Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
TKI-resistant ALK-rearranged lung adenocarcinoma with secondary CTNNB1 p.S45V and tertiary ALK p.I1171N mutations
Authors Ouseph MM, Taber A , Khurshid H, Madison R , Aswad BI, Resnick MB, Yakirevich E, Ali SM, Patel NR
Received 16 April 2019
Accepted for publication 4 June 2019
Published 15 August 2019 Volume 2019:10 Pages 81—86
DOI https://doi.org/10.2147/LCTT.S212406
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Madhu M Ouseph,1 Angela Taber,2 Humera Khurshid,3 Russell Madison,4 Bassam I Aswad,1 Murray B Resnick,1 Evgeny Yakirevich,1 Siraj M Ali,4 Nimesh R Patel1
1Department of Pathology, Rhode Island Hospital and Alpert Medical School at Brown University, Providence, RI 02903, USA; 2Division of Medical Oncology, Miriam Hospital and Alpert Medical School at Brown University, Providence, RI 02906, USA; 3Division of Medical Oncology, Rhode Island Hospital and Alpert Medical School at Brown University, Providence, RI 02903, USA; 4Foundation Medicine, Inc., Cambridge, MA 02141, USA
Abstract: Anaplastic lymphoma kinase (ALK)-rearranged non-small cell lung cancer (NSCLC) is an important molecular subgroup of tumors that are typically sensitive to tyrosine kinase inhibitors (TKIs). Although a substantial portion of patients benefit from TKIs, this approach is complicated by intrinsic and acquired resistance. We report a patient with ALK-rearranged NSCLC who showed an initial response to targeted therapy, but developed resistance to multiple TKIs. Serial comprehensive genomic profiling (CGP) was performed at four independent points during the clinical course. We review the pathology and clonal progression of the tumor, with CGP identifying a secondary CTNNB1 p.S45V mutation after the initiation of targeted therapy, followed by tertiary ALK p.I1171N. The presence of an alteration in a second oncogenic driver gene suggests a possible mechanism for resistance, and a secondary therapeutic target. Due to the involvement of Wnt signaling in the pathogenesis of many tumors and its association with immune evasion, a variety of therapeutic strategies are being developed to target this pathway. This case exemplifies the challenges of targeted therapeutics in the face of tumor progression, as well as the increasing role of genomics in understanding tumor biology.
Keywords: cancer, genomics, profiling, beta-catenin, resistance
Introduction
Anaplastic lymphoma kinase (ALK) gene-rearrangement is seen in 2–7% of non-small cell lung cancer (NSCLC).1 Constitutive kinase activity leads to activation of cellular pathways involved in cell growth and proliferation that drive tumorigenesis. This characterizes a molecular subgroup of NSCLC, with other oncogenic drivers considered mutually exclusive. Tyrosine kinase inhibitors (TKIs) are now first-line therapy for such tumors. Although a substantial portion of patients benefit from TKIs, this approach is complicated by intrinsic and acquired resistance.2,3 We report a patient with ALK-rearranged NSCLC who showed an initial response to targeted therapy, but developed resistance to multiple TKIs. Serial comprehensive genomic profiling (CGP) revealed a secondary CTNNB1 p.S45V mutation after the initiation of targeted therapy, followed by tertiary ALK p.I1171N, suggesting possible mechanisms for resistance. Written informed consent was provided by the patient to have the case details published. Institutional approval was not required to publish the case details.
Case presentation
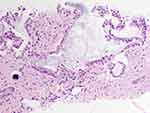
A 59-year-old female with passive smoke exposure presented in October 2015 with progressive cough. She had a family history of lung cancer (mother diagnosed at the age of 78 years; and paternal grandfather); urinary bladder cancer (mother diagnosed at the age of 74 years; maternal uncle at the age of 82 years; and maternal grandfather at the age of 80 years); colon cancer (father diagnosed at the age of 62 years); salivary gland cancer (maternal uncle); and pituitary adenoma (sister at the age of 45 years). A computed tomography scan revealed a 3.7 cm left lower lobe lung lesion. Biopsy and fine needle aspirate of the lung mass demonstrated moderately differentiated adenocarcinoma (Figure 1). Initially, no somatic mutations were identified by a 26-gene mass spectrometry-based mutation panel on the Agena MassARRAY (Agena Bioscience; San Diego, CA, USA). Fluorescence in situ Hybridization (FISH) was negative for ROS1-rearrangement (ROS1 testing performed at Quest Diagnostics; Chantilly, VA, USA) and did not meet validated criteria for ALK-rearrangement. Consequently, the patient was initiated on carboplatin, paclitaxel, and bevacizumab for Stage IV (pT2aN3M1a) disease.
Subsequent CGP by FoundationOne (Foundation Medicine, Inc.; Cambridge, MA, USA) demonstrated EML4-ALK fusion and RICTOR amplification. To resolve the discrepancy between CGP and FISH, ALK immunohistochemistry (Novocastra 5A4; Leica Biosystems; Buffalo Grove, IL, USA) was performed to assess for protein expression; testing was positive, revealing increased ALK protein expression (Figure 2). The patient remained stable on four cycles of chemotherapy, then transitioned to targeted therapy with crizotinib 250 mg twice per day (BID) in January 2016.
After an initial clinical response to crizotinib therapy, the disease clinically progressed with brain metastases in June 2016. Repeat CGP on pleural fluid from June 2016 (while on crizotinib) again showed EML4-ALK fusion, but also a secondary CTNNB1 p.S45V mutation, which was not identified in the original sample. Of note, this sample showed copy gain in the RICTOR gene, but at a level below the threshold for reporting as amplification. The patient was switched to alectinib 600 mg BID in July 2016 with a positive clinical response. However, due to myalgias and creatine kinase (CK) elevation, the dose was reduced to 450 mg BID. She had sustained clinical and radiologic response on alectinib until May 2017, when she showed evidence of worsening pleural and brain metastases. She showed increasing thoracocentesis requirement; therefore, right thoracoscopy with talc pleurodesis was performed in May 2017.
PD-L1 (pharmDx 22C3; Agilent; Santa Clara, CA, USA) immunohistochemical staining was performed on a pleural fluid cell block from May 2017, showing no PD-L1 expression (testing performed at PhenoPath; Seattle, Washington, USA). A third CGP profile was performed on a right parietal pleural excision from May 2017, again showing EML4-ALK fusion and the CTNNB1 p.S45V mutation. In this sample, RICTOR amplification was reported as a variant of unknown significance due to a non-focal pattern. In addition, tumor mutation burden (TMB) was reported as low at 4 mutations/Mb. (TMB information was not available on the two prior specimens.) Figure 3 shows a comparison of the next generation sequencing data for the first three samples at CTNNB1 codon 45.
In June 2017, the patient was switched to brigatinib 90 mg (this dosage was selected in order to limit CK elevation). However, she developed increasing disease, for which she received two cycles of carboplatin/pemetrexed. In March 2018, she was switched to lorlatinib (dose reduced to 75 mg).
The disease continued to progress, and a 4th CGP (FoundationOne CDx) was performed on a lung biopsy from December 2018, showing EML4-ALK fusion, CTNNB1 p.S45V, RICTOR amplification, and a new tertiary ALK p.I1171N mutation. TMB was again reported to be 4 mutations/Mb, and the tumor was reported to be microsatellite stable. PD-L1 showed high expression by immunohistochemistry (Ventana SP263; Roche Diagnostics; Tucson, AZ, USA). FISH for the MET gene was negative for high-level amplification (performed at Quest Diagnostics; Chantilly, VA, USA). Based on the CTNNB1 mutation and RICTOR amplification in the patient’s tumor, she was evaluated for clinical trials with therapeutic agents targeting GSK-3b or PI3K. However, in March 2019, the patient died due to complications of the disease. Figure 4 summarizes the patient’s clinical course, and Table 1 summarizes the duration and best response of each treatment.
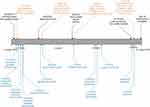 |
Figure 4 Timeline of clinical course, including comprehensive genomic profiling results and therapy. |
 |
Table 1 Duration and best response of each treatment |
Discussion
In 2011, the TKI crizotinib was approved by the US Food and Drug Administration for the treatment of ALK-rearranged NSCLC. Subsequently, multiple TKIs have been developed. However, several mechanisms of resistance to TKIs have also been identified. This includes secondary ALK mutations, downstream activation, and activation of alternative signaling pathways.2–4 Acquired resistance usually develops over a period of several months, suggesting a clonal evolution process where resistant clones overgrow the sensitive ones. ALK p.I1171N, which was seen in our patient, is a well-described secondary resistance mutation that typically occurs after disease progression2 and is located in the tyrosine kinase domain. Our patient’s tumor also showed RICTOR amplification upon initial CGP, and this alteration has been identified in up to 13% of all lung cancers.5 Activation of RICTOR-mTORC2 promotes cell proliferation and survival,5 and tumors showing RICTOR amplification have shown sensitivity to mTORC1/2 inhibitors.
However, the patient presented herein showed an initial clinical response to crizotinib. After the development of resistance, repeat tumor profiling showed a secondary CTNNB1 p.S45V variant. To our knowledge, concomitant CTNNB1 mutations are extremely rare in ALK-rearranged NSCLC. In a study of Finnish NSCLC patients with ALK gene fusion and concomitant driver gene mutations, one patient was reported to have CTNNB1 p.S45P, as well as a mutation in MET; however, the clinical course and treatment information were not available.6
CTNNB1 mutations are seen in only 3–4% of NSCLC (cbioportal.org, accessed March 2019). CTNNB1 encodes β-catenin, a critical effector of Wnt signaling and cellular adhesion.7 Exon 3 mutations, including the p.S45 mutational hotspot, are early events in the development of multiple cancers.7 Activating mutations result in altered cellular signaling, driving tumorigenesis. Furthermore, alterations in molecules involved in other signaling pathways, such as PI3K/AKT signaling, can modulate the Wnt pathway.7 In cell lines harboring EML4-ALK, the fusion has been shown to drive the phosphorylation of AKT and ERK, and this effect has been reduced through ALK inhibition.8 This suggests a possible mechanism for interaction with CTNNB1.9–11 The CTNNB1 p.S45V mutation is exceedingly rare, but occurs at an important phosphorylation site for casein kinase-1 that is centrally involved in Wnt signaling.7
For a variety of cancers, overexpression of immune checkpoint molecules in the tumor microenvironment has been shown to play a critical role in both antitumor immunity evasion and cancer progression.12 Our patient’s tumor showed increased PD-L1 expression using the SP263 antibody. Although immune checkpoint inhibitors have been approved for use in a variety of tumors, some patients do not respond to these agents, and this de novo resistance may be related to immunosuppressive mechanisms within the tumor microenvironment.12 Wnt/β-catenin signaling has been associated with immune evasion12 and activating mutations in CTNNB1 may result in modulation of the tumor microenvironment. Therefore, in addition to its potential impact on ALK inhibition, CTNNB1 mutations have the potential to impact response to immunotherapy treatments. Due to the involvement of Wnt signaling in the pathogenesis of many tumors, a variety of therapeutic strategies are being developed to target this pathway.12 This includes inhibitors of Wnt signaling, antibodies against frizzled receptors and Dkk-1, and inhibitors of Wnt ligand secretion.12
Recently, it was shown that constitutively activated ALK phosphorylates SMAD4, impairing SMAD4 DNA-binding and TGF-β tumor suppressor signaling.13 Furthermore, TKIs may have a role in reversing this effect.13 As previously discussed, β-catenin activation is associated with immunosuppression in the cancer microenvironment7 and resistance to immunotherapy. Therefore, immunomodulation by Wnt signaling may interfere with the effects of TKIs on TGF-β signaling, possibly leading to TKI-resistance.
Conclusion
We present a patient with lung adenocarcinoma showing ALK-rearrangement with resistance to multiple TKIs. Serial CGP showed multiple genomic alterations developing over time through a process of clonal evolution. Although subsequent profiling showed a known resistance mutation in the ALK gene, the development of a possible activating mutation in the CTNNB1 gene after initial clinical response suggests a possible biological mechanism for the patient’s initial resistance. This case reflects the challenges of targeted therapeutics and highlights the importance of comprehensive genomic testing for utilizing targeted therapy in the context of clonal evolution. The CTNNB1 mutation is particularly relevant to targeted therapy, given the role of β-catenin in the cancer microenvironment. However, further investigation, including functional analysis, is required to define the role of CTNNB1 p.S45V mutation in ALK-driven NSCLC and TKI-resistance. This may aid in the continuing development of therapeutic strategies for NSCLC.
Disclosure
Russell Madison is employed by Foundation Medicine, Inc.; and has stock in Roche. Siraj M Ali, MD, PhD is employed by Foundation Medicine, Inc.; has equity interest in Roche; is on the Scientific Advisory Board for Incysus Therapeutics; and is a Consultant for Revolution Medicines. Murray B Resnick, MD, PhD reports personal fees from PathAI, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703.
2. Asao T, Takahashi F, Takahashi K. Resistance to molecularly targeted therapy in non-small-cell lung cancer. Respir Investig. 2019;57(1):20–26.
3. van der Wekken AJ, Saber A, Hiltermann TJ, Kok K, van den Berg A, Groen HJ. Resistance mechanisms after tyrosine kinase inhibitors afatinib and crizotinib in non-small cell lung cancer, a review of the literature. Crit Rev Oncol Hematol. 2016;100:107–116. doi:10.1016/j.critrevonc.2016.01.024
4. Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi:10.1158/1078-0432.CCR-11-2906
5. Cheng H, Zou Y, Ross JS, et al. RICTOR amplification defines a novel subset of patients with lung cancer who may benefit from treatment with mTORC1/2 Inhibitors. Cancer Discov. 2015;5(12):1262–1270. doi:10.1158/2159-8290.CD-14-0971
6. Tuononen K, Kero M, Maki-Nevala S, et al. ALK fusion and its association with other driver gene mutations in Finnish non-small cell lung cancer patients. Genes Chromosomes Cancer. 2014;53(11):895–901. doi:10.1002/gcc.22198
7. Gao C, Wang Y, Broaddus R, Sun L, Xue F, Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: a review. Oncotarget. 2018;9(4):5492–5508. doi:10.18632/oncotarget.23695
8. Bayliss R, Choi J, Fennell DA, Fry AM, Richards MW. Molecular mechanisms that underpin EML4-ALK driven cancers and their response to targeted drugs. Cell Mol Life Sci. 2016;73(6):1209–1224. doi:10.1007/s00018-015-2117-6
9. Jeong WJ, Ro EJ, Choi KY. Interaction between Wnt/beta-catenin and RAS-ERK pathways and an anti-cancer strategy via degradations of beta-catenin and RAS by targeting the Wnt/beta-catenin pathway. NPJ Precis Oncol. 2018;2(1):5. doi:10.1038/s41698-018-0049-y
10. Fang D, Hawke D, Zheng Y, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi:10.1074/jbc.M611871200
11. Yamaguchi H, Hsu JL, Hung MC. Regulation of ubiquitination-mediated protein degradation by survival kinases in cancer. Front Oncol. 2012;2:15. doi:10.3389/fonc.2012.00015
12. Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10(1):101. doi:10.1186/s13045-017-0471-6
13. Zhang Q, Xiao M, Gu S, et al. ALK phosphorylates SMAD4 on tyrosine to disable TGF-beta tumour suppressor functions. Nat Cell Biol. 2019;21(2):179–189. doi:10.1038/s41556-018-0264-3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.