Back to Journals » International Medical Case Reports Journal » Volume 17
Todd Paralysis in a Pregnant Mother Presenting as Acute Stroke: Case Report
Authors Aksu Selman B, Sheikh Hassan M , Rahimov R, Mert S, Köksal A
Received 12 January 2024
Accepted for publication 19 April 2024
Published 23 April 2024 Volume 2024:17 Pages 367—370
DOI https://doi.org/10.2147/IMCRJ.S459256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Beria Aksu Selman,1,* Mohamed Sheikh Hassan,2,3,* Rahim Rahimov,1 Saltanat Mert,1 Ayhan Köksal1
1University of Health Science, Neurology Department Başakşehir Çam and Sakura City Hospital, Istanbul, Turkey; 2Department of Neurology, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia; 3Faculty of Medicine and Surgery, Mogadishu University, Mogadishu, Somalia
*These authors contributed equally to this work
Correspondence: Mohamed Sheikh Hassan, Department of Neurology Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia, Email [email protected]
Abstract: Todd’s paralysis (TP) is relatively uncommon condition that can occur immediately after an epileptic seizure. It is a heterogeneous clinical syndrome that presents with acute-onset neurological findings, such as paralysis, paresthesia, aphasia, hemianopsia, and an altered state of consciousness. This may be accompanied by cytotoxic edema on diffusion MRI. This case illustrates a 28-week pregnant patient with TP who presented with acute stroke-like clinical and radiological findings. The patient was presented to the emergency room with left side weakness following focal onset generalized seizure. Magnetic resonance imaging demonstrated diffusion restriction which led to the initial consideration of acute stroke. However, after the disappearance of the neurologic deficit and the resolution of the diffusion restriction in the control MRI, the diagnosis shifted away from acute stroke to the postictal TP. It is important to keep in mind that TP may mimic acute stroke even in the presence of an acute brain lesion in the brain MRI. The differentiation is necessary as each of them has completely different treatment and etiology.
Keywords: Todd paralysis, acute stroke, magnetic resonance imaging, hemiplegia
Introduction
Todd’s paralysis is a neurological condition - a temporary weakness or paralysis and loss of sensation over part of the body experienced by individuals with epilepsy, in which a seizure is followed by a brief period of temporary paralysis. The paralysis may be partial or complete but usually occurs on just one side of the body. It can last from 30 minutes to 36 hours, with an average of 15 hours, before it resolves completely. Todd’s paralysis may also affect speech and vision. This transient focal neurological deficit seen after epileptic seizures was described by British neurologist Robert Todd, and as a result, the condition is often referred to as TP.1,2
The incidence of Todd paralysis following a seizure is variable and ranges between 0.6% and 13.4%.3 However, the clinical manifestations are heterogeneous and may include paresthesia, aphasia, hemianopsia, and impaired consciousness.4 An episode of TP can last anywhere from minutes to days, depending on the type of seizure, other accompanying structural damage, and any other signs that develop following the seizure.5
It is vital to distinguish Todd’s paralysis from stroke, which has a completely different treatment.6 However, this can be challenging when the paralysis is accompanied by imaging abnormalities such as diffusion restrictions seen in the acute stroke. This present case illustrated a 28-week pregnant female patient presented a Todd paralysis admitted as acute stroke following diffusion restriction seen in her brain MRI.
Case Presentation
A 27-year-old female patient who was 28 weeks pregnant was admitted to our facility during the postictal phase due to a focal-onset generalized tonic-clonic seizure she had 1 hour ago. According to the anamnesis, she initially experienced fainting in the 19th week of her pregnancy, which led to her first generalized tonic-clonic type seizure. At that time, Levetiracetam 500 BID was prescribed as a treatment. An EEG performed in the 19th week did not reveal any abnormal cerebral activity.
The patient’s neurological examination, conducted when she arrived at our emergency department, found that her left upper extremity was fully plegic at 0/5 (based on MRC); in the left lower extremity, she had 0/5 muscle strength in the proximal muscles and 2/5 muscle strength in the distal muscles. The plantar reflex was mute bilaterally (negative Babinski).
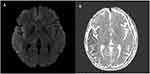
In the patient’s first MRI, acute diffusion restriction areas were observed in the right temporoparietal region, hypointense in ADC, and hyperintense in DWI (see Figure 1). After further examination, preeclampsia was excluded by the relevant department physicians. In order not to exceed the therapeutic window and detect a possible large vessel occlusion, non-contrast brain MR angiography and MR venography were performed. There was not any evidence of a major artery obstruction following the brain MR angiography. Furthermore, hypoplastic left transverse sinus was noted based on MRI venography results. Therefore, intravenous tissue plasminogen activator (TPA) or mechanical thrombectomy was not considered.
After the first examination of the patient, who was initially considered to have had an acute ischemic stroke, the neurological examination at the 45th minute showed that there was almost a complete recovery in the patient’s paresis. As a result, a control MRI was performed, and it was seen that the hyperintense areas of acute diffusion restriction in DWI and hypointensity in the ADC disappeared (see Figure 2). In line with these data, the patient’s muscle strength deficit was interpreted in favor of Todd’s paralysis, which is a complication of the postictal period.
 |
Figure 2 (A-C) control MRI showing resolution of the diffusion restriction seen in the initial MRI after the disappearance of the patient’s weakness. |
The levetiracetam dose was increased to 1000 mg BID. On an electroencephalogram performed during her hospitalization in the neurology unit, a spike-slow wave was observed in the right parietal region. The patient, whose seizures did not recur, was discharged in good condition after the fetal well-being examination was completed.
Discussion
Postictal paralysis is one of the wider spectrum of characteristics that accompany paroxysmal seizures; these symptoms are referred to as a variant of TP. Among the conditions that have been documented are postictal aphasia, hemianopsia, ideomotor apraxia, and retrograde non-memory impairment. The most important suggestion of TP is the presence of previous episodes of involuntary facial or limb contractions that may be associated with altered mental status. Therefore, anamnesis is paramount for identifying TP. Todd paralysis usually commences after a focal seizure and involves one or more limbs.7,8
The mechanism underlying the development of TP still remains uncertain. Numerous theories concerning its etiology have been proposed, including neuronal desensitization, neurotransmitter depletion, focal reduction of cerebral blood flow, active suppression, and extensive neuronal firing during seizures.9,10
Although the differential diagnosis of epilepsy and stroke is always easy to make, neurological deficits that develop after seizures should be specially considered in the differential diagnosis of stroke. TP should also be considered a rare etiological agent in cases presenting with acute-onset neurological deficits and accompanied by cytotoxic edema on DWI.6,11
At clinical presentation, the first symptom being a seizure, early complete recovery of the neurologic deficit, and some features of the lesion on DWI may be important clues in the differential diagnosis.5 In this case report, our pregnant patient’s initial imaging was suggestive of stroke due to diffusion restriction, but as the findings occurred following an epileptic seizure and the deficit in her neurological examination showed almost complete recovery 45 minutes later, the diagnosis shifted away from stroke. The control brain MRI, which was performed immediately after the disappearance of the neurologic deficit, demonstrated resolution of the diffusion restriction seen in the initial MRI. In addition, the brain MR angiography revealed normal findings, and the MR venogram did not show any occlusion of the cerebral veins and sinuses. Preeclampsia was ruled out by consultation with the corresponding physicians. An electroencephalogram demonstrated a spike-slow wave in the right parietal region. As a result, Todd’s palsy, which is attributed to transient global hemispheric hypoperfusion in the postictal period, was considered in the differential diagnosis. In this case, in which the beginning stroke was considered, the treatment was shifted to postictal paralysis. The dose of levetiracetam was increased to 1000 mg BID. The patient was discharged from the hospital after it was confirmed that the seizures were not recurring and that the fetal condition was stable.
Conclusion
We believe that our case illustrates the challenges in distinguishing between TP and stroke, which have entirely distinct etiologies and courses of treatment, and that it provides important clues that help the differentiation of the two conditions.
Ethical Approval
Institutional approval was not required to publish the case details.
Consent for Publication
Written informed consent was obtained from the patient to have the case details and any accompanying images published.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
Beria Aksu Selman and Mohamed Sheikh Hassan are co-first authors of this work. The authors declare that they have no competing interests in this work.
References
1. Xu SY, Li ZX, Wu XW, Li L, Li CX. Frequency and pathophysiology of post-seizure todd’s paralysis. Med Sci Monit. 2020;26:e920751. doi:10.12659/MSM.920751
2. Binder DK. A history of Todd and his paralysis. Neurosurgery. 2004;54(2):480–487. doi:10.1227/01.NEU.0000103490.49537.37
3. Kellinghaus C, Kotagal P. Lateralizing value of Todd’s palsy in patients with epilepsy. Neurology. 2004;62(2):289–291. doi:10.1212/01.WNL.0000103287.50054.85
4. Rolak LA, Rutecki P, Ashizawa T, Harati Y. Clinical features of Todd’s post-epileptic paralysis. J Neurol Neurosurg. 1992;55(1):63. doi:10.1136/jnnp.55.1.63
5. Prado M, Fiorelli EM, Wu MA, Sandrone G, Tobaldini E. Nothing as it seems: todd’s paralysis. Int Emerg Med. 2013;8(5):425–426. doi:10.1007/s11739-013-0904-3
6. Brosinski CM. Implementing diagnostic reasoning to differentiate Todd’s paralysis from acute ischemic stroke. Adv Em Nursing J. 2014;36(1):78–86. doi:10.1097/TME.0000000000000007
7. Marashly A, Ewida A, Agarwal R, Younes K, Lüders HO. Ictal motor sequences: lateralization and localization values. Epilepsia. 2016;57(3):369–375. doi:10.1111/epi.13322
8. Blek N. How can we distinguish postictal Todd’s Paralysis from acute ischemic stroke in the prehospital and early hospital setting? J Epileptol. 2022.
9. Yacoub HA, Fenstermacher N, Castaldo J. Postictal Todd’s paralysis associated with focal cerebral hypoperfusion on magnetic resonance perfusion studies. J Vascular Interventional Neurol. 2015;8(2):32. doi:10.1212/WNL.78.1_MeetingAbstracts.P03.116
10. Hassan AE, Cu SR, Rodriguez GJ, Qureshi AI. Regional cerebral hyperperfusion associated with postictal paresis. J Vascular Interventional Neurol. 2012;5(1):40.
11. Lyman KA, Chetkovich D. New insights into postictal paresis: an epilepsy-associated phenomenon that may not be as benign as long thought. Epilepsy Currents. 2017;17(3):167–168. doi:10.5698/1535-7511.17.3.167
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.