Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Topical Application of M89PF Containing Vichy Mineralising Water and Probiotic Fractions Prevents Cutaneous Damage Induced by Exposure to UV and O3
Authors Benedusi M , Kerob D, Guiotto A, Cervellati F , Ferrara F, Pambianchi E
Received 28 March 2023
Accepted for publication 30 June 2023
Published 8 July 2023 Volume 2023:16 Pages 1769—1776
DOI https://doi.org/10.2147/CCID.S414011
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Mascia Benedusi,1 Delphine Kerob,2 Anna Guiotto,3 Franco Cervellati,1 Francesca Ferrara,4 Erika Pambianchi5
1Department of Neuroscience and Rehabilitation, University of Ferrara, Ferrara, Italy; 2L’Oreal Dermatological Beauty, Levallois, France; 3Department of Environmental Sciences and Prevention, University of Ferrara, Ferrara, Italy; 4Department of Chemical, Pharmaceutical and Agricultural Sciences, University of Ferrara, Ferrara, Italy; 5North Carolina Research Campus, Plants for Human Health Institute, Animal Science, North Carolina State University, Kannapolis, NC, 28081, USA
Correspondence: Mascia Benedusi, Department of Neuroscience and Rehabilitation, University of Ferrara, via Borsari 46, Ferrara, 44121, Italy, Tel +390532455462, Email [email protected]
Purpose: Exposure of the skin to ultraviolet radiation (UV) or ozone (O3) results in stressed skin, leading to the alteration of the skin physical barrier and defence functions. In this work, the preventive benefit of a dermocosmetic, M89PF, containing Vichy mineralising water, probiotic fractions, antioxidant vitamins and hyaluronic acid, in the alteration of skin physical barrier and skin defence functions after exposure to O3 and UV, alone or combined, was assessed.
Methods: Untreated and treated (M89PF) skin explants were exposed to O3, to UV rays or to O3+UV. Immunofluorescence was performed for skin barrier, oxidative stress, and inflammatory markers after one and four days of exposure to the pollutants.
Results: M89PF significantly (p≤ 0.05) prevented the decrease of the expression level of different skin barrier markers, and significantly (p≤ 0.05) prevented the induction of OxInflammatory markers and inflammasome components by UV, O3, or both combined.
Conclusion: M89PF prevents skin barrier damage, as well as oxidative stress and inflammatory markers induced by exposome factors, such as UV, O3, or both combined.
Keywords: ultraviolet radiation, ozone, skin barrier, oxidative stress, inflammation
Introduction
The skin is the largest and most diverse organ of the human body with the epidermis being the first line of defence against the skin exposome which includes ultraviolet radiation (UV) and pollution.1–5 The effect of pollutants, such as Ozone (O3) and UV on human skin has been investigated over the past decades. Ferrara et al have studied the combination of UV and pollution, confirming their impact on both the skin barrier and skin defence system through oxidative stress and activation of inflammatory cytokines.6
UVB and UVA significantly impact the skin, causing sunburn and skin aging.7,8 O3 interacts with lipids and proteins present in the stratum corneum, resulting in the production of reactive oxygen species (ROS), secondary mediators such as aldehydes (4-HydroxyNonenal, 4-HNE) and cyclooxygenase 2 (COX-2), and in the activation of inflammasome pathways, such as NLR Family Pyrin Domain Containing (NLRP1).9–15 This process causes oxidative damage throughout the skin and initiates inflammatory processes.11,16–20 A growing set of data demonstrated that different pollutants affect skin expression levels of the transcription factor aryl hydrocarbon receptor (AhR) and that its activation in skin cells parallels that of keratinocyte differentiation and inflammation, resulting in cutaneous damage, skin diseases and premature skin aging.21–23
Moreover, O3 and UV alter the expression of the stratum corneum cornified envelope components such as lipids, proteins (Keratin, Filaggrin, Involucrin), as well as tight junctions.15,24–31 As a result, skin is stressed, which leads to the alteration of the skin physical barrier and defence functions.32,33 Filaggrin (FLG), a marker of the late epidermal differentiation, plays a pivotal role in the formation and maintenance of the skin barrier, as well as in stratum corneum hydration. Involucrin (INV), a protein involved in the assembly of the cornified envelope, the structure formed in the outermost layers of the epidermis, provides a physical barrier against environmental stressors.34 Finally, as mentioned before, the exposome induces the activation of the inflammasome, a cytosolic multiprotein complex involved in skin inflammatory diseases, upon exposome exposure.31,35 Inflammasomes represent a group of protein complexes of the innate immune system, involved in host defence against different harmful stimuli and in repair processes upon the initiation of an inflammatory status. Ferrara et al described the role of inflammasome activation in different skin pathologies and its possible role as a molecular target in the prevention of pollution-induced skin damage.36 Among the different proteins involved in the inflammasome complex, the apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC), can be considered a hallmark of its activation.37
M89PF (Mineral 89 Probiotic Fractions, Laboratoires Vichy, France) contains Vichy mineralizing water (VMW), hyaluronic acid, niacinamide, tocopherol and probiotic fractions. It is hypoallergenic and contains no perfume. VMW originates from the French volcanic region and contains 15 minerals with a total mineral concentration of 5.2g/l.
This ex vivo study assessed the effect of O3 and UV radiation alone or combined on the skin, as well as the preventive effect of M89PF.
Materials and Methods
Maintenance in Culture and Exposure to UV and O3 of ex vivo Skin Explants
All human tissues were obtained from elective abdominoplasty with donor consent under Pearl IRB approval, in accordance with FDA 45 CFR 46.102 and 21 CFR 56.102 regulations (Pearl Pathways. Exemption Determination Submission. IRB Study Number: 21-TENB-101. Study Title: Collection, culture, and distribution of human abdominoplasty skin tissue). Written informed consent was obtained from all subjects or, if subjects were aged less than 18 years, from a parent and/or legal guardian. All donors were healthy volunteers. No identifying information beyond ethnicity, sex and age was provided.
Subcutaneous fat was trimmed from 12 mm punch biopsies, and samples were rinsed with Phosphatase Buffer Solution (PBS) 1X containing antibiotics/antimycotic. Biopsies were cultured in DMEM Media added with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5% CO2 in 6-well plates, with the upper part of the epidermis exposed to the outside environment. The day after, the media was changed, and M89PF was topically applied. After 24 hours, the media was replaced with fresh media, and biopsies were exposed to 200 mJ UVA/UVB light alone (Newport, Oriel1, Sol1ATM, 1600 W, Xenon Lamp, UVC & AM0 filters), to 0.25 ppm of O3 alone for 2 hours, or to a combination of 200 mJ UVA/UVB and 0.25 ppm of O3 for 2 hours. The UVA/UVB ratio used was 21/1 since the air mass filter AM 0 was adopted to adjust the light output to strictly match the solar spectrum when the sun is at a Zenith angle of 0. UV doses were precisely monitored using a radiometer ILT2400 Hand-Held Light Meter / Optometer (International Light Technologies, Inc., Peabody, MA, USA). Biopsies were pre-treated every day with M89PF, exposed to the pollutants, and collected 24 hours after the first exposure (DAY 1) and after four days (DAY 4) for the subsequent evaluations.
Haematoxylin and Eosin Staining
Skin explants were fixed in 10% NBF (neutral-buffered formalin) and embedded in paraffin. For histological observation, sections (4 μm thickness) were deparaffinized in xylene, and rehydrated in a series of alcohol gradients (100%, 90%, 80% and 70%). Sections were stained with Mayer’s hematoxylin solution, washed in tap water, stained with aqueous Eosin Y solution (Sigma Aldrich, St. Louis, MO, USA) for 2–3 min, and dehydrated in a series of increasing concentrations of alcohols and xylene. Finally, sections were mounted onto glass using a toluene based mounting medium and observed under microscope (Nikon Microphot FXA microscope,Nikon Instruments, Amsterdam, Netherlands).
Immunofluorescence
Paraffin sections (4μm thickness) of skin explants were deparaffinized in xylene and rehydrated in alcohol gradients. After that, sections were blocked with 5% BSA in PBS 1X for 45 minutes at room temperature, and incubated overnight at 4°C with the following diluted (in PBS 1X, 2% BSA solution) primary antibodies: 4HNE (diluted 1:500; AB5605, Millipore Corporation, Burlington, MA, USA), AhR (diluted 1:500; 83,200, Cell Signaling, Danvers, MA, USA), COX-2 (diluted 1:400; NB100-868, Novus Biologicals, Littleton, CO, USA), Involucrin (diluted 1:50; sc-21748, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Filaggrin (diluted 1:50; sc-66192, Santa Cruz). After 24 hours, sections were washed three times with PBS 1X, incubated with specific fluorochrome-conjugated secondary antibodies (diluted 1:500 in PBS 1X, 2% BSA; Alexa Fluor 568 A11004 or Alexa Fluor 488 A11055) at RT, and nuclei were stained with DAPI solution (D1306, Invitrogen) for 2 minutes. Sections were again washed three times with PBS 1X, and mounted using PermaFluor mounting media (Thermo Fisher Scientific, Waltham, MA, USA). Images of sections were made using a Nikon Microphot FXA microscope, and images captured were quantified using ImageJ.
Statistical Analysis
All results were expressed as mean±SD values of three different images. For between-group comparisons, an analysis of variance (ANOVA), followed by Bonferroni’s post-hoc test, which compares all pairs of columns under test with a consideration of p value ≤ 0.05 as the significant difference, was conducted. Data were analysed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA) software.
Results
Effect on the Skin Structure
By Haematoxylin and Eosin staining of skin explants, no evident alterations of the skin structure were observed after exposure to pollutants compared to control conditions, with an intact structure of the epidermis presenting all skin layers and a contiguous stratum corneum, validating the assay methodology. No noticeable changes were observed within the dermis when compared to control samples, suggesting that the selected dose of pollutants did not damage skin biopsies. No significant differences were perceived between treated and untreated tissues, suggesting that treatment with M89PF does not affect the skin morphology during the experimental procedure at either DAY 1 or DAY 4 (Figure S1).
Effect on Hydration and Skin Barrier-Associated Proteins, and the Preventive Role of M89PF
A decrease of FLG levels in skin samples exposed to O3 and UV was observed specifically at DAY 4 (Figure S2B). This depletion was prevented by M89PF. M89PF was efficient after the exposure to the combination of O3 and UV at DAY 1 and DAY 4 (Figure S2A and B), with a significant increase of the FLG expression levels (p≤0.05; M89PF+O3 vs O3=+27%, M89PF+UV vs UV=+43% and M89PF+O3+UV vs O3+UV=+94%).
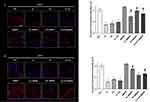
As depicted in Figure 1, the immunofluorescence analysis at DAY 1 (Figure 1A) demonstrated a decrease of the INV expression levels after exposure to pollutants. The topical administration of M89PF prevented this effect not only at DAY 1 but also at DAY 4 (Figure 1B), as confirmed through statistical analysis of the protein quantification (Figure 1, right panels).
Pre-Treatment with M89PF Prevents the Pollutant-Induced Expression of Oxidative Stress Markers
Combined exposure to UV and O3 induced the expression of oxidative markers. However, this effect was counteracted by the pre-treatment of skin tissue with M89PF.
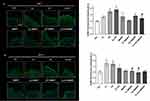
A significant (p≤0.05) increase of the 4-HNE expression levels were observed in untreated skin samples after exposure to UV (+75%) or to UV+ O3 (+90%), compared to the negative control at DAY 1 (Figure 2A). No notable change was observed in M89PF-treated skin samples. Pre-treatment with M89PF prevented 4-HNE increase, observed after the exposure to O3+UV (−35%; p≤0.05 if compared to pollutant-exposed samples).
After 4 days (Figure 2B), an increase of the 4-HNE expression levels for both O3 and UV alone, as well as for the combination of the two pollutants, was observed; this difference was statistically significant (p≤0.05) for O3 (+76%) and for UV (+72) exposure). At DAY 4, the preventive effect of M89PF was even more evident than at DAY 1.
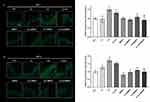
The expression of the AhR levels in untreated skin samples significantly (p≤0.05) increased after exposure to O3 (+48%) or to UV+ O3 (+61%) at DAY 1 (Figure S3A). In M89PF-treated UV+O3–exposed skin samples, AhR expression was significantly reduced compared to untreated samples exposed to UV+ O3 (−50%, p≤0.05). The impact of pollutants was less pronounced, and differences to control samples were statistically insignificant after four days of exposure (Figure S3B). Nevertheless, in this case, M89PF also had a protective role against O3 and UV exposure.
Pre-Treatment with M89PF Prevents the Pollutant-Induced Inflammatory Skin Response
The levels of COX-2 in untreated skin samples exposed to UV (+50%) and UV+O3 (+31%) significantly (p≤0.05) increased at DAY 1 (Figure 3A) compared to control tissues. Topical administration of M89PF resulted in a decrease of COX-2 expression levels in UV and UV+O3 exposed samples (about −30%). Exposure to O3 alone did not alter the COX-2 expression levels after 24 hours, indicating that the effect observed after a combined exposure to UV+O3 was probably due to UV. These data suggest that UV induces inflammatory damage faster than O3. At DAY 4, even if UV induced a higher increase of COX-2 (+72%), O3 altered its expression levels (+27% if compared to control tissues) as well. Between-group differences (M89PF treated samples vs pollutant-exposed samples) were all statistically significant (p≤0.05) in favour of M89PF-treated samples (Figure 3B).
Moreover, at DAY 1, the expression levels of ASC had significantly increased (p≤0.05) in untreated skin samples after exposure to UV, O3 and UV+ O3 (+62%, +102% and +109% respectively if compared to control tissues) (Figure S4A). Pre-treatment with M89PF prevented from pollutant-induced increase in ASC levels. Similarly, between-group differences remained significantly (p≤0.05) in favour of M89PF-treated samples after all exposome exposures and after four days of topical application of M89PF (Figure S4B).
Discussion
Results from this ex vivo study confirm that O3 and UV alone or combined are deleterious to the upper skin layer.6,15,38–40 Exposure to exposome factors significantly (p≤0.05) decrease Filaggrin and Involucrin levels, both markers for the integrity of the natural skin barrier. Moreover, these exposome factors significantly (p≤0.05) increase the levels of oxidative stress markers, such as 4-HNE and AhR, and that of COX-2 and ASC, both being markers of skin inflammation and skin aging.
A single application of M89PF prior to exposure to O3 and UV alone, or both combined, significantly (p≤0.05) reduced the depletion of FLG and INV, thus helping to maintain and protect the natural skin barrier. Moreover, in M89PF-treated samples, the levels of oxidative stress markers triggered by exposure to the exposome factors remained unchanged, compared to negative controls at DAY 1 and DAY 4, with a significant (p≤0.05) difference to untreated exposed samples. Similar results were observed for the skin inflammation markers. As mentioned in the Introduction, M89PF contains different active ingredients; in particular, the minerals contained in M89PF reinforce the natural defences of the skin in restoring the natural skin barrier, stimulating antioxidant activity, and reducing inflammation.41–46 M89PF has been enriched with probiotic fractions, shown to enhance re-epithelization of the skin and help to limit dysbiosis, inflammation and to restore the natural skin barrier.47–49 M89PF has been developed to repair the skin barrier and to reinforce skin defences against exposome factors.
We agree that the present study was conducted ex-vivo and that the sample size may be considered as small. However, results demonstrate that at tissue level, the tested exposome factors have an impact on the upper layers of the skin, and that M89PF provides an unambiguous benefit in protecting these tissues from external aggression. Further research is necessary to confirm the obtained assay results and, potentially, in other skin conditions that may be triggered by exposome, in order to clinically confirm the present observations.
Conclusion
The human body is permanently exposed to O3 and UV radiations, and further research is necessary to assess the impact of the combined exposure and cumulative effects of different air pollutants that affect each other’s response.
M89PF helps to prevent skin barrier damage, as well as oxidative stress and inflammation induced by exposome factors such as UV and O3.
Acknowledgments
The authors acknowledge the writing support of Karl Patrick Göritz, SMWS France.
Funding
The study was funded by Laboratoires Vichy International, a L’Oréal company.
Disclosure
Delphine Kerob is an employee of Cosmetic Active International, a L’Oréal company. The other authors have no conflict of interest to disclose in this work.
References
1. Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci. 2000;23(Suppl 1):S22–S26. doi:10.1016/S0923-1811(99)00077-8
2. Krutmann J, Liu W, Li L, et al. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76(3):163–168. doi:10.1016/j.jdermsci.2014.08.008
3. Krutmann J, Morita A, Chung JH. Sun exposure: what molecular photodermatology tells us about its good and bad sides. J Invest Dermatol. 2012;132(3 Pt 2):976–984. doi:10.1038/jid.2011.394
4. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi:10.1016/j.jdermsci.2016.09.015
5. Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesth Dermatol. 2011;4(9):22–42.
6. Ferrara F, Pambianchi E, Woodby B, et al. Evaluating the effect of ozone in UV induced skin damage. Toxicol Lett. 2021;338:40–50. doi:10.1016/j.toxlet.2020.11.023
7. Kamiya K, Kishimoto M, Sugai J, Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20(18):4347. doi:10.3390/ijms20184347
8. Drakaki E, Dessinioti C, Antoniou C. Air pollution and the skin. Front Environ Sci. 2014;2014:2.
9. Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3s1):S100–S109. doi:10.1016/j.jaad.2016.09.038
10. McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-Contributors and inhibitors. J Cosmet Dermatol. 2018;17(2):124–137. doi:10.1111/jocd.12518
11. Pecorelli A, Woodby B, Prieux R, Valacchi G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. Biofactors. 2019;45(4):536–547. doi:10.1002/biof.1513
12. Araviiskaia E, Berardesca E, Bieber T, et al. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33(8):1496–1505. doi:10.1111/jdv.15583
13. Rembiesa J, Ruzgas T, Engblom J, Holefors A. The impact of pollution on skin and proper efficacy testing for anti-pollution claims. Cosmetics. 2018;5(1):4. doi:10.3390/cosmetics5010004
14. Valacchi G, Sticozzi C, Pecorelli A, Cervellati F, Cervellati C, Maioli E. Cutaneous responses to environmental stressors. Ann N Y Acad Sci. 2012;1271(1):75–81. doi:10.1111/j.1749-6632.2012.06724.x
15. Ferrara F, Woodby B, Pecorelli A, et al. Additive effect of combined pollutants to UV induced skin OxInflammation damage. Evaluating the protective topical application of a cosmeceutical mixture formulation. Redox Biol. 2020;34:101481. doi:10.1016/j.redox.2020.101481
16. Valacchi G, Pagnin E, Corbacho AM, et al. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Rad Biol Med. 2004;36(5):673–681. doi:10.1016/j.freeradbiomed.2003.12.005
17. Packer L, Valacchi G. Antioxidants and the response of skin to oxidative stress: vitamin E as a key indicator. Skin Pharmacol Appl Skin Physiol. 2002;15(5):282–290. doi:10.1159/000064531
18. Valacchi G, Virgili F, Cervellati C, Pecorelli A. OxInflammation: from subclinical condition to pathological biomarker. Front Physiol. 2018;9:858. doi:10.3389/fphys.2018.00858
19. Sticozzi C, Belmonte G, Pecorelli A, et al. Cigarette smoke affects keratinocytes SRB1 expression and localization via H2O2 production and HNE protein adducts formation. PLoS One. 2012;7(3):e33592. doi:10.1371/journal.pone.0033592
20. Valacchi G, Muresan XM, Sticozzi C, et al. Ozone-induced damage in 3D-Skin Model is prevented by topical vitamin C and vitamin E compound mixtures application. J Dermatol Sci. 2016;82(3):209–212. doi:10.1016/j.jdermsci.2016.02.007
21. Fernández-Gallego N, Sánchez-Madrid F, Cibrian D. Role of AHR Ligands in Skin Homeostasis and Cutaneous Inflammation. Cells. 2021;10(11):3176. doi:10.3390/cells10113176
22. Vogel CFA, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530. doi:10.1016/j.redox.2020.101530
23. Kim YJ, Lee JE, Jang HS, et al. Oleanolic acid protects the skin from particulate matter-induced. Aging Biomol Ther. 2021;29(2):220–226. doi:10.4062/biomolther.2020.106
24. De Luca C, Valacchi G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators Inflamm. 2010;2010:321494. doi:10.1155/2010/321494
25. Thiele JJ, Dreher F, Maibach HI, Packer L. Impact of ultraviolet radiation and ozone on the transepidermal water loss as a function of skin temperature in hairless mice. Skin Pharmacol Appl Skin Physiol. 2003;16(5):283–290. doi:10.1159/000072068
26. Moravcová M, Libra A, Dvořáková J, et al. Modulation of keratin 1, 10 and involucrin expression as part of the complex response of the human keratinocyte cell line HaCaT to ultraviolet radiation. Interdiscip Toxicol. 2013;6(4):203–208. doi:10.2478/intox-2013-0030
27. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246. doi:10.1111/j.1600-065X.2011.01027.x
28. Murthy AS, Leslie K. Autoinflammatory skin disease: a review of concepts and applications to general dermatology. Dermatology. 2016;232(5):534–540. doi:10.1159/000449526
29. Sahle FF, Gebre-Mariam T, Dobner B, Wohlrab J, Neubert RH. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharm Physiol. 2015;28(1):42–55. doi:10.1159/000360009
30. Woodby B, Penta K, Pecorelli A, Lila MA, Valacchi G. Skin health from the inside out. Annu Rev Food Sci Technol. 2020;11(1):235–254. doi:10.1146/annurev-food-032519-051722
31. Ferrara F, Pambianchi E, Pecorelli A, et al. Redox regulation of cutaneous inflammasome by ozone exposure. Free Rad Biol Med. 2020;152:561–570. doi:10.1016/j.freeradbiomed.2019.11.031
32. Passeron T, Krutmann J, Andersen ML, Katta R, Zouboulis CC. Clinical and biological impact of the exposome on the skin. J Eur Acad Dermatol Venereol. 2020;34 Suppl 4(S4):4–25. doi:10.1111/jdv.16614
33. Kim BE, Leung DYM. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10(3):207–215. doi:10.4168/aair.2018.10.3.207
34. Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. BioEssays. 2002;24(9):789–800. doi:10.1002/bies.10144
35. Gruber JV, Holtz R. In vitro expression of NLRP inflammasome-induced active Caspase-1 expression in normal human epidermal keratinocytes (NHEK) by various exogenous threats and subsequent inhibition by naturally derived ingredient blends. J Inflamm Res. 2019;12:219–230. doi:10.2147/JIR.S215776
36. Ferrara F, Prieux R, Woodby B, Valacchi G. Inflammasome Activation in Pollution-Induced Skin Conditions. Plast Reconstr Surg. 2021;147(1s–2):15s–24s. doi:10.1097/PRS.0000000000007617
37. Stutz A, Horvath GL, Monks BG, Latz E. ASC speck formation as a readout for inflammasome activation. Methods Mol Biol. 2013;1040:91–101.
38. Fuks KB, Woodby B, Valacchi G. Hautschäden durch troposphärisches Ozon [Skin damage by tropospheric ozone]. Der Hautarzt. 2019;70(3):163–168. German. doi:10.1007/s00105-019-4361-4
39. Battie C, Jitsukawa S, Bernerd F, Del Bino S, Marionnet C, Verschoore M. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol. 2014;23(Suppl 1):7–12. doi:10.1111/exd.12388
40. Cadet J, Douki T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem Photobiol Sci. 2018;17(12):1816–1841. doi:10.1039/c7pp00395a
41. Nusgens BV. Acide Hyaluronique et matrice extracellulaire : une molécule primitive? [Hyaluronic acid and extracellular matrix: a primitive molecule?]. Ann Dermatol Venereol. 2010;137 Suppl 1:S3–S8. French. doi:10.1016/S0151-9638(10)70002-8
42. Burke KE. Mechanisms of aging and development-A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev. 2018;172:123–130. doi:10.1016/j.mad.2017.12.003
43. Nonotte I, Montastier C, Boisnic S, Branchet-Gumila MC, Breton L. Inhibitory effect of Lucas spring water on substance P induced inflammation in organ culture of human skin. Nouv Dermatol. 1998;1:535–542.
44. Moyal D, Tricaud C, Pham DM, Ngyen QL. P2619 efficacy of a spa water in preventing UVA-induced catalase degradation. J Am Acad Dermatol. 2006;54(3):AB190.
45. Mascarenhas NL, Wang Z, Chang YL, Di Nardo A. TRPV4 mediates mast cell activation in cathelicidin-induced rosacea inflammation. J Invest Dermatol. 2017;137(4):972–975. doi:10.1016/j.jid.2016.10.046
46. Tacheau C, Weisgerber F, Fagot D, et al. Vichy Thermal Spring Water (VTSW), a cosmetic ingredient of potential interest in the frame of skin ageing exposome: an in vitro study. Int J Cosmet Sci. 2018;40(4):377–387. doi:10.1111/ics.12470
47. Lombardi F, Palumbo P, Mattei A, et al. Soluble fraction from lysates of selected probiotic strains differently influences re-epithelialization of HaCaT scratched monolayer through a mechanism involving nitric oxide synthase 2. Biomolecules. 2019;9(12):756. doi:10.3390/biom9120756
48. Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29(1):15–21. doi:10.1111/exd.14032
49. Yu Y, Dunaway S, Champer J, Kim J, Alikhan A. Changing our microbiome: probiotics in dermatology. Br J Dermatol. 2020;182(1):39–46. doi:10.1111/bjd.18659
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.