3 inflammasome. SiO2 NPs could activate IgE-sensitized mast cells via enhancing the mitogen-activated protein kinase signaling pathway, which could worsen allergic inflammation.40 These findings provide valuable information on potential therapeutic targets for the treatment of allergic asthma.
Silver and Gold Nanoparticles
Both Ag NPs and Au NPs can play a dual (aggravating or alleviating) role in allergic asthma. In healthy and allergic mice, the inhalation of Ag NPs provoked infiltration of neutrophils, lymphocytes, and eosinophils into the airways and an inflammatory response in the peritoneum.47 Ag NPs also increased the levels of allergic biomarkers such as IgE and leukotriene E-4, Th2 cytokine IL-13, and oxidative stress biomarker 8-hydroxy-2’-deoxyguanosine in both mice. Hence, Ag NPs could pose respiratory risks to not only susceptible but also healthy individuals. Protein profiles of BALF and plasma from mice exposed to Ag NPs were determined, and there were 106 (or 40) and 79 (or 26) unique proteins associated with Ag exposure in BALF (or plasma) of healthy and allergic mice, respectively.48 Additionally, OVA-specific IgE involved in allergic responses and 18 proteins related to systemic lupus erythematosus were commonly expressed in healthy and allergic mice after Ag exposure. Therefore, Ag NPs could provoke immune responses in normal and susceptible individuals. On the contrary, in another study,50 the OVA-induced increases in inflammatory cells, AHR, and levels of ROS, IL-4, IL-5, IL-13, and nuclear factor kappa-B (NF-κB) were dramatically lowered by Ag NPs. It was indicated that Ag NPs moderated asthma symptoms and the antioxidation could be a potential mechanism involved. In another mouse model of asthma induced by OVA, it was pointed out that exposure to Ag NPs decreased the hypersecretion of the mucin protein MUC5AC (a marker of goblet cell metaplasia).49 Additionally, the signaling pathway of vascular endothelial growth factor was suppressed and airway inflammation was alleviated by Ag NPs.
As for Au NPs, they aggravated AHR and pulmonary inflammation, including increased oedema, epithelial damage, and total cells in BALF.53 These findings were also true for TiO2 NPs in this study. On the other hand, a research showed that asthmatic mice could take in more Au NPs into extrapulmonary organs than healthy mice, and the systemic uptake of PEGylated Au NPs is higher than that of citrated Au NPs.51 Both Au NPs attenuated OVA-induced airway inflammation and AHR. The anti-inflammatory effects of Au NPs suggested their therapeutic benefit in asthma, but their potential impacts after uptake need to be considered. Furthermore, Au NPs decreased the OVA-induced accumulation of inflammatory cells, pro-inflammatory cytokines, and ROS in Swiss-Webster (outbred) and A/J (inbred) mice.52 Au NPs also significantly alleviated OVA-induced mucus hypersecretion, airway remodeling, and AHR in the genetically asthma-prone A/J mice. The study suggested that the likely mechanism involved was the reduction of oxidative stress by Au. The multidirectional effect of both Ag and Au NPs may depend on their physicochemical property, the exposure order between them and the allergen, and the mouse species. This information may provide a reference for the treatment of allergic asthma.
Titanium Dioxide Nanoparticles
TiO2 NPs are another type of popular NPs in this research field besides CNTs, and their role is multiple. TiO2 NPs showed an adjuvant effect in mice, promoting allergic sensitization and airway inflammation.62 The TiO2 NPs promoted a Th2-dominated immune response with high levels of OVA-specific IgE and IgG1 in serum and an influx of eosinophils, neutrophils, and lymphocytes in BALF. TiO2 NPs could lead to the systemic uptake of TiO2 NPs in extrapulmonary organs via intranasal exposure.55 These TiO2 NPs caused an increase in Th2 cytokines, including IL-4, IL-5 and IL-13, and exacerbation of allergic airway inflammation. TiO2 NPs also aggravated AHR, lung injury, and immune response in a murine model of asthma.58 The TiO2 elevated the levels of some transcription factors (Stat3, Socs3, and NF-κB) and pro-inflammatory cytokines (IL-6 and TNF-α). Blocking of NF-κB led to the downregulation of Socs3, IL-6, and TNF-α expressions. Therefore, TiO2 NPs could enhance the inflammatory responses in the lung via the NF-κB pathway. Moreover, TiO2 NPs exacerbated AHR and inflammation, and increased ROS levels and IL-1β, IL-18, and caspase-1 expressions in OVA-sensitized mice.57 These results suggested that TiO2 contributed to inflammasome activation in the asthmatic lung. TiO2 NPs aggravated the airway inflammation and responsiveness in OVA mice, and increased the levels of transient receptor potential vanilloid TRPV1 and TRPV4, ATP-gated ion channels P2 × 4 and P2 × 7 in the lung, and ATP, substance P, and calcitonin gene-related peptide in BALF.56 This indicated that neuroinflammation was involved in the pathogenesis of asthma, and TiO2 could exacerbate asthma via the release of neuromediators. Additionally, TiO2 NPs were found to induce toxicological changes in the respiratory tract and exacerbate the development of asthma, characterized by increases in AHR, inflammatory cytokines and responses, and mucus overproduction.54 The possible mechanism involved is the activation of the thioredoxin-interacting protein-apoptosis pathway.
It is suggested that TiO2 NPs could modulate airway inflammation depending on the immunological status of the mice. For example, after exposure to TiO2 NPs, there was pulmonary neutrophilia and increased expression of the chemokine CXCL5 in healthy mice, whereas TiO2 attenuated the Th2 type inflammation in asthmatic mice.61 Acute exposure to TiO2 NPs decreased allergic lung inflammation in rats, which was associated with a drop in both Th2 and Th1 cytokines.60 It has been suggested that the characterization of TiO2 NPs and the duration of exposure may play an important role in this protective effect. Exposure to TiO2 NPs led to an increase in neutrophils and lymphocytes in healthy Brown Norwegian (BN) and Dark Aguoti (DA) rats.59 AHR and Th1 type immune responses were also enhanced in healthy DA rats exposed to TiO2 NPs. However, the OVA sensitization of rats caused significant OVA-specific IgE and IgG responses in BN rats, but only IgG response in DA rats. After the OVA challenge, both strains of the sensitized rats exhibited airway eosinophilia, which was alleviated by TiO2 NPs exposure. Furthermore, neutrophils and lymphocytes were increased by TiO2 in DA rats but not in BN rats. Overall, the effects of TiO2 NPs on immune and airway reactivity in mice may be strain/genetics dependent.
Other Metal Oxide Nanoparticles
Other metal oxide NPs of concern include ZnO, Fe2O3, CeO2, NiO, Al2O3, and CuO NPs. The impact of ZnO NPs on asthma may depend on the route of exposure. Inhalation of ZnO NPs could play a role in the development of allergic airway inflammation in mice.63 However, topically applied ZnO NPs via the skin only played a limited role in the development of allergic airway inflammation in mice. ZnO NPs treatment without OVA application initially increased the levels of Th2 cytokines IL-4, IL-5, and IL-13, and then decreased the levels.64 Moreover, treatment with ZnO NPs and OVA induced airway inflammation with significant neutrophilia and eosinophilia. These suggest that ZnO NPs could induce eosinophilic airway inflammation with or without allergen.
In non-sensitized/healthy mice, Fe2O3 NPs exposure induced an expansion of inflammatory cells with increased numbers of lymphocytes, neutrophils, and eosinophils in the airway.65 While in OVA-sensitized mice, Fe2O3 NPs caused a dramatical cellular decrease in the alveolar space, indicating that the mice with established inflammation underwent cell death when exposed to Fe2O3 NPs. One possible explanation for this was that the acidic environment of the inflamed airways in sensitized mice facilitated the release of Fe ions, thereby promoting ROS generation. OVA triggered allergic Th2 responses with increases in inflammation, eosinophils, OVA-specific IgE, and IL-4.66 These responses were inhibited by Fe2O3 NPs at 250 and 500 μg/mouse, as evidenced by the decrease in eosinophil number and IgE level. However, Fe2O3 NPs at 100 μg/mouse exhibited an adjuvant effect on the Th2 responses. Therefore, the influences of Fe2O3 NPs on allergic responses may hinge on the particle dose.
CeO2 NPs were observed to exacerbate HDM-induced type II airway inflammation, characterized by increases in eosinophils, mast cells, IgE, goblet cell metaplasia, and mucin and inflammatory regulators.70 HDM led to a strong induction of type I interferon and IRF3-dependent gene expression, which was suppressed by co-exposure to CeO2 NPs. It was suggested that regulation of dendritic cells, macrophage functionality, and IRF3 modulation were key early events in how CeO2 directed the lung response to HDM towards type II inflammation. Lower surface area of NiO was associated with an enhanced Th2 profile, whereas higher surface area was associated with a Th1-dominant profile.69 This suggests that NiO surface area is closely correlated with lung injury and inflammation; other physicochemical properties, such as particle size, may contribute to the modulation of immune responses in the lung. Al2O3 NPs could increase ROS levels in lung tissue, decrease glutathione levels, promote the increase of IgE, TGF-β, IL-1β and IL-6, and stimulate the overexpression of transcription factors GATA-3 and RORγt.67 They also decreased IFN-γ and IL-10 levels, increased IL-4 and IL-17A levels, and led to an imbalance in Th1/Th2 and Treg/Th17 immune responses. Vitamin E alleviated asthmatic symptoms by blocking oxidative stress. This study showed that exposure to Al2O3 NPs may exacerbate allergic asthma by promoting the imbalance between Th1/Th2 and Treg/Th17. CuO NPs, as well as their functionalized forms (CuO-COOH and CuO-NH3 NPs), deteriorated the allergic airway inflammation via causing neutrophilia in the lung.68 Whereas, CuO NPs after the surface PEGylation had a significantly lower potential to trigger changes in the lung. Therefore, PEGylation may be a promising approach to inhibit the effects of pristine CuO NPs.
Co-Exposure of NPs with Another Risk Factor and Effects on Asthma
In real-world environments, various risk factors (both chemical and physical factors) often co-exist and jointly exert biological effects.71–74 The co-exposure of NPs with another risk factor has the potential to present a combined hazard to individuals suffering from asthma. For example, the co-exposure of CeO2 NPs and diesel exhaust particles after HDM treatment increased macrophage and IL-17A levels more than levels induced by diesel exhaust particles alone.75 This suggests that CeO2 NPs may alter exhaust particle and HDM-induced inflammatory events in allergic airway disease. Conversely, nitrogen dioxide, a gaseous pollutant, enhanced lung inflammation and airway reactivity to OVA, but the co-exposure of nitrogen dioxide and CB NPs led to a lower level of airway reactivity.76 Specially, both high humidity (a physical risk factor) and CB NPs aggravated the airway remodeling, hyperreactivity, and inflammation in OVA-sensitized Balb/c mice.73 Their co-exposure exhibited an adjuvant effect on the development of asthma, likely via the activation of oxidative stress and TRPV1 pathways and then the facilitation of type I hypersensitivity. Additionally, their single and joint exposures altered the microbial community composition in the gut and attenuated the relevant biological functions, which may interact with the development of asthma. Furthermore, untargeted metabolomics identified the potential lung biomarkers for asthma development and exacerbation caused by CB NPs and high humidity.74 The perturbed metabolic pathways by the exposure of CB NPs and/or high humidity were mainly implicated in asthma symptoms.
Conclusions
In this review, we analyzed studies that investigated the impacts of NPs on allergic asthma and summarized major findings of animal research on adjuvant effects and mechanisms of toxicity induced by NPs. We found that there is diversity in the approaches used to establish asthma models, the characteristics of NPs, and the endpoints selected for toxicity assessment in the available studies. Therefore, there is still insufficient information on how an individual will exactly react to a kind of NPs, and it is nearly impossible to rank different NPs in terms of their toxicity in asthma. Nevertheless, it can be derived that the effects of NPs on the development and exacerbation of asthma may be influenced by the following factors: (1) the physicochemical properties, exposure dose, duration, and route of NPs; (2) the exposure order between NPs and allergen; (3) the genetics/species/strain and immunological status of mice. The mainstream mechanisms underlying the effects of different NPs on allergic asthma can be summarized as follows: (1) changes in oxidative stress; (2) activation or suppression of inflammasomes; (3) interaction with antigen presenting cells; (4) interaction with key Th2 effector immune cells like mast cells and eosinophils; (5) influence on various signaling pathways in the airway.
To reliably identify the concerned properties of NPs, the exploitation and adoption of standardized testing or models to conduct comparative analysis across different NPs and their effects is necessary. Pointing out specific compositions and physicochemical properties associated with the development and deterioration of asthma will pave the way for designing safe nanomaterials. Additionally, detailed mechanistic insights into how NPs may influence asthma at cellular and molecular levels is an ongoing need to advance the safety assessment for disease susceptibility. Co-exposure of NPs with other risk factors can pose deleterious effects on asthma, thus requiring more in-depth and extensive research. The papers reviewed here were based on different animal models exposed to high doses of NPs, and the results were inconsistent and sometimes contradictory. Little is known about the effects of exposure to NPs on the development of asthma in workers in the nanomaterials industry and other industries, and epidemiological data are still sparse. There are currently no clear safe levels of exposure to NPs, nor is there robust evidence on which to base relevant standards. Therefore, epidemiological studies of exposed workers are needed to establish safe levels of exposure to NPs for the management and control of adverse human health effects, including the prevention of asthma.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (42207486) and China Postdoctoral Science Foundation (2021M700580).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dobrovolskaia M, Shurin M, Kagan V, Shvedova A. Ins and outs in environmental and occupational safety studies of asthma and engineered nanomaterials. ACS Nano. 2017;11(8):7565–7571. doi:10.1021/acsnano.7b04916
2. Alsaleh N, Brown J, Hu L, Qiu L, Zhu L. Engineered nanomaterials and type I allergic hypersensitivity reactions. Front Immunol. 2020;11:11. doi:10.3389/fimmu.2020.00222
3. Meldrum K, Guo C, Marczylo E, Gant T, Smith R, Leonard M. Mechanistic insight into the impact of nanomaterials on asthma and allergic airway disease. Part Fibre Toxicol. 2017;14:14. doi:10.1186/s12989-017-0228-y
4. Bara I, Ozier A, de Lara J, Marthan R, Berger P. Pathophysiology of bronchial smooth muscle remodelling in asthma. Eur Respir J. 2010;36(5):1174–1184. doi:10.1183/09031936.00019810
5. Sreedharan S, Zouganelis G, Drake S, Tripathi G, Kermanizadeh A. Nanomaterial-induced toxicity in pathophysiological models representative of individuals with pre-existing medical conditions. J Toxicol Environ Health-Part B-Crit Rev. 2022. doi:10.1080/10937404.2022.2153456
6. Braman S. The global burden of asthma. Chest. 2006;130(1):4S–12S. doi:10.1378/chest.130.1_suppl.4S
7. Sukhanova A, Bozrova S, Sokolov P, Berestovoy M, Karaulov A, Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res Lett. 2018;13:13. doi:10.1186/s11671-018-2457-x
8. Sandhiya S, Dkhar S, Surendiran A. Emerging trends of nanomedicine - an overview. Fundam Clin Pharmacol. 2009;23(3):263–269. doi:10.1111/j.1472-8206.2009.00692.x
9. Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34(3):J234–J246. doi:10.1016/j.jaut.2009.11.009
10. Solano R, Patino-Ruiz D, Tejeda-Benitez L, Herrera A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: a review on the applications, nanotoxicological effects, and risk control strategies. Environ Sci Pollut Res. 2021;28(14):16962–16981. doi:10.1007/s11356-021-12996-6
11. Elsaesser A, Howard CV. Toxicology of nanoparticles. Adv Drug Deliv Rev. 2012;64(2):129–137. doi:10.1016/j.addr.2011.09.001
12. Yang W, Wang L, Mettenbrink EM, DeAngelis PL, Wilhelm S. Nanoparticle toxicology. Annu Rev Pharmacol Toxicol. 2021;61:269–289.
13. Verdon R, Stone V, Murphy F, et al. The application of existing genotoxicity methodologies for grouping of nanomaterials: towards an integrated approach to testing and assessment. Part Fibre Toxicol. 2022;19(1). doi:10.1186/s12989-022-00476-9
14. Ierodiakonou D, Zanobetti A, Coull B, et al. Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J Allergy Clin Immunol. 2016;137(2):390–399. doi:10.1016/j.jaci.2015.05.028
15. Xia T, Zhu Y, Mu L, Zhang Z, Liu S. Pulmonary diseases induced by ambient ultrafine and engineered nanoparticles in twenty-first century. Natl Sci Rev. 2016;3(4):416–429. doi:10.1093/nsr/nww064
16. Li N, Georas S, Alexis N, et al. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J Allergy Clin Immunol. 2016;138(2):386–396. doi:10.1016/j.jaci.2016.02.023
17. Kim K, Jahan S, Kabir E. A review on human health perspective of air pollution with respect to allergies and asthma. Environ Int. 2013;59:41–52. doi:10.1016/j.envint.2013.05.007
18. Chen CM. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol. 2006;57(3):359–377. doi:10.1002/asi.20317
19. Tan L, Wang X, Yuan K, et al. Structural and temporal dynamics analysis on drug-eluting stents: history, research hotspots and emerging trends. Bioact Mater. 2023;23:170–186. doi:10.1016/j.bioactmat.2022.09.009
20. Wang M, Yao G, Sun Y, Yang Y, Deng R. Exposure to construction dust and health impacts - A review. Chemosphere. 2023;311(Pt 1):136990. doi:10.1016/j.chemosphere.2022.136990
21. de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine carbon black particles cause early airway inflammation and have adjuvant activity in a mouse allergic airway disease model. Toxicol Sci. 2005;87(2):409–418. doi:10.1093/toxsci/kfi255
22. Ihrie MD, Taylor-Just AJ, Walker NJ, et al. Inhalation exposure to multi-walled carbon nanotubes alters the pulmonary allergic response of mice to house dust mite allergen. Inhal Toxicol. 2019;31(5):192–202. doi:10.1080/08958378.2019.1643955
23. Carvalho S, Ferrini M, Herritt L, Holian A, Jaffar Z, Roberts K. Multi-walled carbon nanotubes augment allergic airway eosinophilic inflammation by promoting cysteinyl leukotriene production. Front Pharmacol. 2018;9. doi:10.3389/fphar.2018.00585
24. Shipkowski KA, Taylor AJ, Thompson EA, et al. An allergic lung microenvironment suppresses carbon nanotube-induced inflammasome activation via stat6-dependent inhibition of caspase-1. PLoS One. 2015;10(6):e0128888. doi:10.1371/journal.pone.0128888
25. Thompson EA, Sayers BC, Glista-Baker EE, et al. Role of signal transducer and activator of transcription 1 in murine allergen-induced airway remodeling and exacerbation by carbon nanotubes. Am J Respir Cell Mol Biol. 2015;53(5):625–636. doi:10.1165/rcmb.2014-0221OC
26. Ronzani C, Casset A, Pons F. Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma. Arch Toxicol. 2014;88(2):489–499. doi:10.1007/s00204-013-1116-3
27. Sayers BC, Taylor AJ, Glista-Baker EE, et al. Role of cyclooxygenase-2 in exacerbation of allergen-induced airway remodeling by multiwalled carbon nanotubes. Am J Respir Cell Mol Biol. 2013;49(4):525–535. doi:10.1165/rcmb.2013-0019OC
28. Mizutani N, Nabe T, Yoshino S. Exposure to multiwalled carbon nanotubes and allergen promotes early- and late-phase increases in airway resistance in mice. Biol Pharm Bull. 2012;35(12):2133–2140. doi:10.1248/bpb.b12-00357
29. Ichiro IK, Koike E, Yanagisaw R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237(3):306–316. doi:10.1016/j.taap.2009.04.003
30. Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong BA, Bonner JC. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am J Respir Cell Mol Biol. 2009;40(3):349–358. doi:10.1165/rcmb.2008-0276OC
31. Li J, Li L, Chen H, et al. Application of vitamin E to antagonize SWCNTs-induced exacerbation of allergic asthma. Sci Rep. 2014:4. doi:10.1038/srep04275
32. Nygaard UC, Samuelsen M, Marioara CD, Lovik M. Carbon nanofibers have IgE adjuvant capacity but are less potent than nanotubes in promoting allergic airway responses. Biomed Res Int. 2013;2013:1–12. doi:10.1155/2013/476010
33. Inoue K, Yanagisawa R, Koike E, Nishikawa M, Takano H. Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: possible role of oxidative stress. Free Radic Biol Med. 2010;48(7):924–934. doi:10.1016/j.freeradbiomed.2010.01.013
34. Lindner K, Webering S, Stroebele M, et al. Low dose carbon black nanoparticle exposure does not aggravate allergic airway inflammation in mice irrespective of the presence of surface polycyclic aromatic hydrocarbons. Nanomaterials. 2018;8(4):213. doi:10.3390/nano8040213
35. Kroker M, Sydlik U, Autengruber A, et al. Preventing carbon nanoparticle-induced lung inflammation reduces antigen-specific sensitization and subsequent allergic reactions in a mouse model. Part Fibre Toxicol. 2015;12:12. doi:10.1186/s12989-015-0093-5
36. Shang S, Li J, Zhao Y, et al. Oxidized graphene-aggravated allergic asthma is antagonized by antioxidant vitamin E in Balb/c mice. Environ Sci Pollut Res. 2017;24(2):1784–1793. doi:10.1007/s11356-016-7903-7
37. Shurin MR, Yanamala N, Kisin ER, et al. Graphene oxide attenuates th2-type immune responses, but augments airway remodeling and hyperresponsiveness in a murine model of asthma. ACS Nano. 2014;8(6):5585–5599. doi:10.1021/nn406454u
38. Norton SK, Wijesinghe DS, Dellinger A, et al. Epoxyeicosatrienoic acids are involved in the C-70 fullerene derivative-induced control of allergic asthma. J Allergy Clin Immunol. 2012;130(3):761–+. doi:10.1016/j.jaci.2012.04.023
39. Ryan JJ, Bateman HR, Stover A, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179(1):665–672. doi:10.4049/jimmunol.179.1.665
40. Yang YS, Cao MD, Wang A, et al. Nano-silica particles synergistically IgE-mediated mast cell activation exacerbating allergic inflammation in mice. Front Immunol. 2022;13:911300. doi:10.3389/fimmu.2022.911300
41. Ko JW, Shin NR, Je-Oh L, et al. Silica dioxide nanoparticles aggravate airway inflammation in an asthmatic mouse model via NLRP3 inflammasome activation. Regul Toxicol Pharmacol. 2020:112. doi:10.1016/j.yrtph.2020.104618
42. Han H, Park YH, Park HJ, et al. Toxic and adjuvant effects of silica nanoparticles on ovalbumin-induced allergic airway inflammation in mice. Respir Res. 2016;17:17. doi:10.1186/s12931-016-0376-x
43. Park HJ, Sohn JH, Kim YJ, et al. Acute exposure to silica nanoparticles aggravate airway inflammation: different effects according to surface characteristics. Exp Mol Med. 2015:47. doi:10.1038/emm.2015.50
44. Brandenberger C, Rowley NL, Jackson-Humbles DN, et al. Engineered silica nanoparticles act as adjuvants to enhance allergic airway disease in mice. Part Fibre Toxicol. 2013;10:10. doi:10.1186/1743-8977-10-26
45. Yoshida T, Yoshioka Y, Fujimura M, et al. Promotion of allergic immune responses by intranasally-administrated nanosilica particles in mice. Nanoscale Res Lett. 2011;6:6. doi:10.1186/1556-276X-6-195
46. Han B, Guo J, Abrahaley T, et al. Adverse effect of nano-silicon dioxide on lung function of rats with or without ovalbumin immunization. PLoS One. 2011;6(2). doi:10.1371/journal.pone.0017236
47. Chuang HC, Hsiao TC, Wu CK, et al. Allergenicity and toxicology of inhaled silver nanoparticles in allergen-provocation mice models. Int J Nanomedicine. 2013;8:4495–4506. doi:10.2147/IJN.S52239
48. Su CL, Chen TT, Chang CC, et al. Comparative proteomics of inhaled silver nanoparticles in healthy and allergen provoked mice. Int J Nanomedicine. 2013;8:2783–2799. doi:10.2147/IJN.S46997
49. Jang S, Park JW, Cha HR, et al. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int J Nanomedicine. 2012;7:1329–1343. doi:10.2147/IJN.S27159
50. Park HS, Kim KH, Jang S, et al. Attenuation of allergic airway inflammation and hyperresponsiveness in a murine model of asthma by silver nanoparticles. Int J Nanomedicine. 2010;5:505–515. doi:10.2147/IJN.S11664
51. Omlor AJ, Le DD, Schlicker J, et al. Local effects on airway inflammation and systemic uptake of 5 nm pegylated and citrated gold nanoparticles in asthmatic mice. Small. 2017;13(10):1603070. doi:10.1002/smll.201603070
52. Barreto E, Serra MF, Dos Santos RV, et al. Local administration of gold nanoparticles prevents pivotal pathological changes in murine models of atopic asthma. J Biomed Nanotechnol. 2015;11(6):1038–1050. doi:10.1166/jbn.2015.2024
53. Hussain S, Vanoirbeek JAJ, Luyts K, et al. Lung exposure to nanoparticles modulates an asthmatic response in a mouse model. Eur Respir J. 2011;37(2):299–309. doi:10.1183/09031936.00168509
54. Lim JO, Lee SJ, Kim WI, et al. Titanium dioxide nanoparticles exacerbate allergic airway inflammation via TXNIP upregulation in a mouse model of asthma. Int J Mol Sci. 2021;22(18):9924. doi:10.3390/ijms22189924
55. Harfoush SA, Hannig M, Le DD, et al. High-dose intranasal application of titanium dioxide nanoparticles induces the systemic uptakes and allergic airway inflammation in asthmatic mice. Respir Res. 2020;21(1). doi:10.1186/s12931-020-01386-0
56. Kim BG, Park MK, Lee PH, et al. Effects of nanoparticles on neuroinflammation in a mouse model of asthma. Respir Physiol Neurobiol. 2020:271. doi:10.1016/j.resp.2019.103292
57. Kim BG, Lee PH, Lee SH, Park MK, Jang AS. Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol Res. 2017;9(3):257–264. doi:10.4168/aair.2017.9.3.257
58. Mishra V, Baranwal V, Mishra RK, Sharma S, Paul B, Pandey AC. Titanium dioxide nanoparticles augment allergic airway inflammation and Socs3 expression via NF-kappa B pathway in murine model of asthma. Biomaterials. 2016;92:90–102. doi:10.1016/j.biomaterials.2016.03.016
59. Gustafsson A, Jonasson S, Sandstrom T, Lorentzen JC, Bucht A. Genetic variation influences immune responses in sensitive rats following exposure to TiO2 nanoparticles. Toxicology. 2014;326:74–85. doi:10.1016/j.tox.2014.10.004
60. Scarino A, Noel A, Renzi PM, et al. Impact of emerging pollutants on pulmonary inflammation in asthmatic rats: ethanol vapors and agglomerated TiO2 nanoparticles. Inhal Toxicol. 2012;24(8):528–538. doi:10.3109/08958378.2012.696741
61. Rossi EM, Pylkkanen L, Koivisto AJ, et al. Inhalation exposure to nanosized and fine TiO2 particles inhibits features of allergic asthma in a murine model. Part Fibre Toxicol. 2010;7. doi:10.1186/1743-8977-7-35
62. Larsen ST, Roursgaard M, Jensen KA, Nielsen GD. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin Pharmacol Toxicol. 2010;106(2):114–117. doi:10.1111/j.1742-7843.2009.00473.x
63. Huang KL, Chang HL, Tsai FM, Lee YH, Wang CH, Cheng TJ. The effect of the inhalation of and topical exposure to zinc oxide nanoparticles on airway inflammation in mice. Toxicol Appl Pharmacol. 2019;384. doi:10.1016/j.taap.2019.114787
64. Huang KL, Lee YH, Chen HI, Liao HS, Chiang BL, Cheng TJ. Zinc oxide nanoparticles induce eosinophilic airway inflammation in mice. J Hazard Mater. 2015;297:304–312. doi:10.1016/j.jhazmat.2015.05.023
65. Gustafsson A, Bergstrom U, Agren L, Osterlund L, Sandstrom T, Bucht A. Differential cellular responses in healthy mice and in mice with established airway inflammation when exposed to hematite nanoparticles. Toxicol Appl Pharmacol. 2015;288(1):1–11. doi:10.1016/j.taap.2015.07.001
66. Ban M, Langonne I, Huguet N, Guichard Y, Goutet M. Iron oxide particles modulate the ovalbumin-induced Th2 immune response in mice. Toxicol Lett. 2013;216(1):31–39. doi:10.1016/j.toxlet.2012.11.003
67. Cui H, Huang J, Lu M, et al. Antagonistic effect of vitamin E on nAl(2)O(3)-induced exacerbation of Th2 and Th17-mediated allergic asthma via oxidative stress. Environ Pollut. 2019;252:1519–1531. doi:10.1016/j.envpol.2019.06.092
68. Ilves M, Kinaret PAS, Ndika J, et al. Surface PEGylation suppresses pulmonary effects of CuO in allergen-induced lung inflammation. Part Fibre Toxicol. 2019;16. doi:10.1186/s12989-019-0309-1
69. Roach KA, Anderson SE, Stefaniak AB, et al. Surface area- and mass-based comparison of fine and ultrafine nickel oxide lung toxicity and augmentation of allergic response in an ovalbumin asthma model. Inhal Toxicol. 2019;31(8):299–324. doi:10.1080/08958378.2019.1680775
70. Meldrum K, Robertson SB, Roemer I, et al. Cerium dioxide nanoparticles exacerbate house dust mite induced type II airway inflammation. Part Fibre Toxicol. 2018;15:15. doi:10.1186/s12989-018-0261-5
71. Deng R, Lin D, Zhu L, et al. Nanoparticle interactions with co-existing contaminants: joint toxicity, bioaccumulation and risk. Nanotoxicology. 2017;11(5):591–612. doi:10.1080/17435390.2017.1343404
72. Deng R, Yang K, Lin D. Pentachlorophenol and ciprofloxacin present dissimilar joint toxicities with carbon nanotubes to Bacillus subtilis. Environ Pollut. 2021;270. doi:10.1016/j.envpol.2020.116071
73. Deng R, Ma P, Li B, Wu Y, Yang X. Development of allergic asthma and changes of intestinal microbiota in mice under high humidity and/or carbon black nanoparticles. Ecotoxicol Environ Saf. 2022;241:113786. doi:10.1016/j.ecoenv.2022.113786
74. Wang M, Deng R. Effects of carbon black nanoparticles and high humidity on the lung metabolome in Balb/c mice with established allergic asthma. Environ Sci Pollut Res. 2022;29(43):65100–65111. doi:10.1007/s11356-022-20349-0
75. Meldrum K, Robertson S, Romer I, et al. Diesel exhaust particle and dust mite induced airway inflammation is modified by cerium dioxide nanoparticles. Environ Toxicol Pharmacol. 2020:73. doi:10.1016/j.etap.2019.103273
76. Layachi S, Rogerieux F, Robidel F, Lacroix G, Bayat S. Effect of combined nitrogen dioxide and carbon nanoparticle exposure on lung function during ovalbumin sensitization in brown Norway rat. PLoS One. 2012;7(9). doi:10.1371/journal.pone.0045687
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




