Back to Journals » Drug Design, Development and Therapy » Volume 15
Total Flavone of Abelmoschus manihot Ameliorates Stress-Induced Microbial Alterations Drive Intestinal Barrier Injury in DSS Colitis
Authors Wang R , Chen T, Wang Q, Yuan XM, Duan ZL, Feng ZY, Ding Y, Bu F, Shi GP, Chen YG
Received 30 March 2021
Accepted for publication 19 June 2021
Published 8 July 2021 Volume 2021:15 Pages 2999—3016
DOI https://doi.org/10.2147/DDDT.S313150
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Rong Wang,1,* Tuo Chen,2,* Qiong Wang,3 Xiao-Min Yuan,1 Zheng-Lan Duan,1 Ze-Yu Feng,1 Yang Ding,1 Fan Bu,1 Guo-Ping Shi,4 Yu-Gen Chen1
1Department of Colorectal Surgery, The Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210029, People’s Republic of China; 2Department of General Surgery, Affiliated hospital of Yangzhou university, Yangzhou, Jiangsu, 225000, People’s Republic of China; 3Central Laboratory, Jiangsu Province Hospital of Chinese Medicine, Nanjing, Jiangsu, 210029, People’s Republic of China; 4Collaborative Innovation Center for Cancer Medicine, The Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, 210029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu-Gen Chen; Guo-Ping Shi Email [email protected]; [email protected]
Purpose: Total flavone of Abelmoschus manihot (TFA), the effective constituents extracted from Flos Abelmoschus Manihot, has been reported to inhibit inflammation. However, the effect of TFA on ulcerative colitis (UC) progression in patients with depression is unknown. The purpose of our research was to explore the anti-UC effects of TFA in the context of depression in mice with UC by regulating the gut microbiota to drive the intestinal barrier.
Methods: In this study, chronic stress (CS) and dextran sodium sulfate (DSS) were used to induce depression and UC, respectively, in C57BL/6J mice. Fecal microbiota transplantation (FMT) was used to evaluate how treating mice modeling UC and depression with TFA effected their gut microbiota.
Results: Our results showed that TFA effectively improved UC aggravated by CS. In addition, TFA treatment improved the depression-like phenotype, the disturbed gut microbiota, and the intestinal barrier function in CS mice. It is worth noting that FMT from the CS mice to the receptor group further aggravated the damage of the intestinal barrier and the disturbance of the gut microbiota in the recipient DSS mice, thus further aggravating UC, however, treatment of the intervention of TFA in the CS fecal microbiota transplant with TFA also played its therapeutic outcome.
Conclusion: Taken together, our results show that CS disrupts the gut microbiota, triggers intestinal barrier injury and aggravates DSS colitis, while TFA is a promising drug for the treatment of UC in patients with depression.
Keywords: total flavone of Abelmoschus manihot, ulcerative colitis, depression, gut microbiota, intestinal barrier
Graphical Abstract:
Introduction
Inflammatory bowel disease (IBD) is a group of chronic inflammatory diseases that includes Crohn’s disease (CD) and ulcerative colitis (UC). The chronic, unpredictable, and uncertain nature of UC is an important feature that can trigger anxiety and depression.1,2 Recent clinical-research evaluated depression in individuals who subsequently develop UC.3 Compared with those without depression, patients with depression had an approximately 41% increased risk of UC, an increased risk of the relapse of UC, and an increased number of surgeries and hospitalizations, which affect the prognosis of UC.4–7 UC patients with depressive symptoms have a lower quality of life and the course of disease is worse in patients with depression.8,9 However, the underlying mechanisms by which depression aggravates UC remain unclear.
A study showed that in patients with UC, gut microbial diversity decreases, the number of potentially pathogenic bacteria increases, and their biodiversity decreases, which can aggravate colitis.10 In addition, experiments have found that changes in the gut microbiota can aggravate or alleviate colitis.11 Therefore, alleviating gut microbiota disorders may be a potential treatment for UC. The microbiota plays an important role in maintaining the intestinal barrier. The composition of the gut microbiota can influence the development of the immune system and regulate immune mediators, thus affecting the intestinal barrier. Dysbiosis impinges on the epithelial barrier, leading to so-called intestinal leakage, which brings the intestinal contents into contact with the periphery of the host, inducing an inflammatory response.12–14 Members of the gut microbiota coexists to regulate the maturation of the mucosal immune system, and pathogenic bacteria cause immune dysfunction, while the intestinal mucosal immune system can protect the integrity of the intestinal barrier.15 In addition, a large number of clinical trials have found that the gut microbiota structure of depressed individuals is significantly different from that of healthy individuals, generally manifested as decreases in the diversity and richness of gut microbiota.16,17 These results suggest that gut microbes regulate the intestinal barrier and may be the link between depression and colitis.
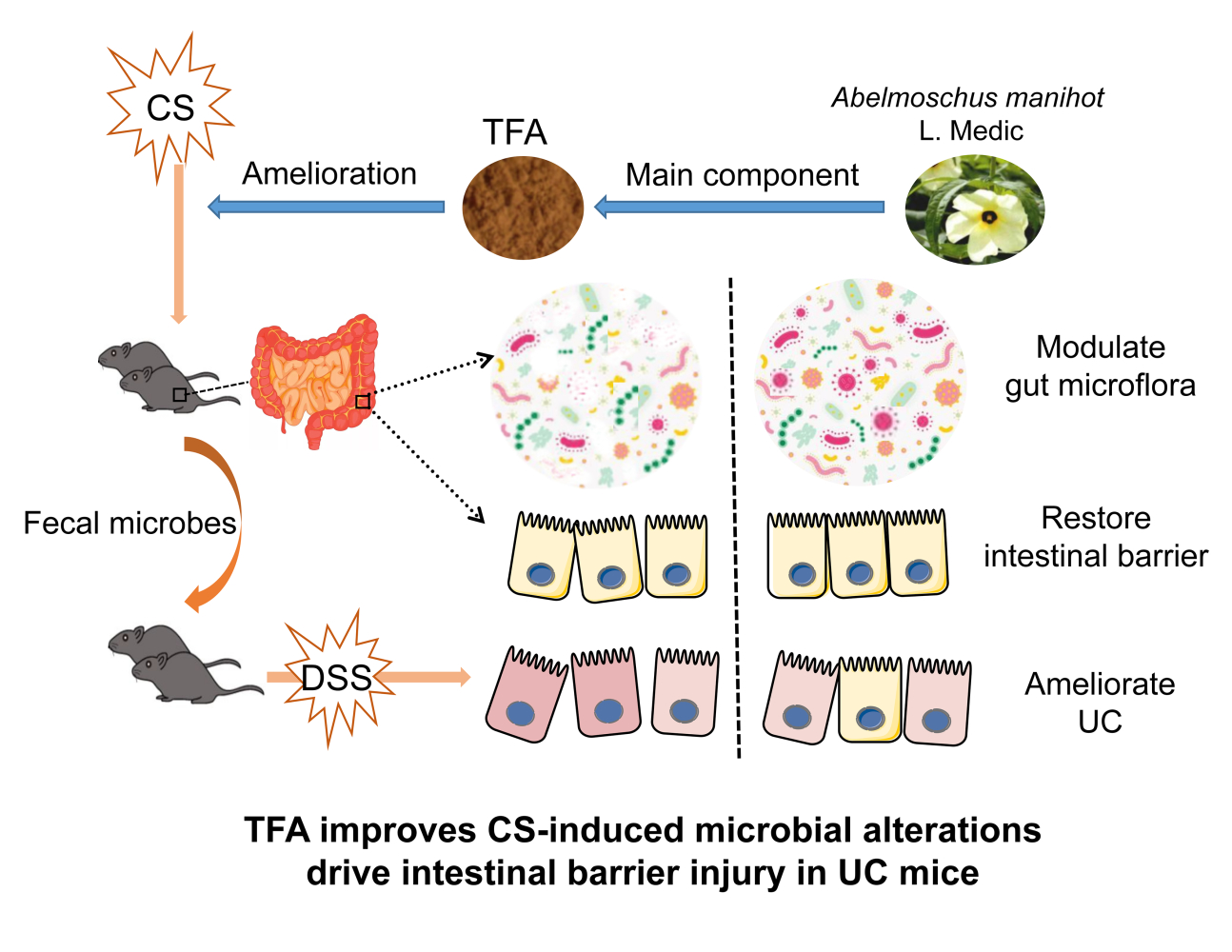
As a traditional Chinese medicine, Flos Abelmoschus manihot is often used as the main drug of the Huang Kui Lian Chang Decoction in the treatment of UC.18 The total flavone of Abelmoschus manihot (TFA) is an effective constituent extracted from Flos Abelmoschus manihot that has significant therapeutic effects on IBD, chronic glomerulonephritis and poststroke depression.19–21 High performance liquid chromatography (HPLC) analysis showed that TFA is mainly composed of eight flavonoid glycosides, including quercetin-3-O-robinobioside, gossypetin-3-O-glucoside, quercetin-3ʹ-O-glucoside, isoquercetin, hyperoside, myricetin, gossypetin and quercetin.20 In this study, we verified that TFA has therapeutic effects on dextran sodium sulfate (DSS) induced colitis with depression. Then, we used fecal microbiota transplantation (FMT) to provide evidence that the gut microbiota drives the intestinal barrier and is involved in the susceptibility of chronic stress to DSS colitis (Graphical Abstract).
Materials and Methods
Drugs
TFA was extracted from the flowers of Abelmoschus manihot at the Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China. Flos Abelmoschus manihot was immersed in 75% ethanol for 60 min. The mixture was refluxed for 60 min at 90°C and then filtered with analytical filter paper. Finally, rotary evaporation was used to evaporate the extracts under vacuum at 60°C.22 The purity of TFA was 90%. TFA was suspended in 1% carboxymethyl cellulose solution at different concentrations for oral administration (62.5 and 125 mg/kg).
TFA was analyzed by HPLC. There were eight standards (purity >98%) purchased from Shanghai Yuanye Bio‑Technology Co., Ltd. A Waters 2694 series HPLC instrument (Waters Corporation) was used for examination with TFA. The sample was separated on a C18 column (4.6x250 mm, 5 µm) and the mobile phase gradient contained acidified water with acetonitrile (solvent A) as well as phosphoric acid (solvent B, 0.2%). The gradient program was performed as follows: 0‑10 min, 86% B; 10‑15 min, 92% B; 15‑25 min, 92% B; 25‑30 min, 81% B; 30‑65 min, 81% B; 65‑70 min, 86% B. Chromatography was performed at a flow rate of 1.0 mL/min at 30°C and aliquots of 10 µL were analyzed.20
Animals and Reagents
Six-week-old male C57BL/6J mice were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd (Jiaxing, Zhejiang, China). The mice were raised in the laboratory of Basic Pharmacology, Affiliated Hospital of Nanjing University of Chinese Medicine (Nanjing, Jiangsu, China). Sterilized standard rodent chow food and sterilized water were not restricted during the experiment, the temperature was controlled at 23 ± 1°C, the humidity was controlled at 50 ± 5%, and the light system was set at 12 hours/day. Dextran sulfate sodium (DSS, molecular weight of 36–50 kDa) was provided by German MP Biopharmaceutical Company, and UC was induced by administering 2.5% DSS through the drinking water for 7 days, while the control group was given normal drinking water.23 Ampicillin, neomycin, metronidazole and vancomycin were purchased from Sigma-Aldrich Company (USA). The experiments were approved by the Animal Ethics Committee of The Affiliated Hospital of Nanjing University of Chinese Medicine. The project identification code of the ethical statement is 2020DW-30-01. The experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chronic Stress (CS)
A depression model of chronic stress was induced by the chronic restraint stress (CRS) method. Mice were subjected to chronic stress after acclimation to the facility for 7 days. Mice were restrained in a 50 mL Falcon tube that was fitted to allow them to breathe. Mice were immobilized for 3 hours once daily for 30 consecutive days to induce depressive-like behavior. The mice could not access water and food during the chronic restraint stress period but could freely access them at the end of it.24
Behavioral Tests
The open field test (OFT) was used to assess the locomotor and depression-like behaviors of mice. Each mouse was individually and gently placed in a chamber (50 cm×50 cm×30 cm) for one minute of adaptation. The distance moved was measured using a video tracking system over 5 min.25
The tail suspension test (TST) and forced swimming test (FST) were both used to assess behavioral despair. In the TST, each mouse was individually placed on a tail suspension monitor 2 cm from the tail tip in a suspended state, and the head was more than 10 cm from the bottom; this lasted for a total of 6 min. After 2 min of adaptation, the immobility time was recorded over 4 min. In the FST, each mouse was individually placed in an organic glass drum (height: 40 cm, diameter: 30 cm) filled with water (depth of 20 cm, water temperature 23 ± 2°C) and was forced to swim in the tank for 6 min, and the immobility duration in the last 4 min was recorded.
Fecal Microbiota Transplantation (FMT)
Briefly, the mice in the recipient group were freely given an antibiotic mixture for 4 weeks. Mixed antibiotics were prepared proportionally as follows: ampicillin (1 g/L), neomycin (1 g/L), metronidazole (1 g/L) and vancomycin (0.5 g/L).26 Mice in the donor group were euthanized at the end of modeling, and the cecum contents were collected and weighed. The contents of the cecum were placed into sterile PBS, and the contents were fully shaken with a suspension instrument for homogenization and then centrifuged at 600 g for 3 min. The supernatant was taken and prepared as a bacterial solution at a ratio of 100 g/L. Mice in the recipient group were given 0.1 mL of bacterial solution by gavage every day for 14 consecutive days.27
Assessment of the Disease Activity Index (DAI)
Mice were monitored daily for body weight. The DAI was recorded every day during DSS treatment using three parameters: body weight loss (0 points = no loss, 1 point = 1–5% loss, 2 points = 5–10% loss, 3 points = 10–15% loss, 4 points = over 15% loss), fecal consistency (0 points = normal, 2 points = loose stools, 4 points = watery diarrhea), and hematochezia (0 points = no bleeding, 2 points = slight bleeding, 4 points = gross bleeding). The DAI was the total score of these three parameters.28
Histopathological Assessment
Distal colon specimens were fixed in Carnoy’s solution after mice were sacrificed. Then, distal colon specimens were paraffin-embedded. Finally, the tissues were sectioned and stained with hematoxylin and eosin, and pathological changes were observed with a light microscope. The following parameters were assessed: infiltration neutrophils, extent of crypt damage, crypt abscesses, submucosal edema, loss of goblet cells and reactive epithelial hyperplasia.29
Staining of PAS-AB
Paraffin sections were dewaxed with water, stained with Alcian blue, periodic acid, Schiff’s reagent and hematoxylin solution, dehydrated and sealed, and finally observed under a microscope. The colonic epithelium was stained positive, and the PAS-AB cells were stained blue. Three high-power fields (200×) were randomly selected from each section, and the number of intestinal villi under each microscope was divided to obtain the number of PAS-AB-positive cells in the colon of each mouse.
Immunohistochemical Staining
Immunohistochemistry was performed based on the procedure described in an earlier study.30 The following antibodies were used in the immunohistochemistry assay: MUC2 (Abcam, GB11344), KLF4 (Abcam, 11880-1-A), and ZO-1 (Abcam, GB111981). Digital images at 200X magnification were acquired using a light microscope.
Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Total RNA was extracted from colon tissues using TRIzol reagent, the concentration of the RNA was measured, and the RNA was then reverse transcribed according to the manufacturer’s instructions using 5x PrimerScript. The primer sequences are shown in Table 1. GAPDH was used as a reference gene. The ΔΔCt method was used to compute the relative gene expression levels.
 |
Table 1 Primers Used in the Real-Time PCR Assays |
16S rDNA Gene High-Throughput Sequencing
The V3-V4 variable region of the bacterial 16S rRNA gene was amplified by F338 (5ʹ-ACTCCTACGGGAGGCAGCA-3ʹ) and R806 (5ʹ-GGACTACHV GGGTWTCTAAT-3ʹ). Using the Illumina MiSeq platform, the extracted PCR products were analyzed by isomolecular 250-bp double-terminal sequencing. The original pyrophosphate sequence was uploaded to the NCBI Data Center database SRA (Sequence Read Archive). High-quality sequence merge overlap generated fastq files. QIIME (version 1.9.1) software was used to multichannel decode and quality control filter the fastq file output.
Statistical Analysis
GraphPad Prism 8.0.2 statistical software was utilized to analyze the above experimental data. Measurement data are represented as the mean ± standard deviation; Student’s t-test, one-way ANOVA or two-way ANOVA was applied to compare differences between multiple groups. A value of P < 0.05 indicated that the difference was statistically significant.
Results
TFA Ameliorated UC Aggravated by Depression in Mice
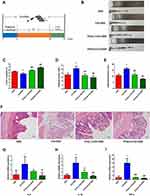
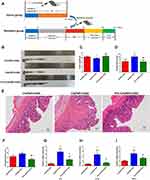
As shown in Figure 1A, we treated depression UC mice with TFA(L) (low-dose, 62.5 mg/kg) or TFA(H) (high-dose, 125 mg/kg) for 37 days to investigate the protective role of TFA on intestinal inflammation in depression UC. In this study, the CS+DSS mice had shorter colon lengths than DSS mice, and this change was obviously ameliorated by TFA treatment in a dose-dependent manner (Figure 1B and xC). The CS+DSS mouse group had a higher DAI score than the DSS mice, while TFA administration markedly decreased the DAI score (Figure 1D). Therefore, TFA treatment significantly ameliorated the disease symptoms of intestinal inflammation in depression UC mice.
Histological analyses revealed obvious inflammatory cell infiltration, mucosal thickening, goblet cell depletion, structural destruction and crypt loss in colonic tissues in the CS+DSS group, with a markedly high histological score. However, the histological score decreased with increasing TFA dose (Figure 1E and F). The expression levels of the proinflammatory cytokines IL-6, IL-1β and TNF-α were significantly increased in the colons of mice with depression UC, as shown by qPCR, while the expression levels of proinflammatory cytokines were efficiently decreased by high-dose TFA administration (Figure 1G-I). Overall, TFA treatment significantly improved the inflammation of colonic tissues in depression UC mice.
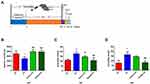
TFA Treatment Rescued Depressive-Like Behaviors of CS Mice
The depression model was induced by exposing mice to chronic stress for 30 days (Figure 2A). The results showed that TFA treatment restored CS-induced depression in an extended range of motion in the OFT (Figure 2B). Moreover, the immobility duration reduction caused by CS in the TST and FST was restored by TFA treatment in a dose-dependent manner (Figure 2C and D).
TFA Treatment Improved the Intestinal Barrier Function of CS Mice
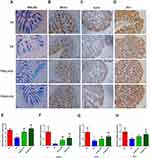
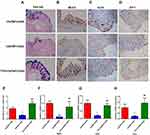
To explore the differences in the colonic physical barrier between the experimental groups, the expression profiles of PAS-AB, MUC2, KLF4 and ZO-1 were investigated. Goblet cell loss, as a hallmark of UC, can lead to a reduction in mucus secretion and can exacerbate colitis. To determine whether TFA treatment could increase colonic goblet cell differentiation in CS mice, mature goblet cells were examined by PAS-AB staining. PAS-AB staining revealed that the abundance of PAS-AB-positive cells was downregulated in CS mice. However, PAS-AB-positive cells were improved with TFA supplementation in a dose-dependent manner. (Figure 3A and E). Additionally, immunohistochemical staining showed that the MUC2, KLF4 and ZO-1 proteins were observably suppressed in CS colitis mice compared with those of control check (CK) mice. In contrast, TFA promoted the expression of the above three proteins in a dose-dependent manner (Figure 3B-D). Consistently, the mRNA expression levels of MUC2, KLF4 and ZO-1 were also significantly upregulated in the CS group and were downregulated following TFA treatment in a dose-dependent manner (Figure 3F-H). Collectively, these data indicate that TFA might prevent CS-induced disruption of the intestinal barrier.
TFA Attenuated CS-Induced Gut Microbial Dysbiosis
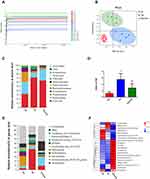
To determine whether TFA (high-dose, 125 mg/kg) attenuated CS-induced gut microbial dynamics, high-throughput gene-sequencing analysis of 16S rRNA in fecal bacterial DNA was conducted. As shown in Figure 4A, the Shannon curve indicated that most of the diversity in the CS group was lower than those in the control and TFA groups. Beta-diversity to generate a principal coordinate analysis (PCoA) was used to extend our understanding of the role of microbiome diversity. A significant clustering separation between OTUs revealed the different community structures among the three groups (Figure 4B).
Subsequently, the most abundant phyla, which included Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, were detected in the fecal microbiota by 16S rRNA sequencing analysis. Firmicutes was the most abundant phylum in these three groups., The relative abundance of Bacteroidetes was decreased remarkably in the CS group compared with the CK group, while that of Firmicutes was significantly increased. After administration of TFA, these changes were markedly reversed (Figure 4C). A reduction was also observed in the ratio of Firmicutes to Bacteroidetes (F/B) after TFA therapy (Figure 4D). There were 10 major genera in the three groups at the genus level (Figure 4E). The abundance of Lactobacillus increased, while those of Desulfovibrio, Enterorhabdus and Bacteroides decreased in the CS group. TFA treatment reversed the depletion of Bacteroides and the overgrowth of Lactobacillus. At the same time, TFA treatment decreased the abundance of levels Helicobacter, Candidatus_Saccharimonas, Enterorhabdus and Lachnospiraceae_UCG-006 and increased the proportions of Roseburia, Alistipes, Oscillibacter, Rikenellaceae_RC9_gut_group, Ruminiclostridium_9, and Alloprevotella (Figure 4F). Overall, these data indicate that TFA corrected CS-induced gut microbiota dysbiosis.
Correlations Between Gut Microbes and Microenvironmental Factors
To investigate whether the changes in intestinal microflora in mice were related to intestinal barrier indicators, correlation analysis was conducted between genera with richness greater than 0.1% and intestinal barrier indexes (MUC2, KLF4, ZO-1). Bacteroides abundance exhibited positive correlations with MUC2, KlF4 and ZO-1, while Lactobacillus abundance was negatively correlated with MUC2, KlF4 and ZO-1. Alistipes abundance, which was increased in the TFA-treated groups, was positively correlated with KLF4 and ZO-1. Desulfovibrio abundance, which was lower in the CS group, was significantly positively correlated with MUC2 and KlF4 levels (Figure 5). These results revealed that TFA improved intestinal barrier function and was related to increases in Alistipes, Desulfovibrio and Bacteroides abundance and decreases in Lactobacillus abundance.
TFA Fecal Microbiota Transplantation Reduces Inflammation in Depression UC Mice
To investigate whether the intestinal protective effects of TFA (high-dose, 125 mg/kg) against colitis injury aggravated by depression are dependent on the presence of the gut microbiota, we created a “germ-free” mouse model. Then, the corresponding feces from mice treated with bacteria were transplanted into “germ-free” mice. Finally, DSS colitis was modeled (Figure 6A). Analysis of the FMT mice indicated that CS fecal microbiota transplants decreased the colon length and increased the DAI score. However, TFA treatment reversed these changes (Figure 6B-D). In addition, histopathological examination of colon tissue revealed exacerbation of inflammatory infiltrates and mucosal injury with CS fecal microbiota transplants. Consistently, TFA intervention achieved remission (Figure 6E and F). As shown in Figure 6G-I, TFA treatment of fecal microbiota transplants also significantly decreased the mRNA expression levels of IL-6, IL-1β and TNF-α in the colonic tissues of depression UC mice.
TFA Fecal Transplantation Attenuated Intestinal Barrier Injury in Depression UC Mice
As shown in Figure 7A and E, PAS-AB staining revealed the decreased abundance of mature PAS-AB-positive cells in the colons of CS(FMT)+DSS mice compared with CK(FMT)+DSS mice, however, TFA treatment significantly upregulated PAS-AB goblet cells. Additionally, immunohistochemical staining with MUC2, KLF4 and ZO-1 showed that CS(FMT)+DSS mice had reduced numbers of these positive cells, while TFA treatment reversed these changes (Figure 7B-D). Accordingly, the mRNA expression levels of MUC2, KLF4 and ZO-1 were also significantly lower in the CS(FMT)+DSS group and were increased in the TFA+CS(FMT)+DSS group (Figure 7F-H). Collectively, these data show that TFA fecal transplantation corrected intestinal barrier injury in depression UC mice.
TFA Fecal Microbiota Transplantation Modulates Gut Microbiota in Depression UC Mice
To confirm that TFA fecal microbiota transplantation promotes remodeling of microbiota, 16S rRNA from isolated fecal bacterial DNA was analyzed. The Shannon index showed that the CS(FMT)+DSS group had decreased microbial diversity compared to the CK(FMT)+DSS group. TFA treatment induced ascending microbial diversity (Figure 8A). PCoA analysis was used to expand our understanding of the role of microbiome diversity. In the OTU cluster separation table, the three groups were different in terms of community composition structure (Figure 8B). We evaluated the gut microbiota landscapes of available samples from all FMT groups to further investigate potential differences in composition. As illustrated in Figure 8C, a total of 10 phyla were detected in these three groups, with Firmicutes, Bacteroidetes and Proteobacteria as the most predominant phylum. More importantly, a higher abundance of Proteobacteria and lower abundance levels of Firmicutes and Bacteroidetes were observed in the CS(FMT)+DSS group than in the CK(FMT)+DSS group, and TFA treatment almost modulated these differences. Moreover, the F/B ratio was higher in the CS(FMT)+DSS group than in the CK(FMT)+DSS group. However, TFA treatment decreased the F/B ratio (Figure 8D). At the genus level, the CS(FMT)+DSS group had a lower abundance of Alistipes and higher abundance levels of Escherichia-Shigella and Enterococcus than the CK(FMT)+DSS group. TFA treatment successfully reversed these changes. In addition, the TFA+CS(FMT)+DSS group had higher abundance levels of Bacteroides, Helicobacter, Mucispirillum and Ruminiclostridium_9 than the CS(FMT)+DSS group (Figure 8E and F). Overall, these results indicated that the TFA microbiota alleviated gut microbiota disorders in depression UC mice.
Correlations Between Gut Microbes and Microenvironmental Factors in FMT Groups
As summarized in Figure 9, the relationships between selected genera with relative abundance levels greater than 0.1% and intestinal barrier indicators (MUC2, KLF4 and ZO-1) and the expression levels of three inflammatory cytokines (IL-6, IL-1β, and TNF-α) were analyzed. Escherichia_Shigella, Enterococcus and Klebsiella abundance levels exhibited significant positive correlations with IL-6, IL-1β and TNF-α and obvious negative correlations with MUC2, KLF4 and ZO-1. Additionally, the abundance levels of Alistipes, Turicibacter, Ruminiclostridium_9, Dubosiella, Family_XIII_AD3011_group and Mucispirillum were similarly positively correlated with MUC2, KLF4, and ZO-1 and negatively correlated with IL-6, IL-1β and TNF-α. These results indicated that TFA fecal microbiota transplantation ameliorated intestinal barrier and intestinal inflammation in depression UC mice and was associated with increases in Alistipes, Turicibacter, Ruminiclostridium_9, Dubosiella, Family_XIII_AD3011_group and Mucispirillum abundance and decreases in Escherichia_Shigella, Enterococcus and Klebsiella abundance.
Discussion
Recent research suggests that individuals with UC have a higher prevalence of depression in the years prior to UC diagnosis, and depression has been identified to worsen the clinical course of UC.2 In addition, CS-induced depression may disturb the gut microbiota, and the resulting injury to the intestinal barrier can then facilitate UC in mice.24,31 In this study, we investigated the effect of the gut microbiota on the intestinal barrier and the anti-UC effects of TFA in depression UC mice. Although the anti-UC effects of Flos Abelmoschus manihot extract are accomplished through regulating gut microbiota and Th17/Treg balance,32 the role of TFA in depression UC mice remains unclear. We first demonstrated the anti-UC effects of TFA in depression UC mice. To clarify whether the anti-inflammatory mechanism of TFA in depression UC can be achieved by regulating its susceptibility factor depression, we verified that TFA can improve depression, the intestinal barrier and the gut microbiota in CS mice. Fecal microbiota transplantation precisely indicated that TFA ameliorates stress-induced microbial alterations that drive intestinal barrier injury in DSS colitis.
Consistent with previous research, we found that the expression levels of IL-6, IL-1β and TNF-α were significantly increased in the CS+DSS group compared with the DSS group, but were suppressed by TFA intervention. Additionally, the severity of inflammation was also closely related to the DAI score, colon length, and histopathology.33 This study suggested that TFA treatment effectively ameliorated intestinal inflammation in CS-aggravated mice with UC, and through FMT experiments, we confirmed that TFA can achieve the effect of treating UC in the presence of depression by regulating the gut microbiota.
In our results, depression behavioral detection was performed with three detection models (OFT, FST and TST), which are in line with the internationally recognized animal detection model of affective disorder.34 Studies have shown that quercetin, a major component of TFA, has antidepressant effects by improving oxidative stress, and hyperoside has antidepressant effects by regulating HPA axis activity and antioxidant activity.35,36 Consistently, our research showed that TFA treatment rescued depressive-like behaviors in CS mice.
In addition to the dysregulation of inflammatory cytokines, the impairment of intestinal barrier function also induces UC.37 The intestinal mucus barrier is the first physical barrier to the invasion of pathogenic organisms in the intestinal lumen. The MUC2 protein secreted by goblet cells is the most important substance constituting the intestinal mucus layer.38 The destruction of the mucus layer and pathological changes in goblet cells are closely related to the progression of enteritis.39 KLF4 plays an important role in the differentiation and maturation of goblet cells.40 ZO-1 is associated with intestinal barrier dysfunction and increased permeability.41 It has been shown that intestinal flora can regulate intestinal barrier function by affecting the tight junctions of intestinal epithelial cells and the secretion of lipopolysaccharide (LPS) endotoxin.42,43 Consistently, we found that intestinal barrier function was impaired in a CS-induced mouse model of depression. Through the FMT experiment, we verified that CS could damage the intestinal barrier in a manner mediated by the gut microbiota.
Intestinal barrier function can be triggered by the gut microbiota,44 and the microbiomes of UC patients are altered, with decreased beneficial bacteria and increased pathogenic bacteria.45 In this study, depression also caused these changes in the gut microbiome. A distinct microbial landscape indicated an observably changed composition of the microbiota in each group. Our results showed that TFA and TFA-microbiota treatment decreased the abundance of Firmicutes and increased the abundance of Bacteroidetes. Notably, as a dominant bacterial group in the gastrointestinal tract of mammals, Bacteroides plays a fundamental role in the processing of complex molecules in the host intestinal tract, which is reduced in CS and CS(FMT)+DSS mice and is enriched in TFA+CS and TFA+CS(FMT)+DSS mice.46 Interestingly, although Lactobacillus is probiotic, our research has shown an increase in its abundance under CS, consistent with a previous study.47 Moreover, the abundance of Escherichia-Shigella is positively correlated with the severity of UC, and its increase is one of the basic characteristics of mucosal inflammation in UC patients.48,49 In this study, Escherichia-Shigella abundance was decreased in TFA+CS(FMT)+DSS mice and was negatively correlated with MUC2, KLF4 and ZO-1 and positively correlated with IL-6, IL-1β and TNF-α in correlation analysis in FMT groups. In particular, as a relatively newly described genus of bacteria, Alistipes has been reported to protect against UC in patients and mice.50–52 Alistipes is an anaerobe and has a good protective effect on mucus barrier function.53,54 To our surprise, Alistipes was not only enriched in TFA+CS and TFA+CS(FMT)+DSS mice but also showed positive correlations with intestinal barrier indexes and negative correlations with proinflammatory factors in the corresponding correlation analyses. Alistipes may be an important genus that is negatively correlated with UC aggravated by depression, and TFA treatment might relieve UC by increasing Alistipes abundance in depression UC mice.
Our current study demonstrates that TFA treatment can improve the intestinal inflammation, gut microbial disturbance, and intestinal barrier damage that are aggravated by depression. Through FMT, we observed that the gut microbiota affected the anti-UC effects of TFA in depression UC mice. TFA may achieve a therapeutic effect by regulating these bacterial genera related to UC. We will further investigate the roles of these genera, particularly Alistipes, in TFA therapy.
Conclusion
In conclusion, TFA treatment ameliorated intestinal inflammation exacerbated by depression, and this effect could be achieved by regulating the intestinal barrier in a gut microbiota-dependent manner. Therefore, TFA might be a potential drug candidate for the treatment of UC in patients with depression.
Abbreviations
CS, chronic stress; CK, control check; DSS, dextran sodium sulfate; FMT, fecal microbiota transplantation; TFA, total flavone of Abelmoschus manihot; IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; OFT, open-field test; TST, tail suspension test; FST, forced swimming test; DAI, disease activity index; PCoA, principal coordinate analysis.
Data Sharing Statement
The data used to support the findings of this study are available from the Yu-gen Chen upon request.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 81873309), the Top academic talents of Jiangsu Provincial Hospital of Traditional Chinese Medicine (No. y2018rc06) and the Key Talents Training Program of Chinese Medicine in Jiangsu Province (No. SLJ0203). This study was also supported by Graduate student scientific research innovation projects in Jiangsu Province (No. SJCX20_0509).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Sandes S, Figueiredo N, Pedroso S, et al. Weissella paramesenteroides wpk4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res Int. 2020;137:
2. Guo C, Wu K, Liang X, Liang Y, Li R. Infliximab clinically treating ulcerative colitis: a systematic review and meta-analysis. Pharmacol Res. 2019;148:
3. Blackwell J, Saxena S, Petersen I, et al. Depression in individuals who subsequently develop inflammatory bowel disease: a population-based nested case-control study. Gut. 2020. doi:10.1136/gutjnl-2020-322308
4. Frolkis AD, Vallerand IA, Shaheen AA, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68(9):1606–1612. doi:10.1136/gutjnl-2018-317182
5. Kim MC, Jung YS, Song YS, et al. Factors associated with anxiety and depression in korean patients with inactive inflammatory bowel disease. Gut Liver. 2016;10(3):399–405. doi:10.5009/gnl15188
6. Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC. Bi-directionality of brain-gut interactions in patients with inflammatory bowel disease. Gastroenterology. 2018;154(6):1635–1646.e3. doi:10.1053/j.gastro.2018.01.027
7. Kochar B, Barnes EL, Long MD, et al. Depression is associated with more aggressive inflammatory bowel disease. Am J Gastroenterol. 2018;113(1):80–85. doi:10.1038/ajg.2017.423
8. Bennebroek EF, Thijssens NA, Stokkers PC, et al. Do inflammatory bowel disease patients with anxiety and depressive symptoms receive the care they need? J Crohns Colitis. 2012;6(1):68–76. doi:10.1016/j.crohns.2011.07.006
9. Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis. 2009;15(7):1105–1118. doi:10.1002/ibd.20873
10. Sandes S, Figueiredo N, Pedroso S, et al. Weissella paramesenteroides wpk4 plays an immunobiotic role in gut-brain axis, reducing gut permeability, anxiety-like and depressive-like behaviors in murine models of colitis and chronic stress. Food Res Int. 2020;137:
11. Singh V, Yeoh BS, Walker RE, et al. Microbiota fermentation-nlrp3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut. 2019;68(10):1801–1812. doi:10.1136/gutjnl-2018-316250
12. Patterson E, Ryan PM, Cryan JF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016;92(1087):286–300. doi:10.1136/postgradmedj-2015-133285
13. Hiippala K, Jouhten H, Ronkainen A, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10:8. doi:10.3390/nu10080988
14. Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–1526. doi:10.1136/gutjnl-2019-318427
15. Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:
16. Kelly JR, Borre Y, O’ BC, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:
17. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi:10.1016/j.bbi.2015.03.016
18. He Z, Zhou Q, Wen K, et al. Huangkui lianchang decoction ameliorates dss-induced ulcerative colitis in mice by inhibiting the nf-kappab signaling pathway. Evid Based Complement Alternat Med. 2019;2019:1040847. doi:10.1155/2019/1040847
19. Zhou L, An XF, Teng SC, et al. Pretreatment with the total flavone glycosides of flos abelmoschus manihot and hyperoside prevents glomerular podocyte apoptosis in streptozotocin-induced diabetic nephropathy. J Med Food. 2012;15(5):461–468. doi:10.1089/jmf.2011.1921
20. Zhang D, Zhu P, Liu Y, et al. Total flavone of abelmoschus manihot ameliorates crohn’s disease by regulating the nfkappab and mapk signaling pathways. Int J Mol Med. 2019;44(1):324–334. doi:10.3892/ijmm.2019.4180
21. Liu M, Jiang QH, Hao JL, Zhou LL. Protective effect of total flavones of abelmoschus manihot l. Medic against poststroke depression injury in mice and its action mechanism. Anat Rec (Hoboken). 2009;292(3):412–422. doi:10.1002/ar.20864
22. Yang BL, Zhu P, Li YR, et al. Total flavone of abelmoschus manihot suppresses epithelial-mesenchymal transition via interfering transforming growth factor-beta1 signaling in crohn’s disease intestinal fibrosis. World J Gastroenterol. 2018;24(30):3414–3425. doi:10.3748/wjg.v24.i30.3414
23. Ito R, Kita M, Shin-Ya M, et al. Involvement of il-17a in the pathogenesis of dss-induced colitis in mice. Biochem Biophys Res Commun. 2008;377(1):12–16. doi:10.1016/j.bbrc.2008.09.019
24. Gao X, Cao Q, Cheng Y, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci U S A. 2018;115(13):E2960–E2969. doi:10.1073/pnas.1720696115
25. Song W, Guo Y, Jiang S, et al. Antidepressant effects of the ginsenoside metabolite compound k, assessed by behavioral despair test and chronic unpredictable mild stress model. Neurochem Res. 2018;43(7):1371–1382. doi:10.1007/s11064-018-2552-5
26. Jimeno R, Brailey PM, Barral P. Quantitative polymerase chain reaction-based analyses of murine intestinal microbiota after oral antibiotic treatment. J Vis Exp. 2018;141. doi:10.3791/58481.
27. Yoshikawa K, Kurihara C, Furuhashi H, et al. Psychological stress exacerbates nsaid-induced small bowel injury by inducing changes in intestinal microbiota and permeability via glucocorticoid receptor signaling. J Gastroenterol. 2017;52(1):61–71. doi:10.1007/s00535-016-1205-1
28. Sann H, Erichsen J, Hessmann M, Pahl A, Hoffmeyer A. Efficacy of drugs used in the treatment of ibd and combinations thereof in acute dss-induced colitis in mice. Life Sci. 2013;92(12):708–718. doi:10.1016/j.lfs.2013.01.028
29. Wang L, Xie H, Xu L, et al. Rsj16 protects against dss-induced colitis by inhibiting the ppar-alpha signaling pathway. Theranostics. 2017;7(14):3446–3460. doi:10.7150/thno.20359
30. Hu J, Chen L, Zheng W, et al. Lactobacillus frumenti facilitates intestinal epithelial barrier function maintenance in early-weaned piglets. Front Microbiol. 2018;9:
31. Abautret-Daly A, Dempsey E, Parra-Blanco A, Medina C, Harkin A. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr. 2018;30(5):275–296. doi:10.1017/neu.2017.3
32. Zhang W, Cheng C, Han Q, et al. Flos abelmoschus manihot extract attenuates dss-induced colitis by regulating gut microbiota and th17/treg balance. Biomed Pharmacother. 2019;117:
33. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–342. doi:10.1038/nri3661
34. Yan K, Chen YB, Wu JR, Li KD, Cui YL. Current rapid-onset antidepressants and related animal models. Curr Pharm Des. 2018;24(22):2564–2572. doi:10.2174/1381612824666180727115222
35. Bhutada P, Mundhada Y, Bansod K, et al. Reversal by quercetin of corticotrophin releasing factor induced anxiety- and depression-like effect in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):955–960. doi:10.1016/j.pnpbp.2010.04.025
36. Wang YS, Shen CY, Jiang JG. Antidepressant active ingredients from herbs and nutraceuticals used in tcm: pharmacological mechanisms and prospects for drug discovery. Pharmacol Res. 2019;150:
37. Ottman N, Reunanen J, Meijerink M, et al. Pili-like proteins of akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. 2017;12(3):e0173004. doi:10.1371/journal.pone.0173004
38. Johansson ME. Mucus layers in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20(11):2124–2131. doi:10.1097/MIB.0000000000000117
39. Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. curr gastroenterol rep. 2010;12(5):319–330. doi:10.1007/s11894-010-0131-2
40. Chen W, Zhuo M, Lu X, et al. Src-3 protects intestine from dss-induced colitis by inhibiting inflammation and promoting goblet cell differentiation through enhancement of klf4 expression. Int J Biol Sci. 2018;14(14):2051–2064. doi:10.7150/ijbs.28576
41. Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70(4):631–659. doi:10.1007/s00018-012-1070-x
42. Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and fabp2 correlated with plasma lps and altered gut microbiome in anxiety or depression. Gut. 2018;67(8):1555–1557. doi:10.1136/gutjnl-2017-314759
43. Alam A, Neish A. Role of gut microbiota in intestinal wound healing and barrier function. Tissue Barriers. 2018;6(3):1539595. doi:10.1080/21688370.2018.1539595
44. Li C, Ai G, Wang Y, et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: impact on intestinal epithelial barrier, gut microbiota profile and tlr4-myd88-nf-kappab pathway. Pharmacol Res. 2020;152:
45. Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:
46. Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):593–621. doi:10.1128/CMR.00008-07
47. Li H, Wang P, Huang L, Li P, Zhang D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol Motil. 2019;31(10):e13677. doi:10.1111/nmo.13677
48. Xu J, Chen N, Wu Z, et al. 5-aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front Microbiol. 2018;9:
49. Zhu Y, He C, Li X, et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 2019;54(4):347–358. doi:10.1007/s00535-018-1529-0
50. Cheung SG, Goldenthal AR, Uhlemann AC, Mann JJ, Miller JM, Sublette ME. Systematic review of gut microbiota and major depression. Front Psychiatry. 2019;10:
51. Wu M, Li P, An Y, et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. 2019;150:
52. Cruz B, Conceicao L, Mendes T, Ferreira C, Goncalves RV, Peluzio M. Use of the synbiotic vsl#3 and yacon-based concentrate attenuates intestinal damage and reduces the abundance of candidatus saccharimonas in a colitis-associated carcinogenesis model. Food Res Int. 2020;137:
53. Shkoporov AN, Chaplin AV, Khokhlova EV, et al. Alistipes inops sp. Nov. And coprobacter secundus sp. Nov., Isolated from human faeces. Int J Syst Evol Microbiol. 2015;65(12):4580–4588. doi:10.1099/ijsem.0.000617
54. Jakobsson HE, Rodriguez-Pineiro AM, Schutte A, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16(2):164–177. doi:10.15252/embr.201439263
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.