Back to Journals » Orthopedic Research and Reviews » Volume 11
Total elbow replacement – patient selection and perspectives
Authors Pooley J
Received 22 April 2018
Accepted for publication 18 October 2018
Published 25 January 2019 Volume 2019:11 Pages 23—40
DOI https://doi.org/10.2147/ORR.S134719
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Clark Hung
Video abstract presented by Joseph Pooley
Views: 2840
Joseph Pooley
Department of Orthopaedics, The Queen Elizabeth Hospital, Gateshead, UK
Abstract: Total joint replacements for elbow arthritis were developed in the late 1960s at the same time as total joint replacements for knee joint arthritis. Since then, there has been a continuing annual increase in the number of patients treated with total knee joint replacement for arthritis, in line with replacement arthroplasty of the other major limb joints, but in contrast to total elbow joint replacement which is falling, since reaching a peak in the 1990s. Which raises the question, why? Continuing controversy about implant design, the relatively high reported complication rates associated with total elbow replacement (TER) and the difficulties encountered in revision surgery are identified as reasons together with changes in the patient population currently treated with TER. The purpose of this review is to explore the reasons for this in the context of the patient population requiring implant surgery for elbow arthritis and our current perspective of elbow pathology requiring treatment. This is not a systematic review of the whole of the literature concerning total elbow joint replacement arthroplasty but is drawn largely from the supporting literature that reflects my own clinical experience and illustrated with teaching materials I have commissioned together with radiographs and intraoperative photographs of patients I have treated.
Keywords: elbow, arthritis, fractures, arthroscopy, arthroplasty
History of total elbow replacement (TER) for arthritis
The elbow joint is widely regarded as homologous with the knee joint, and the original uniaxial hinge design of the joint replacements developed for TER in the late 1960s was exactly the same as those developed for total knee replacement (TKR).
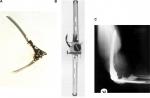
The outcome of these designs used for the treatment of elbow and knee joint arthritis in the late 1960s was also the same, in that the overall satisfactory initial results1 were then followed by disappointing early failures due to implant wear and loosening (Figure 1).
The need to develop less constrained designs was then recognized to more closely replicate the pattern of movement in the elbow and knee, which is not that of a simple uniaxial hinge.2
During the 1970s, designs of TKR evolved from uniaxial hinges briefly into unlinked components that relied entirely upon the intrinsic and extrinsic ligaments for stability, and then into the more intrinsically stable condylar shaped designs, which by the end of the 1970s had become entirely similar to those used today. The development of TER is, however, distinctly different.
In the 1970s, the development of implants for TER diverged along two separate paths. Two different patterns of implants were developed, both of which were less constrained than the original uniaxial hinge designs.
Less constrained hinge (linked) designs, so-called sloppy hinges, which incorporated an axle mechanism allowing a greater degree of freedom of movement than the fully constrained uniaxial hinges were introduced. “Unlinked” designs were also developed in which the components were not joined by an axle mechanism (Figure 2).
  | Figure 2 (A) Linked designs of TER. Left: Stanmore, right: Triaxial. (B) Unlinked design of TER. Left: Souter-Strathclyde, right: Kudo (type IV). Abbreviation: TER, total elbow replacement. |
TER first began to be used in the University Department of Orthopaedic Surgery, Newcastle Upon Tyne in which I was then working, in the early 1980’s, exclusively for patients with advanced degenerative changes due to rheumatoid disease. Initially, we used the Souter-Strathclyde TER, an unlinked system in which the components were fixed with cement,3 but later we began to use the Kudo TER that offered an uncemented option and appeared to be more bone sparing.4
Our preference for unlinked components as our primary implant option was based on our belief that, as had been believed to be the case in the knee joint, unlinked components were more likely to replicate normal elbow joint movement than linked components, and therefore less likely to wear and loosen.
We also felt that, as the stems of the linked components were in the main much longer than those of unlinked components, the unlinked designs were likely to provide better future revision options.
Those advocating linked designs however pointed to the fact that these implants were unlikely to dislocate or disassociate. This had become a recognized complication of unlinked designs, particularly in the inherently unstable severely eroded rheumatoid elbow joints, which was at that time the main indication for TER.
I was led to believe in the 1970s and 1980s that by the time a patient had developed widespread severe erosive degenerative changes due to a rheumatoid disease (“arthritis mutilans”) requiring TER, they were nearing the end of their lives and consequently revision surgery would probably not be required.
Our subsequent experience of TER however demonstrated that this was not the case, and therefore, future revision options are an important consideration when choosing an implant for primary TER. We studied the survivorship of a consecutive group of 27 patients (33 elbows) with rheumatoid arthritis (RA) who had undergone Souter-Strathclyde TER between 1985 and 1989.5 The average age of disease onset was 45 years, and the average age at the time of surgery was 65 years. After a mean follow-up of 17 years, there were nine surviving patients (33%), average age 73 years. All the patients had undergone revision TER, four had undergone one revision, four had undergone two revisions, and one had undergone three revisions. However, none of the initial group of patients had required either arthrodesis or excision arthroplasty, which at that time were considered appropriate treatment for a failed TER.
The early success of TER in the rheumatoid population throughout the 1970s and 1980s and the profound effect a successful implant could make on the quality of a patient’s life by restoring their ability to independently feed themselves and attend to their toileting needs, particularly as elbow involvement was often bilateral, had several consequences. A considerable number of different linked and unlinked implant designs for TER were developed, which however varied considerably in shape and size, unlike the then established basic design for TKR (Figure 3).
The increasing availability of both shoulder and elbow replacement implants coincided with an increased interest in shoulder and elbow surgery in general. This in turn led to the development of shoulder and elbow surgery as a subspecialty of orthopedic surgery, and by the end of the 1980s, national societies such as the British Elbow and Shoulder Society (1987) were becoming established.
Those who attended “Shoulder and Elbow” meetings in those days will recall that “linked vs unlinked TER” was a consistent topic for debate. Furthermore, no consensus was ever reached, despite that the implants being discussed had by then been in regular clinical use for 15–20 years.
It is however perhaps easier to understand in retrospect why this debate persisted, as a review of the literature will confirm that satisfactory results of both the linked and the unlinked designs were obtained during these years in the mainly rheumatoid population into which they were inserted.4,6–12
While the “linked vs unlinked” debate continued during the late 1980s till early 1990s, the nature of the elbow pathology in the patient population treated with TER began to change, for two unrelated reasons. Again, this is something which is perhaps easier to understand in retrospect.
The first reason is that, probably in response to the overall good clinical results observed in the rheumatoid population, TER began to be carried out more often in the more active patients with primary and secondary osteoarthritis (OA).13
The second reason is that the newer disease-modifying drugs for rheumatoid disease14,15 effectively preserved the bony architecture of joints. The radiological appearance of the elbow joints of patients with treated rheumatoid disease became similar to those patients with hypotrophic OA. Consequently, we rarely then encountered the severely eroded elbow joints in which TER had proved to be so successful in the medium to long term in previous years. By the mid-1990s/early 2000s, it was becoming increasingly recognized that the outcome of TER in patients with OA was much less satisfactory than the outcome of the TER in patients with RA.16–19
We had however completed the development of a TER, known as the instrumented bone preserving (IBP) elbow (Biomet, Bridgend, UK), which we began to use clinically in 1997. Although the IBP is an unlinked system, it subsequently provided the degree of implant stability, which we had postulated in the design stages.20,21 Nevertheless, by the early 2000s, it had become evident to us that the results we were then observing following TER were not as good as the results we had observed in those patients with severe erosive rheumatoid disease we had treated in the past. We therefore became increasingly reluctant to recommend TER for the treatment of elbow arthritis.
Our increasing reluctance appears to have been shared by others, and this is reflected in the findings of a recent review of the Norwegian Arthroplasty Register.22 The author’s found that 838 TERs were carried out in Norway between 1994 and 2016, but noted that the annual number of TERs performed decreased annually during the study period and commented that the opposite trend had been documented for hip and knee arthroplasties. Furthermore, their results demonstrate a steep fall in the annual number of TERs carried out for inflammatory arthropathy in Norway during the study period, particularly from its peak in the mid-1990s to the early 2000s and the decline has continued since.
Complications of TER
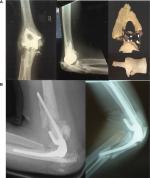
The reported significant complication rate following TER ranges from 20% to 45% and is therefore very much higher than the complication rate associated with any other major limb joint replacement.23,24 Ferlic and Clayton in 1995 commented, “every series of total elbow arthroplasties has a number of failures. Some series contain a high percentage of revisions.”23 Gschwend et al in the following year published a review of the world literature 1986–1992, in which they analyzed 22 publications reporting 828 cases of elbow arthroplasty.17 They found an overall complication rate of 43%, which included aseptic loosening, infections, ulnar nerve complications, instability, disassembly, dislocation, subluxation, intraoperative fractures, mechanical failure of prosthetic components, and ectopic bone formation.
Subsequently, Voloshin et al in 2011 noted that despite advances in prosthetic design and surgical technique for TER over the previous decade, there had been no further systematic review of the literature reporting the complications of TER since the mid-1990s.24 They therefore used Medline PubMed interface to identify all English language articles pertaining to elbow arthritis, elbow arthroplasty, and elbow replacement published between January 1993 and March 2009. They identified 64 studies describing a total of 2,938 TER procedures and concluded that the overall complication rate was likely lower when compared with the reports in the early literature. However, they found identical findings to those of Gschwend et al, in that the most frequent complications were implant loosening, instability, and infection (Figures 4 and 5).
Voloshin et al noted that the rates of clinically significant loosening were similar between linked and unlinked designs. They confirmed, as might have been expected, that the instability rate associated with unlinked devices was significantly greater than linked implants, but they also noted that linked implants were subject to bushing wear and disassembly (Figure 6).
More recently in 2015, Plaschke et al reported a retrospective case-controlled study of 167 TER procedures carried out between 1980 and 2008 based on data provided by the Danish National Patient Register.25 They also did not find any difference in the results between linked and unlinked TERs but commented that revision TER is “complicated surgery, which yields acceptable but poorer results than after primary TER.”
In early 2018, Pham et al reported their results using the Coonrad-Morrey TER in 46 rheumatoid patients (54 elbows) between 1997 and 2012 with an average follow-up of 7 years (range 2–16 years).26 Bushing wear was found in 16 elbows (29%), there were 14 complications (26%). Revision surgery was required in 7 (13%). They concluded that these results were “satisfactory” but pointed out that in their series, “the rate of complications remains high even if the rate of implant revision stayed low.”
We think it is likely therefore that the relatively high rate of complications that continue to be associated with TER, together with the technical difficulties and poor results of revision surgery, has resulted in an understandable degree of resistance on the part of many orthopedic surgeons to recommend this as a surgical option in the treatment of elbow arthritis. This in turn may therefore be a factor that has contributed to the decline in the annual number of TERs now carried out.
Surgical approaches for TER
In his comprehensive textbook, The Elbow and Its Disorders, Morrey begins his description of surgical exposure of the elbow by stating “Few joints require familiarity with as many surgical exposures as does the elbow. The intricate anatomy and range of pathological processes account for the numerous techniques that have been described to provide appropriate surgical access to the elbow region.”27
We consider therefore that the perceived complexity of elbow anatomy compared with the other major limb joints may also be a factor in continuing reluctance of otherwise experienced orthopedic surgeons to undertake TER.
Whereas, we would entirely agree that familiarity with the surgical anatomy of the elbow is a prerequisite to undertaking TER, we consider that this is also the case when undertaking prosthetic replacement of the other major limb joints. Furthermore, as familiarity with one surgical approach is sufficient to competently and confidently carry out replacement arthroplasty of the other major limb joints, we see no reason why this should not also be the case in the elbow joint.
We developed a posterior surgical approach based on studies of the blood supply to the triceps muscle.28,29 This provides us with a symmetrical exposure of the elbow joint, which subsequent cadaver studies carried out by others have also demonstrated, providing a wider exposure of the articular surfaces when compared with other surgical approaches, including olecranon osteotomy.30
The description of the anatomy of the triceps insertion in the classical anatomical textbooks which refer to a “common tendon inserted into the posterior part of the superior surface of the olecranon” does not appear to correlate entirely with the anatomy we see in surgical procedures, during which the muscle fibers can be seen to insert directly into bone.31 We wonder therefore if a lack of clarity about the nature of the triceps insertion has contributed to the lack of standardization of surgical exposures for TER when compared with the “standard” surgical approaches used for other joint replacements.
The surgical approach we use recognizes that the distal triceps, designated as the “triceps tendon” in anatomical texts, is a flattened aponeurosis that is continuous with the fascia covering anconeus and can be reflected to expose the mainly muscular insertion (Figure 7A and B). Anconeus can then be detached from its ulnar insertion together with the lateral triceps. Continuing the dissection proximally by separating the lateral triceps from a longitudinally disposed fibrous intramuscular septum enables lateral triceps and anconeus to be mobilized in continuity, and then retracted to expose the radiocapitellar joint and lateral supracondylar region of the humerus (Figure 7C and D). The intramuscular septum can be divided proximal to its insertion into the tip of the olecranon, which then enables medial triceps to be reflected to expose the olecranon fossa and medial supracondylar ridge of the humerus (Figure 7E). Medial capsulotomy (while protecting the ulnar nerve) (Figure 7F) and subperiosteal elevation of the origin of the radial collateral ligament, if needed in stiff elbows (Figure 7G), then enable dislocation by distraction and flexion of the joint and provide a wide exposure of the articular surfaces (Figure 7H). Closure begins by repairing the intramuscular septum of triceps, which can be used to adjust muscle tension if required (Figure 7I). Completion of the closure of the muscle envelope begins by suturing the detached edge of anconeus to its insertion into the proximal ulna with sutures passed first through the muscle tissue, then the deep fascia, and then back again through the muscle. The deep closure is completed by suturing the reflected triceps fascia back to its cut edge (Figure 7J).
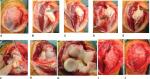  | Figure 7 A posterior surgical approach suitable for elbow arthroplasty. Notes: (A) The skin and subcutaneous tissues have been reflected to reveal the distal triceps designated “triceps tendon.” The ulnar nerve has been mobilized and decompressed. (B) A longitudinal incision through the deep fascia beginning proximally in the midline has been directed around the radial aspect of the tip of the olecranon ending at the subcutaneous border of the ulna. The fascia covering triceps and anconeus has been reflected exposing the muscular insertion of triceps. (C) Anconeus is detached from the subcutaneous border of the ulna together with lateral triceps from the tip of the olecranon. (D) Lateral triceps is separated from a longitudinally disposed intramuscular septum and retracted with anconeus exposing the radiocapitellar joint and lateral supracondylar ridge of the humerus. (E) The intermuscular septum is divided proximal to its insertion into the tip of the olecranon enabling triceps to be reflected to expose the olecranon fossa and medial supracondylar ridge. (F) Medial capsulotomy is performed and (G) subperiosteal elevation of the origin of the radial collateral ligament in stiff elbows. (H) Dislocation by distraction and flexion provides a wide exposure of the articular surfaces. (I) Soft tissue closure begins with repair of the intermuscular septum. (J) The remaining soft tissue envelope is then closed. Reproduced with permission from Pooley J. Unicompartmental elbow replacement: development of a lateral replacement (LRE) arthroplasty. “https://journals.lww.com/shoulderelbowsurgery/Abstract/2007/12000/Unicompartmental_Elbow_Replacement__Development_of.7.aspx” Techniques in Shoulder & Elbow Surgery. 2007;8:204–212.60 |
Whatever surgical approach is used, we consider that it is important to isolate, decompress, and protect the ulnar nerve during TER. We reviewed a consecutive series of 27 of our patients with RA who underwent Kudo TER.32 We measured a mean length increase of 8.6 mm across the elbow joint on the postoperative radiographs compared with the preoperative radiographs following insertion of the prosthetic components into these previously eroded joints.
We noted that ulnar nerve paresis complicating TER had earlier been reported to occur in as many as 31%–65% of cases in some series,33,34 but we found no case of ulnar nerve dysfunction in our series.
We think therefore that our practice of mobilizing and decompressing the ulnar nerve during surgery may have prevented a chronic traction injury to the nerve as a consequence of the inevitable lengthening across a previously eroded degenerate joint by inserting the components of a TER. This would then explain the high incidence of postoperative ulnar nerve dysfunction reported earlier by others.
van Rheenen et al recently reviewed 2,759 surgical operations on the elbow comprising both arthroscopic and open procedures and found neurological deficit in only ten patients.35 They concluded that an awareness of anatomical landmarks, identification and marking of the ulnar nerve, and exposing nerves during open surgery were the main contributors to avoiding neurological complications in this series.
We would therefore agree with the conclusions of these authors that it is much safer to routinely expose the ulnar nerve when performing TER than to attempt to avoid ulnar nerve injury by leaving it “undisturbed.”
Use of TER for the treatment of distal humeral fractures
Whereas there has been a sharp decline in the number of TERs performed for the treatment of elbow arthritis since the late 1990s,22 the opposite has been the case for TER carried out as the primary treatment for comminuted, unreconstructable distal humeral fractures, particularly in elderly patients.
We performed a retrospective study of elderly patients treated in our unit in whom TER had been used as the primary treatment for distal humeral fractures, and we carried out a pragmatic review of the literature published between the mid-1990s and the present day, by entering the search terms “elbow arthroplasty” and “treatment of distal humeral fractures” into the Scopus database.36
Our literature review revealed that there had been a gradual change in clinical opinion over the previous 10–15 years. Ray et al in 2000 commented that TER had been proposed as a “last-ditch” attempt and “salvage” procedure for the treatment of technically difficult distal humeral fractures in the elderly.37 Twelve years later, however, Argintar et al considered that by then, TER had become the “gold standard” in the management of “unreconstructable” distal humeral fractures in elderly patients.38
We were able to review the case notes of a consecutive series of eleven “elderly” (ie, aged 60 years or older) patients who had sustained a comminuted fracture of the distal humerus treated primarily by TER between 1997 and 2011 and interview the five surviving patients. When assessed with the Mayo elbow performance index during their most recent follow-up at an average of 3.5 years postoperatively (range: 2–6 years), seven patients were classified as excellent and four were classified as good. We noted that none of the eleven patients had experienced complications, which required further procedures. All the five surviving patients reported that they were satisfied with the function of their TER.
We examined our photographic records of the pathological anatomy seen at the time of surgery for these distal humeral fractures, all of which corresponded to AO type C fractures, together with the bone fragments removed during each of these procedures. We found that articular cartilage covered most of the surface area of all the fragments. Furthermore, most of the fragments had either no attachment to soft tissues or minimal attachment to soft tissues to the extent that they were unlikely to have a viable blood supply. Consequently, even if it had been technically possible to achieve firm internal fixation of these avascular bone fragments, fracture healing was unlikely to occur.
Our observations of the morphology of these AO type C fractures in the elderly at the time of surgery revealed that the degree of comminution was always far more extensive than that demonstrated by the preoperative radiographs and, in any event therefore, were not amenable to firm open reduction and internal fixation (ORIF).
Consequently, we consider that AO type C fractures of the distal humerus are entirely comparable with the Neer four-part fracture of the proximal humerus, for which it is generally accepted that replacement arthroplasty is required.39,40
We concluded from our literature review that the early functional results of TER in the treatment of comminuted distal humeral fractures in the elderly are superior to that of ORIF. Our review of postoperative radiographs demonstrated that radiographic evidence of implant wear and loosening was no different from that following TER in arthritis. We concluded therefore that the use of TER for primary treatment of distal humeral fractures in the elderly is equally capable of providing satisfactory function as TER used for degenerative changes in this relatively low-demand group of patients, at least in the medium term.
We first began to treat elderly patients with comminuted distal humeral fractures in our unit with TER by inserting components of an IBP elbow (Biomet UK Ltd, Swindon, UK). Although the IBP is an unlinked TER, we found that it was capable of providing joint stability and early satisfactory results. We note that similar results with the IBP system used for the primary treatment of fractures of the distal humerus had been reported by others.20 However, the potential for dislocation of unlinked components is ever present, and we considered that the need for a further major surgical procedure, such as revision TER, was a major concern in this group of frail elderly patients, many of whom had significant comorbidities. We would have similar reservations about joint stability when inserting a humeral hemiarthroplasty for treating comminuted, unreconstructable, distal humeral fractures in the elderly. More recently, therefore, we began to use a linked TER, the “Discovery elbow” (Biomet UK Ltd), in this patient group.
In conclusion, therefore, we would consider that elbow arthroplasty is the logical treatment option for AO type C fractures of the distal humerus rather than ORIF, and we would advise using a linked TER in the elderly to reduce the likelihood of further surgical interventions. We also consider that a linked TER, or possibly a hemi arthroplasty, is appropriate for the treatment of any fracture of the distal humerus for which firm fixation allowing early mobilization cannot be achieved.
It is of course a well-established surgical principle that any primary surgical procedure is much more likely to provide a better outcome and is associated with fewer complications than a revision procedure. Perhaps, therefore, it is not surprising that Frankle et al found this to be the case in their study of immediate TER for the treatment of distal humeral fractures, in which they found that patient satisfaction was far less following revision of a failed ORIF to a TER, than following insertion of primary TER for fractures of the distal humerus.41 In order therefore to provide the best treatment, particularly for an elderly patient who has sustained a potentially disabling comminuted distal humeral fracture, we consider that it is important to have a TER available as an option at the time of surgery.
Alternative procedures to TER for arthritis
We reviewed the literature available in the mid-1990s to better understand the place of surgical treatments other than joint replacement for arthritis of the elbow and found this to be somewhat confusing due to the lack of a standard nomenclature. For example, the terms “excision arthroplasty” and “interposition arthroplasty” were often used interchangeably.42
Synovectomy of the elbow was first carried out in the early 1920s as a treatment for “chronic arthritis.” This was later combined with radial head excision for the treatment of rheumatoid disease of the elbow in the early 1940s. Summers et al in 1987 published a review of the English literature over the preceding 40 years and identified 20 retrospective studies on elbow synovectomy, which provided data on 850 operations.43 Our review performed a decade later identified a further eight studies, all of which were retrospective but nevertheless contributed to an additional 273 patients.42 We found however that evaluating the results of elbow synovectomy reported in these studies was difficult due to considerable variations in the surgical procedures described as “synovectomy.” Some surgeons believed that an adequate synovectomy could be carried out through a single lateral incision. Others however considered that both medial and lateral approaches performed simultaneously were necessary to ensure that a complete synovectomy was achieved and also enable ulnar nerve decompression to be carried out if the patient had reported symptoms of ulnar nerve entrapment preoperatively. We also found that there was considerable difference of opinion as to whether radial head excision should be considered an integral part of the operation of elbow synovectomy for rheumatoid disease. There was however general agreement that it was not appropriate to insert a radial head prosthesis in these patients.
It had become evident to us therefore during the course of our literature review that the term “synovectomy” had been applied to a wide range of procedures. These varied from simple removal of diseased synovium accessible through an incision on the lateral aspect of the elbow, but preserving the radial head, to an extensive debridement procedure, which included radial head excision, decompression of the ulnar nerve, and in some patients, additional procedures on the wrist.
We believe therefore that this will explain why some authors claimed that a late synovectomy of a rheumatoid elbow could be as successful as an early synovectomy and, even more remarkably, that synovectomy performed on the more degenerate joints provided better results than synovectomy of joints, which were less severely affected by the rheumatoid disease.
The inherent limitations of a retrospective study of synovectomy were taken into account in the then largest study by Porter et al. These investigators were able to access the records documenting 282 elbow synovectomy procedures carried out in a single center during a 6-year period (1962–1969). In addition, they were able to review a representative group of 123 patients.44 Based on their clinical and radiological audit, they graded the results as either “satisfactory” or “unsatisfactory” and concluded that 54% of the patients had obtained a satisfactory result. They also concluded that excision of the radial head was a necessary element of the procedure when carrying out elbow “synovectomy” for rheumatoid disease, and they found that if the disease was at an advanced stage at the time of surgery then any benefit was likely to be short lived.
Woods et al were able to compare the results of 45 TERs in a group of 38 patients with rheumatoid disease and compare these with the results of radial head excision combined with synovectomy (RHES) carried out on a group of 45 age-matched patients treated in the same center. The two groups were similar in respect of both the duration of the disease and their preoperative clinical status, although pain had been present for longer and was slightly more severe in the 38 patients treated with TER.45
They found that TER was more reliable in relieving pain in the medium term than RHES and felt therefore that the use of TER was justified even though it was associated with a greater risk of complications. They concluded however that because of the lack of evidence demonstrating good long-term results of TER, RHES could still have a place in the treatment of younger patients or in patients whose symptoms appeared to arise mainly from the radio humeral joint.
Nemoto et al reported their short-term results of arthroscopic synovectomy of the rheumatoid elbow in ten patients (eleven elbows), all of whom presented with severe pain and swelling.46 They noted a significant improvement in pain and a “satisfactory functional result” in each patient. They pointed out that whereas some authors believed that radial head resection is an essential component of elbow synovectomy, others considered that elbow instability is an inevitable consequence of removal of the radial head.47 Nemoto et al believed however that in any event, removal of bone from the radial head should be kept to a minimum in order to reduce the risk of elbow instability.
We have encountered some difficulty in interpreting the literature describing the nonimplant surgical options for OA of the elbow, as with the literature describing the equivalent treatments for inflammatory arthritis, as there is also a lack of standardization of both nomenclature and technique. Terms such as “arthrolysis,” “debridement,” and “ulnohumeral arthroplasty” are used interchangeably without definition, and there is considerable variation in both the surgical approach used and the extent of these procedures between series.
We use the term “arthrolysis” to describe an operation aimed at restoring a functional range of movement to a stiff elbow in which pain is not a significant feature, despite evidence of early degenerative changes, by dividing tight capsuloligamentous structures and intra-articular adhesions. We would then add the term “debridement” if excision of osteophytes and/or removal of loose bodies was required in addition to relieve painful impingement or locking symptoms.
Minami reported a study on the radiographs of 1,012 males and 280 females with OA and concluded, on the grounds of the radiographic appearances, that elbow OA begins with the formation of osteophytes in the coronoid, coronoid fossa, olecranon, and olecranon fossa.48 Minami considered that in the early stages of OA of the elbow, the presence of osteophytes in these four locations resulted in a reduction of the arc of movement of the elbow and terminal motion pain.
Minami et al reported their results of “Outerbridge–Kashiwagi’s” (OK) method of arthroplasty for OA of the elbow in 44 elbows followed up for a period of 8–16 years.49 They explained that Outerbridge had “happened to find” an X-ray of an elbow with a congenital hole connecting the olecranon fossa with the coronoid fossa (personal communication, 1986), which then led to the concept of the OK arthroplasty.50 They found an overall increase in the range of flexion/extension of 17° and an improvement in pain in 27 elbows (61%). However, only seven (9%) were pain free and eight patients continued to experience severe pain. These patients were unable to work and required analgesics.
Antuña et al reviewed the results of the OK procedure to which they referred to as “ulnohumeral arthroplasty,” on 45 patients (46 elbows) with primary OA at an average of 84 months (range 24–164 months) following surgery.51 They found that in 34 elbows (74%) a “satisfactory objective result” had been achieved, but 12 had an “unsatisfactory objective result.” Subjectively, 24 elbows were considered by the patient to be much better, 14 were better, eight were the same, and four were worse postoperatively than they had been preoperatively.
Wada et al reviewed their results of “debridement arthroplasty” for primary OA in 33 elbows (32 patients).52 Their technique differed from the OK procedure, in that osteophytes were removed through a posteromedial approach in 24 elbows, and an additional lateral approach was used in nine elbows. The main aim of surgery appears to have been the relief of elbow stiffness as, although all 33 elbows were painful preoperatively, pain was only mild in 21 elbows and moderate in 11 elbows, no patient had severe pain. It was noted at the time of the most recent follow-up that a mean preoperative flexion contracture of 31° had reduced to 24°, an improvement of 7°. A mean preoperative flexion of 101° had improved to 118°, an improvement of 7°. The average preoperative pain score of 13.9 points had improved to 27.0 points at the time of follow-up (P<0.001).
Savoie et al reported their results of arthroscopic treatment for the arthritic elbow.53 They performed an arthroscopic modification of the OK procedure on 24 patients, average age 59 (range 17–78 years). The radial head was excised arthroscopically in 18 of these patients. The arthritic changes were due to trauma (post-traumatic osteoarthritis [PTOA]) in 15 patients, rheumatoid disease in four patients, juvenile rheumatoid disease in one patient, and primary OA in four patients. When reviewed at a mean of 32 months postoperatively (range 24–60 months), it was noted that there had been an improvement in the arc of movement and a significant decrease in pain. It was concluded that the procedure seemed to be a valuable addition to the other procedures available for treating an arthritic elbow and could be used as an intermediate step between conservative treatment and TER.
We consider therefore that, as with the published results of alternative surgeries to TER for RA, alternative surgeries for OA appear capable of providing “satisfactory” results in some patients. However, long-term prospective data are not available and the outcome for an individual patient in terms of pain relief would appear to be unpredictable.
What is the pathology we are now treating when carrying out surgery for elbow arthritis?
I think that most orthopedic surgeons of my generation, who began their clinical practice in the early 1970s, would probably agree that the most important development they have seen in terms of our understanding of intra-articular pathology, and then planning the most appropriate surgical procedure, has been the introduction of the arthroscope. It must be difficult therefore for our younger colleagues to imagine practicing without the benefit of arthroscopy, which has remained the definitive tool for the diagnosis of intra-articular pathology since the 1970s, despite the subsequent introduction of MRI and continuing improvements in other imaging techniques.
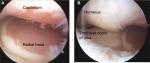
In a consecutive series of 117 arthroscopies on patients with significant elbow pain but little or no radiographic abnormality, often referred with a presumed diagnosis of “resistant lateral epicondylitis,” we found that degenerative changes involving the articular cartilage were present in 68 elbows. In 60 elbows, these changes were confined to the lateral compartment (radiocapitellar joint) and were in sharp contrast to the normal appearances of the articular cartilage of the medial compartment (ulnohumeral joint)54 (Figure 8).
These observations coincided with our increasing use of open arthrolysis and debridement in the early 2000s in preference to TER for patients with painful stiff elbows due to advanced degenerative changes. During these procedures, we became increasingly aware of a similar pattern of articular cartilage wear, and we were surprised to find that this had occurred irrespective of the degree of degenerative changes seen on preoperative radiographs or the cause of the degenerative changes (primary OA, PTOA, or RA). Full-thickness loss of the articular cartilage from the radiocapitellar joint was a consistent finding, whereas the articular surfaces of the ulnohumeral joint appeared to be normal or much better preserved (Figure 9).
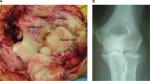  | Figure 9 (A) Intraoperative photograph during elbow arthrolysis on a 54-year-old male patient for pain and stiffness. Full-thickness loss of the articular cartilage in the radiocapitellar joint contrasts with the well-preserved articular surfaces of the ulnohumeral joint. (B) The preoperative radiographs demonstrate little evidence of degenerative change. Notes: Reproduced with permission from Pooley J. Unicompartmental elbow replacement: development of a lateral replacement (LRE) arthroplasty. “https://journals.lww.com/shoulderelbowsurgery/Abstract/2007/12000/Unicompartmental_Elbow_Replacement__Development_of.7.aspx” Techniques in Shoulder & Elbow Surgery. 2007;8:204–212.60 |
Goodfellow and Bullough, almost 40 years earlier, had published their studies of the elbows of elderly subjects examined postmortem.55 They found extensive loss of the articular cartilage from the lateral compartment contrasting with normal appearances of the articular surfaces of the medial compartment, and identical findings on postmortem material have been reported by other groups since.56–58 The clinical significance of these findings remained unknown, as it had not been recorded if the subjects studied postmortem had suffered elbow symptoms during their lifetime. Our arthroscopy findings had however demonstrated that this pattern of degenerative change can develop and become clinically significant, typically patients in their late 40s or early 50s. The postmortem studies suggested that this may then remain the case throughout life.
Therefore, in view of the results of the postmortem studies and our intraoperative observations, we would be unable to agree with the conclusions Minami drew from the pattern of osteophyte formation in the osteoarthritic elbow he found on plain X-ray examinations.48 Minami concluded that OA “begins with the formation of osteophytes in the coronoid, coronoid fossa, olecranon, and olecranon fossa.” This concept then formed the pathological basis of debridement procedures and led to the development of the OK procedure (ulnohumeral arthroplasty).49
This interpretation of the pathology of elbow OA would however appear to reject the long-established understanding of the evolution of OA in any synovial joint, in that the earliest degenerative changes begin in the articular cartilage. Consequently, the earliest radiological evidence for OA is narrowing of the radiological joint space. Osteophyte formation, if it occurs at all, is a secondary development. Furthermore, as is commonly observed in the other major limb joints, the presence or absence and extent of osteophyte formation bear no correlation to either the degree of articular cartilage degeneration or the severity of associated symptoms.
We consider that both the pathological evidence and our clinical observations indicate that elbow OA begins in the lateral compartment (radiocapitellar joint), characteristically become symptomatic in midlife, and may then remain largely confined to the lateral compartment.
The pattern of articular cartilage degeneration observed in the elbow joint postmortem and our intraoperative observations will also therefore explain the findings of Forster et al.59 They reviewed a series of 36 patients with elbow OA who had undergone the OK procedure (ulnohumeral debridement), with a mean follow-up of 36 months. They found improvement in the flexion/extension arc and pain and locking symptoms, but they noted that a significant number had persisting rest pain. They classified the results overall to be only fair or poor in two thirds of the patients. They then postulated that this was due to the fact that the radiocapitellar joint had not been treated by their procedures.
We thought therefore that the development of implants with which to resurface the capitellum and radial head would provide a logical treatment option for our patients with radiologically well-preserved elbows, in whom we had found the lateral compartment to be denuded of articular cartilage on arthroscopy, but who continued to experience intrusive symptoms. We also considered that resurfacing the lateral compartment of the elbow when performing arthrolysis and debridement procedures in patients with radiologically advanced degenerative changes might improve the surgical outcome, particularly in terms of pain relief.
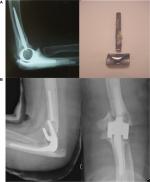
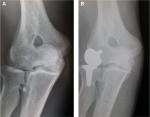
We subsequently developed the components of a lateral resurfacing elbow (LRE) arthroplasty (Formerly Biomet Ltd, , now LRE System Ltd, Oxford, UK), which we began to use in clinical practice in 200560 (Figures 10 and 11). Our encouraging early results with the LRE have been subsequently replicated by others, in a wide range of patients, which included high-demand manual workers.61,62 A recent review of an initial group of our patients (28 patients, 30 elbows, mean follow-up 8.3 years), who underwent LRE arthroplasty, has identified no radiological evidence of component wear or loosening to date which, together with continuing pain relief, has therefore confirmed that our early encouraging results are being maintained in the long-term.63
  | Figure 10 (A) Anteroposterior radiograph demonstrating primary (hypotrophic) osteoarthritis with minimal osteophyte formation but marked narrowing of the radiocapitellar joint space. (B) Intraoperative photograph of this patient demonstrating the pattern of articular cartilage degeneration. The radiocapitellar joint surfaces are denuded of articular cartilage; the articular surfaces of the ulnohumeral joint, however, are well preserved. (C) An early postoperative radiograph following insertion of the components of a lateral resurfacing elbow. Notes: Reproduced with permission from Pooley J. Unicompartmental elbow replacement: development of a lateral replacement (LRE) arthroplasty. “https://journals.lww.com/shoulderelbowsurgery/Abstract/2007/12000/Unicompartmental_Elbow_Replacement__Development_of.7.aspx” Techniques in Shoulder & Elbow Surgery. 2007;8:204–212.60 |
Consequently, the LRE has now replaced TER as our primary implant option in the treatment of elbow arthritis, other than for patients with arthritis mutilans due to untreated rheumatoid disease, which was the main indication for TER when components were first developed in the late 1960s. Since then, however, thanks to the development of effective medications for rheumatoid disease, arthritis mutilans, for which TER has proved to be so effective in providing pain relief and preserving useful elbow function for so many patients, is now becoming a thing of the past.
Summary and conclusion
The aim of this review was to explore the reasons for the annual decrease in the number of TERs carried out for the treatment of arthritis when the annual number of patients treated with replacement arthroplasty of the other major limb joints continue to increase, by considering the patient population requiring implant surgery for elbow pathology and our current perspectives of the elbow pathology requiring treatment.
Patient population
Most of the implants designed for TER, since the early 1970s, have proved to be successful in treating patients with severe degenerative changes due to rheumatoid disease (arthritis mutilans), which was originally the main indication for surgery.
By the mid-1990s, the patient population had changed, in that effective medications had been developed for rheumatoid disease and consequently patients with arthritis mutilans were subsequently then rarely seen. The disease-modifying drugs preserved the normal bony architecture, and consequently, the elbow joints of patients with RA were becoming similar to those with OA.
TER implants have however proved to be less successful in treating patients with OA than patients with erosive arthritis due to rheumatoid disease.
Consequently, there has been an increasing awareness among orthopedic surgeons performing TER on the patient population since the mid-1990s that the results of surgery are less satisfactory than the results they obtained in the past. This has therefore resulted in an increasing reluctance to recommend TER for elbow arthritis despite subsequent improvements in implant design.
Pathology of elbow arthritis
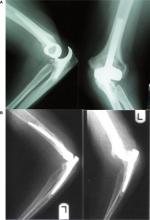
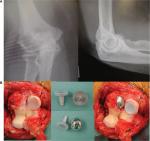
“Untreated” RA results in severe destruction of the joint surfaces of the elbow, which profoundly alters the anatomy of the joint. The definition between the trochlea and the capitellum is lost, the radial head subluxates laterally, the ulna aligns with the midline of the humerus, and the joint takes on a characteristic 1-compartment appearance. This pattern of degeneration is therefore appropriately treated by excising the remnant of the radial head and inserting an ulnohumeral replacement arthroplasty in which the stem of a humeral component aligns with the stem of an ulnar component. This pattern of degeneration is therefore quite distinct from primary OA or OA secondary to trauma or treated rheumatoid disease, in which the two-compartment configuration of the elbow joint is maintained (Figure 12).
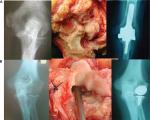  | Figure 12 (A) Left: AP radiograph showing the characteristic appearances of rheumatoid disease involving the elbow before the development of effective disease-modifying drugs. The definition between the trochlea and the capitellum has been lost, and the radial head has subluxated laterally. The elbow joint has effectively become one-compartmental. Middle: intraoperative photograph of this patient demonstrating severe destruction of the joint surfaces. The radial head has been resected prior to the insertion of a TER. Right: postoperative radiograph following insertion of a TER (IBP), which is appropriate reconstruction for this pattern of joint disease. (B) Left: AP radiograph demonstrating the characteristic appearances of rheumatoid disease involving the elbow in a patient treated with disease-modifying drugs. There is loss of joint space (secondary osteoarthritis) but the normal bone architecture and the two-compartmental configuration of the elbow joint are preserved. Middle: intraoperative photograph of this patient. The radial head articular surface and much of the capitellum were denuded of articular cartilage; the ulnohumeral joint surfaces appeared degenerate but better preserved. Right: postoperative AP radiograph following insertion of a lateral resurfacing elbow, which relieved the preoperative symptoms. Notes: (A) Middle and left impages reproduced with permission from Pooley J. Unicompartmental elbow replacement: development of a lateral replacement (LRE) arthroplasty. “https://journals.lww.com/shoulderelbowsurgery/Abstract/2007/12000/Unicompartmental_Elbow_Replacement__Development_of.7.aspx” Techniques in Shoulder & Elbow Surgery. 2007;8:204–212.60 Abbreviations: AP, anteroposterior; IBP, instrumented bone preserving; TER, total elbow replacement. |
Inferring the pattern of degeneration in elbow OA during the late 1970s from the location of osteophytes seen on plain X-rays has been misleading, in that this was taken to indicate that the disease begins in and mainly involves the ulnohumeral articulation. This then led to the development of “anatomic ulnohumeral arthroplasties” such as the OK procedure, the results of which are unpredictable, and the use of the ulnohumeral designs of TER for OA which had proved to be successful in patients with severe erosive RA.
Studies of postmortem material and intraoperative observations made during arthroscopic procedures and open surgery have however demonstrated that in OA, degenerative changes begin in the radiocapitellar joint. Ulnohumeral joint involvement may then develop, usually beginning in the articular cartilage of the radial aspect of the trochlear and trochlear notch, as a result of increased valgus loading due to loss of the articular cartilage from the radiocapitellar joint and consequent narrowing of the “joint space.”
Resurfacing the radiocapitellar joint has therefore proved to be an effective treatment for primary and secondary OA and in our practice has now displaced TER for this condition (Figure 13).
The future for TER
TER is now being increasingly used for treating comminuted fractures of the distal humerus, particularly in the elderly population. TER has proved to be superior to ORIF in this group of patients and is the more logical treatment option particularly for AO type C distal humeral fractures.
The complication rates associated with TER remain much higher than those associated with replacement of the other major limb joints. We consider however that improvements in TER design are likely to reduce complications, particularly implant wear and loosening.
There appears to be a continuing belief that elbow anatomy is more complicated than that of the other major joints, and consequently, surgical procedures on the elbow are more difficult. We can see no justification for this, and we believe that familiarity with one extensile surgical approach makes elbow surgery no more demanding and equally rewarding as surgical procedures on the other major limb joints.
Disclosure
As the surgeon designer of the Lateral Resurfacing Elbow System, I wish to declare an involvement with the current manufacturer of this system, LRE system Ltd. The author reports no other conflicts of interest in this work.
References
Dee R. Total replacement arthroplasty of the elbow for rheumatoid arthritis. J Bone Joint Surg Br. 1972;54(1):88–95. | ||
Morrey BF, Bryan RS. Complications of total elbow arthroplasty. Clin Orthop Relat Res. 1982;170:204–212. | ||
Souter WA. Surgery of the rheumatoid elbow. Ann Rheum Dis. 1990;49 Suppl 2:871–882. | ||
Kudo H, Iwano K. Total elbow arthroplasty with a non-constrained surface-replacement prosthesis in patients who have rheumatoid arthritis. A long-term follow-up study. J Bone Joint Surg Am. 1990;72(3):355–362. | ||
Robinson E, Burke N, Douglas P, Orr J, Pooley J. Mechanism of loosening of the Souter-Strathclyde total elbow replacement evidence from revision surgery. Acta Orthop Belg. 2010;76(1):27–29. | ||
Madsen F, Søjbjerg JO, Sneppen O. Late complications with the Pritchard Mark II elbow prosthesis. J Shoulder Elbow Surg. 1994;3(1):17–23. | ||
Allieu Y, Meyer zu Reckendorf G, Daude O. Long-term results of unconstrained Roper-Tuke total elbow arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 1998;7(6):560–564. | ||
Schneeberger AG, Hertel R, Gerber C. Total elbow replacement with the GSB III prosthesis. J Shoulder Elbow Surg. 2000;9(2):135–139. | ||
Kelly EW, Coghlan J, Bell S. Five- to thirteen-year follow-up of the GSB III total elbow arthroplasty. J Shoulder Elbow Surg. 2004;13(4):434–440. | ||
Willems K, De Smet L. The Kudo total elbow arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2004;13(5):542–547. | ||
Malone AA, Taylor AJ, Fyfe IS. Successful outcome of the Souter-Strathclyde elbow arthroplasty. J Shoulder Elbow Surg. 2004;13(5):548–554. | ||
Thillemann TM, Olsen BS, Johannsen HV, Søjbjerg JO. Long-term results with the Kudo type 3 total elbow arthroplasty. J Shoulder Elbow Surg. 2006;15(4):495–499. | ||
Espag MP, Back DL, Clark DI, Lunn PG. Early results of the Souter-Strathclyde unlinked total elbow arthroplasty in patients with osteoarthritis. J Bone Joint Surg Br. 2003;85(3):351–353. | ||
Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–1593. | ||
Lipsky PE, van der Heijde DM, St Clair EW, et al; Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med Ed. 2000;343(22):1594–1602. | ||
Kraay MJ, Figgie MP, Inglis AE, Wolfe SW, Ranawat CS. Primary semiconstrained total elbow arthroplasty. Survival analysis of 113 consecutive cases. J Bone Joint Surg Br. 1994;76(4):636–640. | ||
Gschwend N, Simmen BR, Matejovsky Z. Late complications in elbow arthroplasty. J Shoulder Elbow Surg. 1996;5(2 Pt 1):86–96. | ||
Wright TW, Hastings H. Total elbow arthroplasty failure due to overuse, C-ring failure, and/or bushing wear. J Shoulder Elbow Surg. 2005;14(1):65–72. | ||
Schneeberger AG, Meyer DC, Yian EH. Coonrad-Morrey total elbow replacement for primary and revision surgery: a 2- to 7.5-year follow-up study. J Shoulder Elbow Surg. 2007;16(3 Suppl):S47–S54. | ||
Kalogrianitis S, Sinopidis C, El Meligy M, Rawal A, Frostick SP. Unlinked elbow arthroplasty as primary treatment for fractures of the distal humerus. J Shoulder Elbow Surg. 2008;17(2):287–292. | ||
Kleinlugtenbelt IV, Bakx PA, Huij J. Instrumented bone preserving elbow prosthesis in rheumatoid arthritis: 2-8 year follow-up. J Shoulder Elbow Surg. 2010;19(6):923–928. | ||
Krukhaug Y, Hallan G, Dybvik E, Lie SA, Furnes ON. A survivorship study of 838 total elbow replacements: a report from the Norwegian Arthroplasty Register 1994-2016. J Shoulder Elbow Surg. 2018;27(2):260–269. | ||
Ferlic DC, Clayton ML. Salvage of failed total elbow arthroplasty. J Shoulder Elbow Surg. 1995;4(4):290–297. | ||
Voloshin I, Schippert DW, Kakar S, Kaye EK, Morrey BF. Complications of total elbow replacement: a systematic review. J Shoulder Elbow Surg. 2011;20(1):158–168. | ||
Plaschke HC, Thillemann TM, Brorson S, Olsen BS. Outcome after total elbow arthroplasty: a retrospective study of 167 procedures performed from 1981 to 2008. J Shoulder Elbow Surg. 2015;24(12):1982–1990. | ||
Pham TT, Delclaux S, Huguet S, Wargny M, Bonnevialle N, Mansat P. Coonrad-Morrey total elbow arthroplasty for patients with rheumatoid arthritis: 54 prostheses reviewed at 7 years’ average follow-up (maximum, 16 years). J Shoulder Elbow Surg. 2018;27(3):398–403. | ||
Morrey BF. Surgical exposures of the elbow. In: Morrey BF, editor. The Elbow and Its Disorders. Philadelphia: WB Saunders; 1993:139–166. | ||
Pooley J, Singh R. Elbow Arthroplasty. A Guide for Orthopaedic Surgeons Using the iBP Elbow System. Biomet UK; 2000. | ||
Rajeev AS, Pooley J. A posterior approach to the elbow joint based on the blood supply to the triceps muscle. Eur J Orthop Surg Traumatol. 2009;19(7):467–472. | ||
Amirfeyz R, Clark D, Quick T, Blewitt N. Newcastle approach to the elbow, a cadaveric study. Arch Orthop Trauma Surg. 2011;131(6):747–751. | ||
Romanes GJ. Cunningham’s Manual of Practical Anatomy. 13th ed. London: Oxford University Press; 1966. | ||
Blewitt N, Pooley J. Elbow lengthening after total prosthetic arthroplasty: observations and clinical implications. J Shoulder Elbow Surg. 1994;3:200–206. | ||
Hodgson SP, Parkinson RW, Noble J. Capitellocondylar total elbow replacement for rheumatoid arthritis. J R Coll Surg Edinb. 1991;36(2):133–135. | ||
Ruth JT, Wilde AH. Capitellocondylar total elbow replacement. A long-term follow-up study. J Bone Joint Surg Am. 1992;74(1):95–100. | ||
van Rheenen TA, van den Bekerom MP, Eygendaal D. The incidence of neurologic complications and associated risk factors in elbow surgery: an analysis of 2759 cases. J Shoulder Elbow Surg. 2015;24(12):1991–1997. | ||
Pooley J, Salvador Carreno J. Total elbow joint replacement for fractures in the elderly--functional and radiological outcomes. Injury. 2015;46 (5):S37–S42. | ||
Ray PS, Kakarlapudi K, Rajsekhar C, Bhamra MS. Total elbow arthroplasty as primary treatment for distal humeral fractures in elderly patients. Injury. 2000;31(9):687–692. | ||
Argintar E, Berry M, Narvy SJ, Kramer J, Omid R, Itamura JM. Hemiarthroplasty for the treatment of distal humerus fractures: short-term clinical results. Orthopedics. 2012;35(12):1042–1045. | ||
Muller ME, Nazarian S, Koch P, Schatzker J. Comprehensive Classification of Fractures of Long Bones. Berlin: Springer-Verlag; 1990. | ||
Neer CS 2nd. Displaced proximal humeral fractures. 1. Classification and evolution. J Bone Joint Surg. 1970;52(6):1077–1089. | ||
Frankle M, Virani N, Fisher D, Mighell M. Immediate total elbow arthroplasty for distal humerus fractures. Tech Orthop. 2006;21(4):363–373. | ||
Pooley J, Prasad MG. Arthritis surgery of the elbow: surgical treatment other than joint replacement. Curr Orthop. 1997;11(4):236–241. | ||
Summers GD, Webley M, Taylor AR. A reappraisal of synovectomy and radial-head excision in rheumatoid arthritis. Br J Rheumatol. 1987;26(1):59–61. | ||
Porter BB, Richardson C, Vainio K. Rheumatoid arthritis of the elbow: the results of synovectomy. J Bone Joint Surg Br. 1974;56B(3):427–437. | ||
Woods DA, Williams JR, Gendi NS, Mowat AG, Burge PD, Carr AJ. Surgery for rheumatoid arthritis of the elbow: a comparison of radial-head excision and synovectomy with total elbow replacement. J Shoulder Elbow Surg. 1999;8(4):291–295. | ||
Nemoto K, Arino H, Yoshihara Y, Fujikawa K. Arthroscopic synovectomy for the rheumatoid elbow: a short-term outcome. J Shoulder Elbow Surg. 2004;13(6):652–655. | ||
Copeland SA, Taylor JG. Synovectomy of the elbow in rheumatoid arthritis: the place of excision of the head of the radius. J Bone Joint Surg Br. 1979;61(1):69–73. | ||
Minami M. Roentgenological studies of osteoarthritis of the elbow joint. J Jpn Orthop Assoc. 1977;51:1223–1226. | ||
Minami M, Kato S, Kashiwagi D, Sadatoshi K, Daiji K. Outerbridge-Kashiwagi’s method for arthroplasty of osteoarthritis of the elbow – 44 elbows followed for 8–16 years. J Orthop Sci. 1996;1(1):11–15. | ||
Kasiwagi D. Osteoarthritis and Outerbridge-Kashiwagi method. In: Orthopaedic Mook 54. Tokyo: Kanehara; 1988:45–58. | ||
Antuña SA, Morrey BF, Adams RA, O’Driscoll SW. Ulnohumeral arthroplasty for primary degenerative arthritis of the elbow: long-term outcome and complications. J Bone Joint Surg Am. 2002;84-A(12):2168–2173. | ||
Wada T, Isogai S, Ishii S, Yamashita T. Débridement arthroplasty for primary osteoarthritis of the elbow. J Bone Joint Surg Am. 2004;86-A(2):233–241. | ||
Savoie FH 3rd, Nunley PD, Field LD. Arthroscopic management of the arthritic elbow: indications, technique, and results. J Shoulder Elbow Surg. 1999;8(3):214–219. | ||
Rajeev A, Pooley J. Lateral compartment cartilage changes and lateral elbow pain. Acta Orthop Belg. 2009;75(1):37–40. | ||
Goodfellow JW, Bullough PG. The pattern of ageing of the articular cartilage of the elbow joint. J Bone Joint Surg Br. 1967;49(1):175–181. | ||
Murata H, Ikuta Y, Murakami T. An anatomic investigation of the elbow joint, with special reference to aging of the articular cartilage. J Shoulder Elbow Surg. 1993;2(4):175–181. | ||
Debouck C, Rooze M. A topographical study of cartilaginous lesions to the elbow. Surg Radiol Anat. 1995;17(4):301–305. | ||
Ahrens PM, Redfern DR, Forester AJ. Patterns of articular wear in the cadaveric elbow joint. J Shoulder Elbow Surg. 2001;10(1):52–56. | ||
Forster MC, Clark DI, Lunn PG. Elbow osteoarthritis: prognostic indicators in ulnohumeral debridement--the Outerbridge-Kashiwagi procedure. J Shoulder Elbow Surg. 2001;10(6):557–560. | ||
Pooley J. Unicompartmental elbow replacement: development of a lateral replacement (LRE) arthroplasty. Tech shoulder Elbow Surg. 2007;8:204–212. | ||
Giannicola G, Angeloni R, Mantovani A, et al. Open debridement and radiocapitellar replacement in primary and post-traumatic arthritis of the elbow: a multicenter study. J Shoulder Elbow Surg. 2012;21(4):456–463. | ||
Heijink A, Morrey BF, Eygendaal D. Radiocapitellar prosthetic arthroplasty: a report of 6 cases and review of the literature. J Shoulder Elbow Surg. 2014;23(6):843–849. | ||
Watkins CEL, Elson DW, Harrison JWK, Pooley J. Long-term results of the lateral resurfacing elbow arthroplasty. Bone Joint J. 2018;100-B(3):338–345. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.