Back to Journals » Nutrition and Dietary Supplements » Volume 9
Tocotrienol-rich mixture inhibits cell proliferation and induces apoptosis via down-regulation of the Notch-1/NF-κB pathways in NSCLC cells
Authors Rajasinghe LD , Gupta SV
Received 10 December 2016
Accepted for publication 23 February 2017
Published 15 December 2017 Volume 2017:9 Pages 103—114
DOI https://doi.org/10.2147/NDS.S129891
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chandrika J Piyathilake
Lichchavi D Rajasinghe, Smiti V Gupta
Department of Nutrition and Food Science, Wayne State University, Detroit, MI, USA
Abstract: Lung cancer is one of the leading causes of cancer deaths. Non-small cell lung cancer (NSCLC), with a 5-year survival rate of 5% at stage IIIB, accounts for 80%–85% of all lung cancers. Aberrant Notch-1 expressions have been reported in lung cancer patients and could potentially be a beneficial molecular/therapeutic target against NSCLC. Tocotrienols, isomers of vitamin E, have been shown to exhibit antitumor activity via inhibition of different signaling pathways in tumor cells. Previously, we reported that delta-tocotrienol downregulates Notch-1 via NF-κB. However, the pure isomers are presently not available in quantities required for animal or clinical studies. Therefore, the objective of this study was to investigate the interactions and effects of commercially available tocotrienols (a mixture of isomers) on the Notch-1 pathway in NSCLC, adenocarcinoma (A549) and squamous cell lung cancer (H520) cell lines. A dose-dependent decrease in all growth, cell migration, and tumor invasiveness was observed in both cancer cell lines with the addition of tocotrienols. A significant induction of apoptosis was also observed using Annexin V stain in flow cytometry analysis. Since tocotrienols significantly affected proliferation, apoptosis, migration, and invasiveness, reverse transcription polymerase chain reaction and Western blot analysis were used to explore the molecular mechanisms responsible for the regulations by testing the expression of Notch-1 and its downstream genes. A dose-dependent decrease in expression of proteins was observed in Notch-1, Hes-1, Survivin, and Bcl-XL. In addition, we found a mechanism linking the NF-κB pathway and Notch-1 down-regulation from NF-κB DNA-binding activities. Thus, our data suggest that commercially available tocotrienols inhibits cell growth, migration, and tumor cell invasiveness via downregulation of Notch 1 and NF-κB while inducing apoptosis. Hence, these commercially available tocotrienol-rich mixture could potentially be an effective supplementation for lung cancer prevention.
Keywords: vitamin E, lung cancer, tocotrienol, NF-KB, apoptosis, proliferation, Notch
Introduction
Lung cancer is one of the leading causes of death among cancers with non-small cell lung cancer (NSCLC) accounting for 87% of all lung cancer cases.1 The common types of NSCLC include squamous cell carcinoma, large cell carcinoma, and adenocarcinomas. The 5-year survival rate is only 5% at stage IIIB due to the aggressiveness of this type of cancer.1 This rate has not improved over the past 4 decades despite advances in cancer therapy treatment regimens in NSCLC.
Despite new findings in molecular pathways involved in lung cancer biology and the application of new therapeutic, NSCLC remains one of the foremost reasons for cancer deaths worldwide.2 The treatment methods currently available for lung cancer include surgery, target therapy, and different modalities of chemotherapy and radiation therapy. However, these treatment methods have not significantly impacted the 5-year survival rate of NSCLC over the past 4 decades.3 Poor survival rates are mainly attributed to late diagnosis, tumor metastasis and current chemotherapeutic drugs that are accompanied with several adverse effects, drug resistance, and recurrence among treated NSCLC patients.3 Therefore, new therapeutic modalities with minimal adverse side effects are needed to improve the treatment outcome, including better long-term survival of patients diagnosed with lung cancer.
Cell signaling transduction pathways convert environmental stimuli to changes in cell behavior and are thus central to the control of all biological processes.4 Most of the signaling pathways controlling cell growth and differentiation, including Notch, are commonly altered in various cancers. Notch is an evolutionarily conserved family of transmembrane receptors, which are connected with 5 Notch ligands. In mammals, 4 Notch receptors (Notch-1, -2, -3, and -4) and 5 ligands, including delta-like ligands 1, 3, and 4, and Jagged 1 and 2, have been identified. Once a ligand binds to the receptor, 2 proteolytic enzymes, namely ADAM metalloprotease and a presenilin–γ-secretase complex, make 2 proteolytic cleavages. Then Notch receptor releases to the Notch intracellular domain (NICD).5 The activated form of Notch, NICD, translocates to the nucleus and binds to the transcriptional repressor to induce transcription of Notch downstream target genes such as the Hes family, Hey family, nuclear factor (NF)-kB, vascular endothelial growth factor, BcL family, c-myc, and cyclin D1.6,7
The Notch transmembrane receptors and their ligands play a vital role in cancer development,8 and their dysregulation has been found to contribute to many types of human cancers,9 including NSCLC.10,11 Although the role of different Notch transmembrane receptors in NSCLC development is not completely understood, Notch-1 is considered to play a vital role in cancer development12–15 compared with other Notch transmembrane receptors. For instance, Notch-1 shows a growth-promoting function on NSCLC, whereas in SCLC, it plays a tumor-suppressive role.12 Baumgart et al13 reported that Notch-1 expression from excessive ADAM17 activities leads to subsequent regulation of the epidermal growth factor receptor expression and tumorigenicity of NSCLC cells. Overexpression of Notch-1 has also been reported to inhibit apoptosis in lung adenocarcinoma.14 Inhibiting Notch signaling by Gamma secretase inhibitors also prompted apoptosis in lung squamous cell carcinoma cells.16 Additionally, Notch-1 gene mutations are more frequently recognized than other Notch receptor genes in tumors with Notch sequencing data.17 Taken together, these reports suggest that modification of Notch-1 signaling may be a preferred beneficial therapeutic target for NSCLC.
Notch-1 has been reported to cross talk with NF-kB, which plays a major role in numerous biological processes, including cell proliferation, cell death, inflammation, apoptosis regulation, and immune response in cancer cell transformation and development.18–20 Moreover, constitutive levels of Notch activity are vital in maintaining NF-κB activity in various cell types.21 Reduced Notch expression levels in mice have been shown to significantly lower NF-κB activity.21 Therefore, Notch-1-mediated cell growth inhibition and induction of apoptosis could be partly mediated via inactivation of NF-κB activity.
Vitamin E is composed of isomers of tocopherols and their unsaturated counterparts, the tocotrienols. However, the most commercially available vitamin E supplements contain tocopherols as their key ingredient with little or no tocotrienols. Recent studies point toward the higher potency of tocotrienols in their antioxidant and antitumor properties compared with the tocopherols.22 Tocotrienol isomers, namely α, β, γ, and δ, are found naturally in cereal grains, vegetable oils, and palm oil, and have demonstrated a strong association with the prevention of cancer and inhibition of tumors, both in vitro and in vivo.23 Tocotrienols have displayed antitumor effects on different human cancer cells, including prostate, breast, colon, melanoma, and lung, via induction of apoptosis by inhibiting multiple signaling pathways, including the Notch and NF-κB pathways. Our previous study clearly showed that delta-tocotrienol inhibits NF-κB signaling pathways via downregulation of Notch-1, thereby inhibiting the proliferation, metastatic/invasive potential while inducing apoptosis of NSCLC adenocarcinoma cells in a dose-dependent manner.10,24–26 However, overall effects of tocotrienols on NSCLC are still not well understood.
Using delta-tocotrienol to treat cancer is not viable since it is difficult to isolate and expensive. Additionally, individual isomers are not currently available in quantities required for animal or clinical studies. Thus, it becomes necessary to investigate the therapeutic targets of naturally available tocotrienol-rich mixtures. The present study aims to investigate the effect of commercially available tocotrienol-rich mixture in capsules (TRMCs) extracted directly from palm oil with the working hypothesis that this treatment would inhibit NSCLC cell proliferation and induce apoptosis by inhibition of Notch-1 signaling via the NF-kB pathway.
Materials and methods
Cell culture and treatment with tocotrienols
Two different NSCLC cell lines, representing squamous cell carcinoma (H520) and adenocarcinoma (A549), were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI) medium (Mediatech, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin in 5% CO2 and 37°C. Tocotrienol-rich capsules provided by Carotino (Kuala Lumpur, Malaysia), containing 21.3% tocopherols and 78.7% tocotrienols, were used in this study. The tocotrienols in the capsule contained 26.7% α-, 3.3% β-, 38.1% γ-, and 10.6% δ-tocotrienol isomers, whereas the remainder 21.3% is composed of the α-tocopherol isomer. The media containing dimethyl sulfoxide (DMSO) (vehicle control) or different concentrations of TRMC diluted from a 100 mg/mL stock solution were used as experimental treatment media for cell culture. The final concentration of treatment media is expressed as the amount of TRMC (mg) in l mL of RPMI media (mg/mL).
Anti-proliferative effects of TRMC
The anti-proliferative effects of TRMC on NSCLC cell lines were analyzed using MTS assay. A549 and H520 cells were seeded at the density of 5×105 cells in a 96-well plate and incubated overnight. After incubation, the medium was replaced, and cells were treated with fresh medium containing <0.10% DMSO (control) and different concentrations of TRMC (treatment). After 72 hours of treatment, 20 µL of Cell Titer 96 Aqueous One Solution Reagent from Promega (Madison, WI, USA) was added to each well and incubated for 2 hours at 37°C in a humidified, 5% CO2 atmosphere. Then absorbance at 490 nm was measured using the Bio-Tek EL×800 plate reader (Winooski, VT, USA). Each variant of the experiment was performed in triplicate.
In clonogenic assay, A549 and H520 cells were seeded in a 100 mm dish at the density of 1×105 and 1×106 cells, respectively, and incubated overnight. Subsequently, culturing media were replaced with treatment (different concentrations of TRMC) and control media and then incubated for another 72 hours. The viable cells were counted by an automated cell counter (Logos Biosystems, Annandale, VA, USA), and 2000 cells were transferred per 100 mm dishes with 10 mL growing media. Then, cells were allowed to grow for 25 days at 37°C in a 5% CO2 incubator. After subsequent incubation, all the colonies were fixed in 4% paraformaldehyde and stained with 2% crystal violet.
Cell death detection
Cell death detection histone/deoxyribonucleic acid (DNA) enzyme-linked immunosorbent assay (ELISA) Kit from Roche (Palo Alto, CA, USA) was used to detect apoptosis in NSCLC cells. A549 and H520 cells were seeded into 6-well plates at the density of 1×105 and 1×106 cells, respectively. After an overnight incubation, cells were treated with control medium or treatment medium (different concentrations of TRMC) for 72 hours. Cytoplasmic histone/DNA fragments were extracted from lysed cell extract and incubated in microtiter plate modules coated with anti-histone antibody. Next, peroxidase-conjugated anti-DNA antibody was used to detect the immobilized histone/DNA fragment. Bound antibodies were detected by the intensity of color development in microtiter plate modules, after washing with 2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid) substrate. The absorbance of the samples was measured at 405 nm using the Bio-Tek EL×800 plate reader.
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (BD Biosciences, San Jose, CA, USA) was used for apoptosis analysis. A549 and H520 cells were incubated in the control or treatment (0.6 mg/mL concentration of TRMC) medium for 72 hours. After that, cells were extracted by scraping and collected with ice-cold PBS. Then, cells were spun down and resuspended in 1X binding buffer at a concentration of 105/mL cells in a total volume of 100 µL. Subsequently, 5 µL of Annexin V-FITC and 5 µL of propidium iodide (PI) were added. All cells were kept in the dark for 20 minutes at room temperature. Finally, 400 µL of 1X binding buffer was then added to each tube, and the number of apoptotic cells was analyzed by flow cytometry (BD Biosciences).
Cell migration assay
A549 and H520 cells were seeded in a 6-well plate at the density of 2×105 and 1×106 cells per well, respectively. After the cells had been incubated for 36 hours, the media was removed, and a scratch wound across each well was made using a 100 µL pipette tip. All the wound areas were washed with PBS 3 times to ensure that no loosely held cells were attached. The width of the scratch was imaged and measured by a Nikon H 600 L microscope connected to the camera at five places along the scratch. Subsequently, the cells were cultured in control or treatment medium (different concentrations of TRMC) for 30 hours. Then, the width of the scratch was reimaged and measured to find the progress of cells that had migrated into the wound.
Cell invasive assay
The tumor invasive ability in the aforementiond cell lines was assessed by BD BioCoat Matrigel Invasion Chamber (BD Biosciences). A549 cells 2.5×105 and H520 5×105 were seeded with basal media in each 6-well upper chamber in the presence or absence of treatment media (different concentrations of TRMC). In the meantime, 3 mL of culture medium with 10% FBS was added to each lower chamber of the 6-well plate. After a 20-hour incubation, the cells in the upper chamber were removed using a cotton swab. Then cells were fixed in 4% paraformaldehyde and stained with 2% crystal violet. Then, cell unbound crystal violet was washed with PBS before they became dry. After that, the stained crystal violet (cell bound) was washed with 20% acetic acid, and then the absorbance of the dissolved crystal violet was measured at 405 nm using the Bio-Tek EL×800 plate reader. Each experimental condition was performed in triplicate.
Quantitative real-time polymerase chain reaction (qRT-PCR) for gene expression analysis
One million A549 and H520 cells were seeded in 100 mm dish per plate and incubated for 24 hours. Subsequently, culturing medium was replaced with treatment (different concentrations of TRMC) or control medium and then incubated for another 48 hours. Total RNA was isolated using RNeasy Mini Kit from QIAGEN (Valencia, CA, USA) according to the manufacturer’s protocols. 1000 ng of total RNA from each sample was subjected to the first-strand complementary DNA (cDNA) synthesis using High-Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA, USA) in a total volume of 50 µL.
qRT-PCR was performed to explore the Notch-1 expression. Diluted cDNA (2 µL) and 2 µL each of reverse primer (5′-GTT GTA TTG GTT CGG CAC CAT-3′) and forward primer ( 5′-CAC TGT GGG CGG GTC C-3′), and 12.5 µL of master mix (SYBR GREEN PCR Master Mix; Applied Biosystems, Warrington, UK) were used in each 25 µL of PCR reactions performed in Eppendorf Master Cycler RealPlex 4 (Eppendorf, Hauppauge, NY, USA) at 25°C for 10 minutes, followed by 48°C for 30 minutes and 95°C for 5 minutes. Expression values were normalized with a β-actin (sense [5′-ACCAACTGGGACGACATGGAGAAG-3′]; antisense [5′-TACGACCAGAGGCATACAGGGACT-3′]). Each gene expression was tested in triplicate.
Western blot for protein expression analysis
Western blot analysis was performed as part of a protein expression analysis using the following antibodies: poly (ADP-ribose) polymerase (PARP), β-actin, Survivin, Bcl-XL and Notch-1 (Cell Signaling Technology, Danvers, MA, USA) in cell signaling pathways. One million A549 and H520 cells were seeded in a 100 mm dish per plate and incubated for 24 hours. Then cells were treated for 72 hours with treatment (different concentrations of TRMC) and control media and incubated for 72 hours. Cells were lysed in the cold 1X cell lysis buffer (Cell Signaling Technology) for 30 minutes on ice with 1X Protease inhibitor (Cell Signaling Technology). Then protein concentrations were calculated by using Pierce BSA Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Subsequently, 50 mg of total cell lysates were mixed with equal amounts of 4X lemma buffer (Bio-Rad Laboratories), and samples were loaded on 10% sodium dodecyl sulfate -polyacrylamide gel electrophoresis. After electrophoresis, the gel electrophoretically was transferred to a polyvinylidene difluoride (Trans-Blot Turbo Mini PVDF system; Bio-Rad Laboratories) using Trans-Blot® Turbo™ Transfer System (Holliston, MA, USA). The membranes were incubated for 2 hours at room temperature with 5% Casein. After that, membranes were incubated overnight at 4°C with primary antibodies (1: 1000–4000). The membranes were washed 3 times with Tris-buffered saline with Tween 20 and subsequently incubated with the secondary antibodies (1:5000) containing 2% bovine serum albumin for 2 hours at room temperature. The signal intensity was then measured by a chemiluminescent imager with ChemiDoc XRS (Bio-Rad Laboratories).
NF-κB filter plate assay for measuring NF-κB DNA-binding activity
NF-κB filter plate assay kit was obtained from Signosis (Sunnyvale, CA, USA) and used to determine the NF-κB DNA-binding ability of each sample. A549 and H 520 cells were seeded in Petri dishes and incubated for 24 hours. Cells were then treated with or without different concentrations of TRMC. After 72 hours of treatment, cells were collected and washed, and nuclear protein extraction was performed with a NE-PER® Nuclear and Cytoplasmic Extraction reagent kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocols.
Protein concentrations were determined using the Pierce BCA protein assay kit (Rockford, IL, USA). Standard samples were prepared according to the manufacture’s protocol. The absorbance of both standards and samples was measured at 562 nm using a UV-1800 spectrophotometer from Shimadzu Scientific Instruments (Kyoto, China). The assay was conducted according to the protocol using a biotin-labeled DNA sequence of NF-κB mixed with 3 µg of nuclear extract to form an NF-κB-DNA binding complex. For each sample, 10 µL TF binding buffer mix, 2 µL NF-κB probe, 3 µg of nuclear protein extract and distilled water was added to bring the total volume up to 20 µL. A filter plate was used to retain bound NF-κB probe, while the unbound NF-κB probe was filtered out. The bound, prelabeled NF-κB probe was then eluted from the filter, collected, and transferred to a hybridization plate for quantitative analysis. NF-κB probe was further detected using streptavidin- horseradish peroxidase, and luminescence of the probe was measured using an Ultra Multifunctional Microplate Reader from Tecan (Vienna, VA, USA).
Data analysis
Significant differences between treatment and control groups were analyzed using a 1-way analysis of variance (Chris Rorden’s ezANOVA for windows, version 0.98 ). Values of P<0.05 were considered statistically significant.
Results
Anti-proliferative effect of tocotrienols on A549 and H520 cells
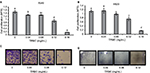
To evaluate and compare the cell viability and proliferative effects after in vitro exposure of tocotrienols, MTS and the traditional clonogenic assays were performed. Results from the MTS assay showed a dose-dependent decrease in cell growth and proliferation for both A549 and H520 cells. A549 cells with treatment of 0.04, 0.08, 0.12, and 0.16 mg/mL concentrations of TRMC demonstrated a 13%, 15%, 38%, and 88% cell growth inhibition, respectively, relative to control, after 72 hours incubation (Figure 1A). Similarly, the H520 cell line with treatment of 0.04, 0.08, 0.12, and 0.16 mg/mL concentrations of TRMC also exhibited a 0%, 12%, 33%, and 84% cell growth inhibition relative to control, under the same conditions, respectively (Figure 1B). Inhibition of cell growth was significant at every concentration for A549 cells, while for H520 cells, inhibition was significant at a concentration of ≥0.08 mg/mL of TRMC.
The clonogenic assay was performed to investigate the enduring proliferative effect of tocotrienols. Exposure of TRMC on A549 and H520 cells for 72 hours irreversibly inhibited 80% clonogenic growth compared with untreated cells (Figure 1C and D). For both cell lines, colony formation was greatly reduced at 0.12 mg/mL of TRMC. In this study, there were similar trends in both MTS and clonogenic assays, suggesting that the available mixture of tocotrienols in the commercially produced capsules significantly inhibited the growth of NSCLC cells.
Tocotrienols induce apoptosis in lung cancer cell lines
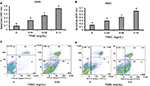
Histone/DNA ELISA assay and Annexin V/PI staining were used to evaluate the apoptotic effects of TRMC on A549 and H520 cells. Results from ELISA showed significantly increased apoptosis with increased concentration of TRMC on A549 cells and H520 cell lines (Figure 2A and B). To further confirm the results from our histone ELISA data, flow cytometry-based quantification was performed after Annexin V/PI staining. Quantitation of apoptotic cells from flow cytometry analysis after treatment with 0.06 mg/mL of TRMC for 72 hours showed increased apoptosis in both cell lines (Figure 2C and D). Thus, it is evident that tocotrienols caused a statistically significant increase in the percentage of apoptotic cells in lung cancer cell lines.
Inhibition of cell invasion and migration by tocotrienols
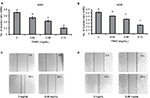
The effect of TRMC on tumor cell invasion and migration was evaluated using Matrigel invasion and wound-healing assays. TRMC concentrations (0.4–0.12 mg/mL) resulted in a significantly decreased penetration of lung cancer cells through the Matrigel-coated membrane as compared to the control cells (Figure 3A and B), confirming that TRMC reduced the invasion capacity of lung cancer cells. For further confirmation of anti-migratory effects of TRMC, the wound-healing assay was performed. The results of the wound-healing assay revealed that there was reduction in cell migration from custom-made wounds with 0.8 mg/mL of TRMC after 30 hours of incubation (Figure 3C and D). In contrast, there was a significant wound healing in the control cells without TRMC, under the same incubation conditions.
Downregulation of the Notch-1 and its target gene expressions by tocotrienols
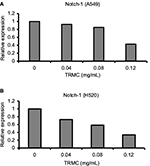
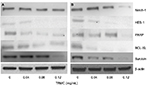
Investigations of molecular mechanisms behind the ability of tocotrienols to inhibit cell growth, cell invasion, and migration and induce apoptotic cell death in NSCLC cells were evaluated using RT-PCR and Western blot analysis. A significant TRMC dose-dependent decrease in Notch-1 mRNA expressions was seen in A549 and H520 cells after incubating for 48 hours (Figure 4A and B). Moreover, results from protein expressions in Notch-1 downstream genes, namely HES 1, BcL-XL, and Survivin, PARP, showed a dose-dependent decrease with TRMC in Western blot analysis (Figure 5).
Inhibition of NF-κB DNA-binding activity with tocotrienols
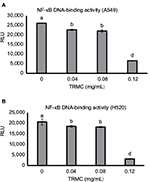
NF-κB and Notch pathways have shown cross talk in many types of cancers, including lung cancer. Thus, we explored whether the downstream effect of Notch-1 downregulation was mechanistically linked to the NF-κB pathway. Nuclear proteins from treated and control A549 and H520 cells were analyzed for NF-κB DNA-binding activity as measured by the NF-κB filter plate assay. As demonstrated in Figure 6A and B, compared with the control, TRMC significantly inhibited the DNA-binding activity of NF-κB for both cell lines.
Discussion
Notch signaling is reported to play important roles in regulating cancer cell proliferation, differentiation, invasion, and apoptosis.21,27–29 Aberrant expression of Notch has been reported in many types of cancer, including pancreatic, colon, lung, cervical, breast, and skin cancers.30–35 Variable expression levels of Notch-1 were observed in a clinical study. High Notch-1 expression in some NSCLC patients was found to be associated with a later TNM stage in histological grading,11 suggesting that Notch-1 may play key roles in the advancement of NSCLC. Interestingly, PCR and Western blot data from our study clearly demonstrated that TRMC targeted and dose-dependently inhibited the expression of Notch-1 in A549 and H520 cell lines. In our previous studies, we established that delta tocotrienol inhibited cell proliferation by impeding different therapeutic targets, including Notch-1.10,24–26 Similarly, we observed that TRMC inhibited the cell proliferation in a dose-dependent manner in MTS and clonogenic assay along with Notch-1 inhibition, suggesting that expression of Notch-1 with TRMC may prevent the expansion of A549 and H520 cells. Therefore, TRMC could potentially provide a Notch-1 target-based therapeutic method in preventing advancement of NSCLC.
Furthermore, earlier studies have shown that blockage of the Notch pathway using γ-secretase inhibitor suppressed osteosarcoma growth in vitro and in vivo.36,37 Preclinical studies have also shown the therapeutic efficacy of Notch inhibitors against NSCLC.38 Stabilized peptides was another approach that interfered with receptor/ligand interactions in Notch signaling pathway.39 Although these approaches have shown potential in inhibiting Notch activations, their inhibitory potential has not been evaluated at a clinical level, warranting the importance of exploring novel natural Notch-1 inhibitors with minimal side effects. In this study, we used TRMC, which is directly isolated from palm oil with minimal processing. Tocotrienols have been widely consumed by humans for a long time, and now it is recognized as a safe substance under US Food and Drug Administration regulations.40 Thus, our approach using TRMC as an inhibitor of Notch-1 expression could be a promising strategy to achieve better treatment outcome with minimal side effects for NSCLC patients.
We further observed that TRMC dose-dependently inhibited the HES-1 expressions in A549 and H520 cell lines. It is well documented that Hes-1 is a transcriptional target of the Notch signaling pathway,41 suggesting TRMC dose- dependently inhibits the Notch-1 pathway in a downstream manner. Further insight into the molecular mechanism of Notch-1 pathway and its target genes, Notch-1/Hes-1 pathways have been reported to be upstream to NF-κB activation in lung cancer and leukemia cells.10,42 According to the current evidence, Notch ligands induced NF-κB activation in leukemia cells and decreased Notch-1 lowered NF-κB DNA-binding activity.43 Another study also reported that mice with reduced Notch activities had a significantly decreased NF-κB activity.44 Schwarzer et al reported Notch had exerted its effects through regulation of NF-κB in human lymphomas.45 Our previous study also demonstrated the cross talk between the Notch-1 pathway and the NF-κB pathway in adenocarcinoma lung cancer cell lines, which were induced by delta tocotrienols.10,24–26 In this study, the results from NF-κB filter plate assay clearly showed a dose-dependent decrease in NF-κB DNA-binding activity in A549 and H520 cells with increased concentrations of TRMC. NF-κB is located at the junction of multiple pathways involved in cell proliferation, survival, and invasion. Inhibition of the key molecule, NF-κB activity by TRMC, reinforces its potential impact as an anticancer agent. The effect of TRMC on the expressions of BCL-2 and Survivin, downstream target genes of NF-κB, responsible for apoptosis,46 were evaluated by Western blot analysis . As shown in Figure 5, the expressions of BCL-2 and Survivin in both A549 and H520 cell lines were significantly inhibited with treatment of TRMC. These results clearly establish that TRMC inhibited NF-κB activity and its target protein expressions, namely BCL-2 and Survivin. Simultaneous inhibition of Notch, NF-κB activity, and NF-κB target proteins such as BCL-2 and Survivin implies that an inhibitory effect passes through Notch-1 to NF-κB downstream target genes via NF-κB.
The NFκB filter assay was used to monitor the activity of NF-κB. In the assay, biotin-labeled DNA-binding sequence of NF-κB was mixed with nuclear extract to allow the formation of NFκB-DNA complex. A filter plate was used to retain the bound NF-κB probe and remove the free DNA probe. The bound prelabeled NFκB probe was then eluted from the filter and hybridized to the hybridization plate for quantitative analysis using a luminometer. The results clearly demonstrate that the reduced binding is not due to the interference of the binding affinity between NF-κB and DNA in the complex. It is, in fact, due to the downstream effect of Notch-1 signaling passed via NF-κB activation to its target genes. Therefore, our data alone and in conjunction with current evidence, strongly support that TRMC inhibits the Notch-1-mediated NF-κB pathway.
TRMC induced apoptosis in A549 and H520 cells, dose dependently in this study. The TRMC consisted of α-tocotrienol, β-tocotrienol, and γ-tocotrienol and the δ-tocotrienol isomers. Some studies have shown that γ-tocotrienol induced apoptosis in neuroblastoma SH-SY5Y cells47 and human gastric cancer cells.48 In our previous study, we clearly showed that delta-tocotrienol induced apoptosis in NSCLC in a dose- and time-dependent manner at 10–30 µM concentrations. In another study, γ- and δ-tocotrienols exerted a more potent anticancer effect on breast cancer cell lines compared with α-tocotrienol.49 Numerous results from recent studies on tocotrienols also indicated that γ- and δ-tocotrienols exhibited greater anticancer activity than α- or β-tocotrienols, whereas δ-tocotrienol shows a higher efficacy and effectiveness in the induction of apoptosis in both A549 and U87MG cancer cells compared with α- and γ-tocotrienols.50 Therefore, induction of apoptosis in A549 and H520 cells with be either the result of individual γ- and δ-tocotrienol isomers or their cumulative effects.
Bcl-2 and Bcl-XL inhibitor proteins play a significant role in apoptosis.51,52 We observed a dose-dependent decrease in Bcl-XL protein expression with TRMC in Western blot analysis. Also, we found inhibition of Survivin protein with TRMC in Western blot analysis where Survivin, a member of the inhibitor of apoptosis, inhibited caspase activation, thereby leading to negative regulation of apoptosis.53 Moreover, a downregulation of Notch-1 was observed to decrease Bcl-XL apoptosis protein expression in pancreatic cancer cells. In breast cancer, downregulation of Notch-1 is associated with the lower expression of Bcl-2 and Bcl-XL.54 Activated Notch-1 pathway can increase the expression of Survivin expression.55 Furthermore, Survivin and Bcl-XL are downstream targets of the NF-κB in several cancer cells. In addition, in our previous study, we clearly showed δ-tocotrienol inhibited Bcl-XL, PARP, and Survivin in NSCLC in a dose-dependent manner at 10–30 µM concentrations. Consistent with aforementioned results, we suggest that TRMC inhibits Survivin, Bcl-2, and Bcl-XL via downregulation of Notch-1 and NF-κB while inducing apoptosis in NSCLC.
In this study, we observed that TRMC is capable of repressing both the mRNA and protein levels of Notch-1, and therefore, the downregulated levels of this protein are likely due to the repressed levels of its mRNA. Therefore, we looked into Notch-regulatory machinery to get a better understanding of Notch regulation. In lung cancer, the deregulation of the Notch is primarily associated with activating missense mutations mostly in ligand-binding domain (EGF repeats 11 and 12) or the ankyrin domains that lead to a ligand-independent activation.50 In addition, as a key component of the Notch-mediated transcription complex, Notch can regulate the expression of a number of microRNAs; at the same time, Notch ligands, Notch receptors, or Notch effectors are also subject to regulation by microRNAs. For instance, miR-34a decreased the expression of Notch-1 and its downstream targets, including Hes-1, cyclin D1, Survivin, and Bcl-2, impairing Notch signaling, cell proliferation, and invasion and inducing apoptosis in NSCLC cells.61 Epigenetic mechanism in Notch expression regulation has not been well studied. Therefore, it is very important to investigate the effect of TRMC on ligand-binding domains, ankyrin domains, and microRNAs as future directions.
One of the limitations of this study was that cell culture experiments were performed at hyperoxic conditions (20% oxygen). Some human solid tumors, including NSCLC, develop the capability to grow in hypoxic conditions due to poor microcirculation within the tumor mass,56,57 and these conditions control its growth and survival56 by regulating transcriptional induction of genes involved in glycolysis, hematopoiesis, angiogenesis, apoptosis, and tissue invasion.58 For instance, aberrant Notch-1 expression also exhibited tumor promotion under hypoxic conditions in lung cancer.59 The hyperoxic condition in these experiments may have an impact on the effective tocotrienol concentration and regulatory mechanism. Similarly, some studies showed that the effect of tocotrienols was found to be more potent under hypoxic than under normoxic conditions in cancer treatment.60 Therefore, it may be useful to perform some experiments under hypoxic conditions before proceeding with in vivo studies. Moreover, bioavailability is always a potential concern for all nutraceuticals. Although in vitro experimental evidence has been very promising, oral supplementation of tocotrienols in animal and human studies has produced varying results.61 Oral absorption of tocotrienols into the circulation is mediated by a carrier transporter system that displays saturation and downregulation when exposed to high concentrations of tocotrienols.10,61 To compensate for these limitations in oral absorption of tocotrienols, investigators have developed new derivatives and nanoparticle delivery systems that significantly enhance tocotrienol bioavailability and, therefore, the therapeutic effects of tocotrienols on cancer.61 In addition to bioavailability, timing and dosage are also concerns, and these factors will be different for cell cultures versus animals versus humans. Further experiments need to be conducted to investigate whether this capsule can show the same results in animals before it can be taken to a human trial, which is the ultimate goal.
Conclusion
Treatment with the tocotrienol mixture resulted in a dose-dependent and significant decrease in cell growth, cell migration, tumor invasiveness, and induction of apoptosis. Mechnistically, a dose-dependent decrease in the expression was observed in Notch-1 and its downstream target Hes-1. In addition, apoptosis-related proteins, namely, Survivin, PARP, Bcl 2, and Bcl-XL, were found to be downregulated. Survivin and Bcl-2 are directly affected by NF-kB, whose activity was decreased with added tocotrienols as well. Synchronized inhibition of Notch-1, NF-κB activity, and NF-κB target proteins indicates that an inhibitory effect passes through Notch-1 to NF-κB and its downstream target genes. Taken together, our data support the potential use of TRMC as a therapeutic agent for treating NSCLC.
Acknowledgment
The authors thank Professor Pramod Khosla for providing the tocotrienol-rich capsules.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Saintigny P, Burger JA. Recent advances in non-small cell lung cancer biology and clinical management. Discov Med. 2012;13(71):287–297. | ||
Wangari-Talbot J, Hopper-Borge E. Drug resistance mechanisms in non-small cell lung Carcinoma. J Can Res Updates. 2013;2(4):265–282. | ||
Kolch W, Halasz M, Granovskaya M, Kholodenko BN. The dynamic control of signal transduction networks in cancer cells. Nat Rev Cancer. 2015;15(9):515–527. | ||
Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393(6683):382–386. | ||
Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66(10):1631–1646. | ||
Xiao YF, Yong X, Tang B, et al. Notch and Wnt signaling pathway in cancer: crucial role and potential therapeutic targets (Review). Int J Oncol. 2016;48(2):437. | ||
Miele L. Notch signaling. Clin Cancer Res. 2006;12(4):1074–1079. | ||
Hansson EM, Lendahl U, Chapman G. Notch signaling in development and disease. Semin Cancer Biol. 2004;14(5):320–328. | ||
Ji X, Wang Z, Geamanu A, Sarkar FH, Gupta SV. Inhibition of cell growth and induction of apoptosis in non-small cell lung cancer cells by delta-tocotrienol is associated with notch-1 down-regulation. J Cell Biochem. 2011;112(10):2773–2783. | ||
Jin MM, Ye YZ, Qian ZD, Zhang YB. Notch signaling molecules as prognostic biomarkers for non-small cell lung cancer. Oncol Lett. 2015;10(5):3252–3260. | ||
Guo H, Lu Y, Wang J, et al. Targeting the Notch signaling pathway in cancer therapeutics. Thorac Cancer. 2014;5(6):473–486. | ||
Baumgart A, Seidl S, Vlachou P, et al. ADAM17 regulates epidermal growth factor receptor expression through the activation of Notch1 in non-small cell lung cancer. Cancer Res. 2010;70(13):5368–5378. | ||
Wang G, Xu Z, Wang R, et al. Genes associated with MUC5AC expression in small airway epithelium of human smokers and non-smokers. BMC Med Genomics. 2012;5:21. | ||
Li Y, Burns JA, Cheney CA, et al. Distinct expression profiles of Notch-1 protein in human solid tumors: implications for development of targeted therapeutic monoclonal antibodies. Biologics. 2010;4:163–171. | ||
Cao H, Hu Y, Wang P, Zhou J, Deng Z, Wen J. Down-regulation of Notch receptor signaling pathway induces caspase-dependent and caspase-independent apoptosis in lung squamous cell carcinoma cells. APMIS. 2012;120(6):441–450. | ||
Sparaneo A, Fabrizio FP, Muscarella LA. Nrf2 and Notch signaling in lung cancer: near the crossroad. Oxid Med Cell Longev. 2016;2016:7316492. | ||
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–348. | ||
Kasibhatla S, Tseng B. Why target apoptosis in cancer treatment? Mol Cancer Ther. 2003;2(6):573–580. | ||
Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45(6):355–361. | ||
Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol Cancer Ther. 2006;5(3):483–493. | ||
Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–261. | ||
Zarogoulidis P, Cheva A, Zarampouka K, et al. Tocopherols and tocotrienols as anticancer treatment for lung cancer: future nutrition. J Thorac Dis. 2013;5(3):349–352. | ||
Ji X, Wang Z, Sarkar FH, Gupta SV. Delta-tocotrienol augments cisplatin-induced suppression of non-small cell lung cancer cells via inhibition of the Notch-1 pathway. Anticancer Res. 2012;32(7):2647–2655. | ||
Rajasinghe L, Pindiprolu R, Razalli N, Wu Y, Gupta S. Delta tocotrienol inhibits MMP-9 dependent invasion and metastasis of Non-Small Cell Lung Cancer (NSCLC) cell by suppressing Notch-1 mediated NF-κb and uPA pathways. FASEB J. 2015;29(Suppl 1):752.718. | ||
Rajasinghe LD, Gupta SV. Delta tocotrienal inhibit mTOR pathway by modulating glutamine uptake and transporters in non-small cell lung cancer. FASEB J. 2016;30(Suppl 1):688.616–688.616. | ||
Leong KG, Gao WQ. The Notch pathway in prostate development and cancer. Differentiation. 2008;76(6):699–716. | ||
Maraver A, Fernandez-Marcos PJ, Cash TP, et al. NOTCH pathway inactivation promotes bladder cancer progression. J Clin Invest.125(2):824–830. | ||
Greife A, Jankowiak S, Steinbring J, et al. Canonical Notch signalling is inactive in urothelial carcinoma. BMC Cancer. 2014;14(1):628. | ||
Baker AT, Zlobin A, Osipo C. Notch-EGFR/HER2 bidirectional crosstalk in breast cancer. Front Oncol. 2014;4:360. | ||
Connolly K, Manders P, Earls P, Epstein RJ. Papillomavirus-associated squamous skin cancers following transplant immunosuppression: one Notch closer to control. Cancer Treat Rev. 2014;40(2):205–214. | ||
Knudsen ES, O’Reilly EM, Brody JR, Witkiewicz AK. Genetic diversity of pancreatic ductal adenocarcinoma and opportunities for precision medicine. Gastroenterology. 2016;150(1):48–63. | ||
Damaskos C, Karatzas T, Kostakis ID, Nikolidakis L, Kostakis A, Kouraklis G. Nuclear receptors in pancreatic tumor cells. Anticancer Res. 2014;34(12):6897–6911. | ||
Bertrand FE, Angus CW, Partis WJ, Sigounas G. Developmental pathways in colon cancer: crosstalk between WNT, BMP, Hedgehog and Notch. Cell Cycle. 2012;11(23):4344–4351. | ||
Tan X, Apte U, Micsenyi A, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129(1):285–302. | ||
Tanaka M, Setoguchi T, Hirotsu M, et al. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br J Cancer. 2009;100(12):1957–1965. | ||
Engin F, Bertin T, Ma O, et al. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum Mol Genet. 2009;18(8):1464–1470. | ||
Purow B. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol. 2012;727:305–319. | ||
Lin L, Mernaugh R, Yi F, Blum D, Carbone DP, Dang TP. Targeting specific regions of the Notch3 ligand-binding domain induces apoptosis and inhibits tumor growth in lung cancer. Cancer Res. 2010;70(2):632–638. | ||
FDA. Agency Response Letter GRAS Notice No. GRN 000307; 2016. Available from: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm209856.htm. Accessed February 1, 2017. | ||
Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377(6547):355–358. | ||
Espinosa L, Cathelin S, D’Altri T, et al. The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer Cell. 2010;18(3):268–281. | ||
Xu X, Zhao Y, Xu M, et al. Activation of Notch signal pathway is associated with a poorer prognosis in acute myeloid leukemia. Med Oncol. 2011;28(1):483–489. | ||
Wang Y, Chan SL, Miele L, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101(25):9458–9462. | ||
Schwarzer R, Dorken B, Jundt F. Notch is an essential upstream regulator of NF-kappaB and is relevant for survival of Hodgkin and Reed-Sternberg cells. Leukemia. 2012;26(4):806–813. | ||
Zhang M, Yang J, Li F. Transcriptional and posttranscriptional controls of survivin in cancer cells: novel approaches for cancer treatment. J Exp Clin Cancer Res. 2006;25(3):391–402. | ||
Tan JK, Then SM, Mazlan M, Raja Abdul Rahman RN, Jamal R, Wan Ngah WZ. Gamma-tocotrienol acts as a BH3 mimetic to induce apoptosis in neuroblastoma SH-SY5Y cells. J Nutr Biochem. 2016;31:28–37. | ||
Sun W, Wang Q, Chen B, Liu J, Liu H, Xu W. Gamma-tocotrienol-induced apoptosis in human gastric cancer SGC-7901 cells is associated with a suppression in mitogen-activated protein kinase signalling. Br J Nutr. 2008;99(6):1247–1254. | ||
Pierpaoli E, Viola V, Pilolli F, Piroddi M, Galli F, Provinciali M. Gamma- and delta-tocotrienols exert a more potent anticancer effect than alpha-tocopheryl succinate on breast cancer cell lines irrespective of HER-2/neu expression. Life Sci. 2010;86(17–18):668–675. | ||
Lim SW, Loh HS, Ting KN, Bradshaw TD, Zeenathul NA. Cytotoxicity and apoptotic activities of alpha-, gamma- and delta-tocotrienol isomers on human cancer cells. BMC Complement Altern Med. 2014;14:469. | ||
Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80(11):1613–1631. | ||
Pandey MK, Prasad S, Tyagi AK, et al. Targeting cell survival proteins for cancer cell death. Pharmaceuticals. 2016;9(1):11. | ||
Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16:49. | ||
Pan H, Zhou W, He W, et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-kappaB activity via the Notch-1 pathway. Int J Mol Med. 2012;30(2):337–343. | ||
Chen Y, Li D, Liu H, et al. Notch-1 signaling facilitates survivin expression in human non-small cell lung cancer cells. Cancer Biol Ther. 2011;11(1):14–21. | ||
Harris AL. Hypoxia [mdash] a key regulatory factor in tumour growth. Nature Rev Cancer. 2002;2(1):38–47. | ||
Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Rev Cancer. 2004;4(6):437–447. | ||
Denko NC, Fontana LA, Hudson KM, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22(37):5907–5914. | ||
Chen Y, De Marco MA, Graziani I, et al. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007;67(17):7954–7959. | ||
Shibata A, Nakagawa K, Tsuduki T, Miyazawa T. δ-Tocotrienol treatment is more effective against hypoxic tumor cells than normoxic cells: potential implications for cancer therapy. J Nutr Biochem. 2015;26(8):832–840. | ||
Sylvester PW, Kaddoumi A, Nazzal S, El Sayed KA. The value of tocotrienols in the prevention and treatment of cancer. J Am Coll Nutr. 2010;29(Suppl 3):324S–333S. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.