Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Tofacitinib for Prurigo Nodularis: A Case Report
Authors Peng C , Li C, Zhou Y, Wang Q , Xie P, Li T, Hao P
Received 7 January 2022
Accepted for publication 1 March 2022
Published 21 March 2022 Volume 2022:15 Pages 503—506
DOI https://doi.org/10.2147/CCID.S354025
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Changlan Peng,1 Chunxiao Li,2 Yingying Zhou,1 Qiuyue Wang,1 Ping Xie,1 Tianhao Li,2,* Pingsheng Hao2,*
1Department of Dermatology, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Dermatology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Tianhao Li; Pingsheng Hao, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Chengdu, Sichuan Province, People’s Republic of China, Tel +86-13880986337 ; +86-13881965024, Fax +86-28-87732407, Email [email protected]; [email protected]
Purpose: Despite recent advances in the treatment of prurigo nodularis, conventional treatment suffers from a dilemma of poor efficacy. The clinical use of Janus kinase (JAK) inhibitors in the treatment of Prurigo nodularis has rarely been explored.
Patients and Methods: We present a case of prurigo nodularis successfully treated with, JAK inhibitor tofacitinib with no adverse effects.
Results: This case report of successful treatment shows a good clinical efficacy of using JAK inhibitor tofacitinib in the treatment of prurigo nodularis. Cytokines may be an important cause of prurigo nodularis.
Conclusion: JAK inhibitor tofacitinib may be a new option for the treatment of prurigo nodularis, especially for patients who have failed conventional treatment.
Keywords: prurigo nodularis, JAK inhibitors, tofacitinib
Introduction
Prurigo nodularis (PN) is an intensely pruritic disease characterized by highly keratinized, itchy papules and nodules symmetrically distributed on the extremities of the limbs.1 Treatment is often challenging and unsatisfying. Recently, Janus kinase (JAK) inhibitors has been successfully used for the treatment of PN.2,3 Here, we report a case of a patient with PN who did not respond to conventional treatment but was successfully treated by the JAK inhibitor tofacitinib.
Case Presentation
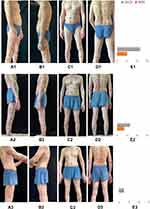
A 65-year-old male patient was admitted due to “ recurrent systemic erythema, papules and itching for more than 2 years” (Figure 1A1–D1). His laboratory examination shows no obviously abnormal (Including humoral immunity, autoimmune antibody profile, tuberculosis, hepatitis B, AIDS, syphilis, etc.) except the eosinophils were 1.47*109/L, and the percentage of eosinophils was 22.1%. Over the past two years, he has undergone many diagnoses and treatments without satisfactory results. Eight months before this admission, the patient received a skin pathological examination in another hospital in the city and was diagnosed as PN. After admission, the patient was diagnosed as PN, and pruritus Numerical Rating Scale (NRS) is 10 (Figure 1E1), Dermatology Life Quality Index (DLQI) is 23 (Figure 1E1). We referred to the guidelines for the treatment of PN and treated the patient with the following:4 topical corticosteroids (topical halogen cream), NB-UVB irradiation, and systemic therapy (glycyrrhizic acid, vitamin C, calcium gluconate, rubastine fumarate, ebastine, pregabalin, and thalidomide). However, the symptoms did not improve significantly. Report on the effectiveness of dupilumab in PN,4–6 we had planned to give him dupilumab, but he refused because of the expensive price. Then, we treated the patient with tofacitinib and stopped other treatments. After 1 week of tofacitinib application, the number of nodules decreased and became smaller, and the NRS and DLQI also improved significantly. Refer to the dosage of tofacitinib in the treatment of pruritic dermatosis,2,7,8 the patient took tofacitinib for a total of 6 weeks at a dosage of 5mg bid for weeks 1–2, 5mg qd for weeks 3–4, and 5mg qod for weeks 5–6. Skin lesions were photographed before, 1 week after, and 6 weeks after tofacitinib treatment (Figure 1A2–D2; Figure 1A3–D3), NRS and DLQI were assessed simultaneously (Figure 1E2; Figure 1E3). In view of previous reports of exacerbations of PN treated with tofacitinib,8 we closely observed the patients and showed good efficacy with no adverse effects during 6 weeks of tofacitinib administration. The patient was followed up for 5 months after drug withdrawal, and no recurrence was found.
Discussion
PN is a refractory skin disease, and pruritus is the main problem that needs to be solved. According to recent studies, the pathogenesis of PN is associated with decreased intraepidermal nerve fiber density and abnormal secretion of cytokines such as IL-4, IL-17, IL-22, and IL-31.1,2 As a non-selective JAK inhibitor, tofacitinib can inhibit the JAK-STAT pathway, block the transcription of IL-4 and IL-31,9,10 increase the density of epidermal nerve fibers, and reduce pruritus. The first reported case of tofacitinib for PN,2 the success of baricitinib for prurigo-type atopic dermatitis,3 both demonstrate the effectiveness of the JAK inhibitor for the treatment of PN.
Conclusion
Therefore, tofacitinib as an inhibitor of JAK is a promising choice for the treatment of PN, in which cytokines are the main inflammatory factors causing pruritus. In future, larger sample sizes and multi-ethnic clinical studies will be needed to provide stronger evidence.
Ethics Statement
Signed consent was obtained from the family member for the publication of the case details and accompanying images. Institutional approval was not required to publish the case details.
Consent Statement
Written informed consent was provided by the patient for publication of images and information.
Disclosure
Tianhao Li and Pingsheng Hao are co-correspondence authors for this study. The authors have no conflicts of interest to declare in this work.
References
1. Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67–77. doi:10.1080/17512433.2021.1852080
2. Molloy OE, Kearney N, Byrne N, Kirby B. Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45:918–920. doi:10.1111/ced.14320
3. He Y, Ji S, Yu Q. Effectiveness of baricitinib in prurigo-type atopic dermatitis: a case report. Dermatol Ther. 2021;34:e14878. doi:10.1111/dth.14878
4. Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
5. Holm JG, Agner T, Sand C, Thomsen SF. Dupilumab for prurigo nodularis: case series and review of the literature. Dermatol Ther. 2020;33(2):e13222. doi:10.1111/dth.13222
6. Toffoli L, Farinazzo E, Zelin E, et al. Dupilumab as promising treatment for prurigo nodularis: current evidences. J Dermatolog Treat. 2021;15:1–6. doi:10.1080/09546634.2021.1886232
7. Xiao Y, Li W. A case of intractable pruritic plaque psoriasis with treated with tofacitinib. Chin J Dermatovenereol. 2021;35(12):1380–1383.
8. Jerjen R, Wall D, Meah N, Sinclair R. Comment on ‘Successful treatment of recalcitrant nodular prurigo with tofacitinib. Clin Exp Dermatol. 2020;45(8):1068–1070. doi:10.1111/ced.14379
9. Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217–228. doi:10.1016/j.cell.2017.08.006
10. Hashimoto T, Sakai K, Sanders KM, et al. Antipruritic effects of Janus kinase inhibitor tofacitinib in a mouse model of psoriasis. Acta Derm Venereol. 2019;99:298–303. doi:10.2340/00015555-3086
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.