Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Titanium dioxide nanoparticles enhance production of superoxide anion and alter the antioxidant system in human osteoblast cells
Authors Niska K, Pyszka K, Tukaj C, Wozniak M, Witold Radomski M, Inkielewicz-Stepniak I
Received 31 August 2014
Accepted for publication 1 November 2014
Published 4 February 2015 Volume 2015:10(1) Pages 1095—1107
DOI https://doi.org/10.2147/IJN.S73557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Thomas Webster
Karolina Niska,1 Katarzyna Pyszka,1 Cecylia Tukaj,2 Michal Wozniak,1 Marek Witold Radomski,3–5 Iwona Inkielewicz-Stepniak1
1Department of Medical Chemistry, 2Department of Electron Microscopy, Medical University of Gdansk, Gdansk, Poland; 3School of Pharmacy and Pharmaceutical Sciences, Trinity Biomedical Sciences Institute, The University of Dublin Trinity College, Dublin, Ireland; 4Kardio-Med Silesia, 5Silesian Medical University, Zabrze, Poland
Abstract: Titanium dioxide (TiO2) nanoparticles (NPs) are manufactured worldwide for a variety of engineering and bioengineering applications. TiO2NPs are frequently used as a material for orthopedic implants. However, to the best of our knowledge, the biocompatibility of TiO2NPs and their effects on osteoblast cells, which are responsible for the growth and remodeling of the human skeleton, have not been thoroughly investigated. In the research reported here, we studied the effects of exposing hFOB 1.19 human osteoblast cells to TiO2NPs (5–15 nm) for 24 and 48 hours. Cell viability, alkaline phosphatase (ALP) activity, cellular uptake of NPs, cell morphology, superoxide anion (O2•-) generation, superoxide dismutase (SOD) activity and protein level, sirtuin 3 (SIR3) protein level, correlation between manganese (Mn) SOD and SIR, total antioxidant capacity, and malondialdehyde were measured following exposure of hFOB 1.19 cells to TiO2NPs. Exposure of hFOB 1.19 cells to TiO2NPs resulted in: (1) cellular uptake of NPs; (2) increased cytotoxicity and cell death in a time- and concentration-dependent manner; (3) ultrastructure changes; (4) decreased SOD and ALP activity; (5) decreased protein levels of SOD1, SOD2, and SIR3; (6) decreased total antioxidant capacity; (7) increased O2•- generation; and (8) enhanced lipid peroxidation (malondialdehyde level). The linear relationship between the protein level of MnSOD and SIR3 and between O2•- content and SIR3 protein level was observed. Importantly, the cytotoxic effects of TiO2NPs were attenuated by the pretreatment of hFOB 1.19 cells with SOD, indicating the significant role of O2•- in the cell damage and death observed. Thus, decreased expression of SOD leading to increased oxidizing stress may underlie the nanotoxic effects of TiO2NPs on human osteoblasts.
Keywords: TiO2NPs, superoxide dismutase, sirtuin 3, nanotoxicity
Introduction
Titanium dioxide nanoparticles (TiO2NPs) are some of the most widely manufactured nanoparticles (NPs) (1–100 nm) in the world and they are very often used in the production of biomedical ceramic and orthopedic implants.1 TiO2NPs, when added to composites, increase their durability to mechanical damage, especially cracking and breaking, and minimize the risk of bacterial infections.1–3 Because of these unique properties TiO2NPs are increasingly used in endoprostheses and scaffolds for bone-tissue reconstruction.2,3 However, nanotoxicological studies are crucial for the safe and sustainable development of emerging and established nanomaterials such as TiO2NPs. Indeed, TiO2NPs may also have harmful/cytotoxic effects. For example, TiO2NPs could induce DNA double-strand breaks in bone-marrow cells after oral administration.4 Zhang et al5 observed that TiO2NPs stimulated pro-inflammatory gene expression in pro-osteoblast cells (MC3T3-E1). Wang et al6 indicated that TiO2NPs are potentially toxic to major organs and cause damage to the knee joints in rabbits.
A number of studies have investigated mechanisms of this cytotoxicity and demonstrated that TiO2NPs induce alterations in redox homeostasis, which includes both impairment of antioxidant defenses and increased production of reactive oxygen species (ROS) in different types of cells.7–9 ROS may be involved in the pathogenesis of bone loss-related diseases. Indeed, decreased plasma antioxidant levels were observed in aged osteoporotic women.10 However, surprisingly little is known about the cellular mechanisms responsible for the effects of TiO2NP-induced oxidative stress on osteoblast functions, in particular, the role of the superoxide anion (O2•−).
Superoxide is formed when oxygen (O2) acquires an additional electron. O2•− is converted by the antioxidant enzyme superoxide dismutase (SOD) into hydrogen peroxide (H2O2) and singlet oxygen.11 SOD is the first enzymatic line of the antioxidant defense system, which scavenges superoxide radicals and prevents lipid peroxidation of the cellular membrane. Antioxidant enzymes are supported by nonenzymatic antioxidants and the total antioxidant capacity (TAC) is often used to estimate the overall antioxidative status in cells. Recently, particular attention has been paid to the link between mitochondrial SOD (SOD2/manganese [Mn] SOD) and sirtuin 3 (SIR3). SIR3 is a member of the sirtuin family and deacetylates SOD2 to increase its catalytic activity. It is localized in mitochondria and regulates mitochondrial function, homeostasis, and oxidative metabolism, as well as oxidative stress and cellular injury.12,13
Understanding the molecular mechanisms underlying TiO2NP-induced oxidative damage in osteoblasts will assist in developing novel strategies for the safe use of TiO2-containing NPs. Therefore, the main objective of the study reported here was to research the role of the O2•–/SOD/SIR3 system on TAC and human osteoblast hFOB 1.19 cytotoxicity following challenge with TiO2NPs.
Materials and methods
Chemicals
TiO2NPs (5–15 nm) were purchased from US Research Nanomaterials, Inc (Houston, TX, USA). The crystal structure of the TiO2 used in our experiments was anatase, per the manufacturer’s product characteristics. The concentrations of the TiO2NPs used in experiments were carefully selected according to the results obtained from a preliminary concentration–response study (Figure S1).
Characterization of nanoparticles
Dynamic light scattering
The size distributions and zeta potentials of the TiO2NPs were obtained using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Measurements were taken six times for concentration 50 μg/mL in serum-free (SF) culture medium at room temperature.
Cell line and procedures
Human fetal osteoblast cell line (hFOB 1.19) was obtained from the American Type Culture Collection ([ATCC] ATCC number: CRL-11372; Manassas, VA, USA) and maintained as a monolayer culture in T-75 cm2 tissue-culture flasks. The cells were grown in a mixture of Dulbecco’s Modified Eagle’s Medium and Ham F12 medium (1:1 ratio) also containing sodium pyruvate 110 mg/L and supplemented with 10% fetal bovine serum, 6 μg/mL penicillin-G, and 10 μg/mL streptomycin. Cells were maintained at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. When confluent cells were detached enzymatically with trypsin-ethylenediaminetetraacetic acid (EDTA) and subcultured into a new cell-culture flask. The medium was replaced every 2 days. These hFOB cells have been indicated to be an excellent model system for the study of osteoblast biology in vitro.14
Treatments
hFOB 1.19 cells were treated with TiO2NPs (5–100 μg/mL) for 24 and/or 48 hours. The TiO2NPs were suspended in SF cell culture and diluted to appropriate concentrations ex tempore every time before adding the cells. Controls were also treated with SF cell-culture medium. The dilutions of TiO2NPs were filtered through a 0.22 μm membrane filter, then sonicated using a sonicator bath at room temperature for 20 minutes to reduce NP agglomeration. According to the manufacturer’s protocol and the literature,5 20 minutes’ sonication is sufficient to reduce NP agglomeration. For some experiments, SOD (50 U/mL) (Sigma-Aldrich Corporation, St Louis, MO, USA) was added 30 minutes before the TiO2NPs were incubated with the cells.
Cytotoxicity
Water-soluble tetrazolium salt 1 assay
Cell viability was measured by water-soluble tetrazolium salt (WST) 1 assay (Hoffman-La Roche Ltd, Basel, Switzerland). hFOB 1.19 cells were seeded in triplicate at a density of 104 cells/100 μL of cell-culture medium into 96-well plate. The following day, the hFOB 1.19 cells were treated with TiO2NPs under SF conditions at the concentrations 5, 25, 50, and 100 μg/mL for 24 and 48 hours.
Mitochondrial activity assay (an index of cell growth and cell death) was performed by adding a premixed optimized dye reagent WST-1 to culture wells.15,16 Absorbance was read at 450 nm (reference: 630 nm) using an Asys Hitech GmbH microplate reader (Biogenet, Eugendorf, Austria). Absorbance values were also corrected with blank NPs. Treated-cell viability was calculated and expressed as a percentage (%) of viability of control cells (100%) based on mean absorbance values at 450 nm.
Transmission electron microscope analysis
Transmission electron microscope (TEM) analysis was performed according to a previously described method17 with slight modification. hFOB 1.19 cells were fixed in 2.5% glutaraldehyde (GA) in 0.1 M sodium (Na)-cacodylate buffer at pH 7.4. Fixation was carried out at 4°C for 1 hour and then cells were rinsed three times in the same buffer. Following fixation in GA the cells were post-fixed in 1% osmium tetroxide in 0.1 M Na-cacodylate buffer for 1 hour. After washing with the same buffer, they were dehydrated in graded series of ethanol, immersed in propylene oxide, embedded in Epon 812, and polymerized. Sectioning was performed with a diamond knife on an OmU2 ultramicrotome (Reichert, Austria). Semi-thin sections (1.5 μm) were stained with toluidine blue and examined under a light microscope. Ultrathin sections were placed on formvar-covered cooper grids, and double-stained with lead citrate and uranyl acetate. Observations were carried out with a JEM-1200 EX II TEM (JEOL, Tokyo, Japan) at an accelerating voltage of 80 kV.
Cell necrosis
Cell viability was measured by lactate dehydrogenase (LDH) assay (Promega Corporation, Fitchburg, WI, USA). hFOB 1.19 cells were seeded in triplicate at a density of 104 cells/100 μL of cell-culture medium into 96-well plate. The following day, the hFOB 1.19 cells were treated with TiO2NPs under SF conditions at the concentrations 5, 25, 50, and 100 μg/mL for 48 hours. LDH release into the surrounding medium was determined according to the manufacturer’s instructions. Absorbance values were also corrected with blank NPs. LDH data were expressed as a percentage of the total LDH released from cells.18
Cell metabolism
The alkaline phosphatase (ALP) activity was determined using a commercially available kit (Abcam plc, Cambridge, England) according to the manufacturer’s instructions. Briefly, hFOB 1.19 osteoblast cells were cultured until they reached 80%–90% confluence and treated as described in the “Treatments” section for 48 hours. After that, the cells were washed with phosphate-buffered saline (PBS) and lysed in buffer containing 10 mM Tris hydrochloric acid (HCl) (pH 7.5), 0.5 mM magnesium chloride (MgCl2), and 0.1% Triton™ X-100. The cell lysates were centrifuged at 2,000 g and the soluble portion was used for enzymatic assay. An aqueous solution of 2 mg/mL of p-Nitrophenyl phosphate was mixed with 0.1 M amino propanol in 2 mM MgCl2, with a pH of 10.4 being prepared. Next, 200 μL of the substrate was added to the 96-well plates and incubated in the dark for about 30 minutes. The enzymatic reaction was stopped by the addition of 50 mM sodium hydroxide (NaOH). The final product (p-Nitrophenol) was quantified at 405 nm using the Asys Hitech GmbH microplate reader. The results were normalized by the amount of cells and by specific activity (nmol p-Nitrophenol/min/mg/of protein). Absorbance values were also corrected with blank NPs.
Superoxide anion production
Superoxide generation was measured using flow cytometry. Briefly, the cells were cultured in six-well plates until they reached 80%–90% confluence and treated as specified in the “Treatments” section for 48 hours. Cells were then split and cultured on coverslips and incubated with 5 mM dihydroethidium (DHE) at 37°C for 30 minutes. The DHE staining detecting O2•− production was quantified by flow cytometer (BD FACSCalibur™, BD Biosciences, San Jose, CA, USA).19 The data were presented as percent control, in which “control” is 100%.
Total antioxidant capacity
The TAC was determined using a commercially available kit (Sigma-Aldrich) according to the manufacturer’s instructions, with modifications. The cells were cultured in 10 cm petri dish until they reached 80%–90% confluence and treated as indicated in the “Treatments” section. For determining cellular TAC, post-treatment cells were washed with PBS and suspended in 200 μL of ice-cold lysis buffer and sonicated. The lysate was centrifuged at 10,000 g for 10 minutes, and the protein concentration of the supernatant fraction was determined by the Bradford method.20 The Trolox-equivalent antioxidant activity was measured by measuring the ability of hydrogen-donating antioxidants to scavenge the radical cations generated by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid). Absorbance was recorded at 570 nm (Asys Hitech GmbH microplate reader). Absorbance values were also corrected with blank NPs. The data were presented as percent control, in which “control” is 100%.
Total superoxide dismutase activity
The SOD activity was measured using a Superoxide Dismutase Assay Kit (Cayman Chemical Company, Ann Arbor, MI, USA). Briefly, 10 μL of cell supernatants that were obtained by scraping, sonicating, and centrifugation (15,000 g, 5 minutes) the cells’ monolayer (2×106 cells) in a cold environment (4°C) was added to 200 μL radical detector (50 μL tetrazolium mixed with 19.95 mL assay buffer – ie, 50 mM Tris HCl, pH 8.0 contained 0.1 mM diethylene triamine pentaacetic acid [DTPA] and 0.1 mM hypoxanthine). The reaction was initiated by adding 20 μL of xanthine oxide in a 96-well plate. The plate was shaken and incubated for 20 minutes at room temperature before the absorbance (450 nm) was recorded using the Asys Hitech GmbH microplate reader. The SOD activities in the samples were calculated according to the formula supplied with the manufacturing kit.
Western blotting of superoxide dismutase and sirtuin 3
Western blotting analysis was used in order to investigate SOD1 (copper [Cu]/zinc [Zn] SOD), SOD2 (MnSOD), and SIR3 according to previously described protocol.17 Western blotting was used in order to investigate the SOD1, SOD2, and SIR3. Briefly, hFOB 1.19 cells were cultured in 10 cm petri dishes until they reached about 90% confluence and treated with TiO2NPs under SF conditions at the concentrations 25, 50, and 100 μg/mL for 48 hours. Afterwards, conditioned media were discharged and attached cells rinsed with PBS, detached, and homogenized. Following electrophoresis, proteins were transferred onto nitrocellulose membrane (Protran®, Schleicher and Schuell BioScience GmbH, Dassel, Germany) and detected using antibodies: anti-Sirt3, anti-SOD1, and anti-SOD2 antibodies (Cell Signaling Technology, Inc, Danvers, MA, USA). Protein bands were quantified using densitometry software (Bio-Rad Laboratories Inc, Hercules, CA, USA), and normalized using β-actin (Sigma-Aldrich) as a loading control.
Lipid peroxidation
hFOB 1.19 cells were plated into 12-well plates at a density of 2.5×106 cells per well in complete medium. Pre-confluent cells were exposed to TiO2NPs as indicated in the “Treatments” section for 48 hours. Malondialdehyde (MDA), a marker of lipid peroxidation, was measured using an Oxiselect™ TBARS Assay Kit (Cell Biolabs, Inc, San Diego, CA, USA) following the manufacturer’s protocol. Spectrophotometric measurements were recorded on the Asys Hitech GmbH microplate reader at 532 nm. The concentration of MDA in samples was calculated using MDA standards as reference. The concentration of MDA in TiO2NP-treated cells was presented as the fold increase of MDA production over the untreated cell control. Absorbance values were also corrected with blank NPs.
Protein content
Protein-content determination was measured by the method of Bradford.20
Statistical analysis
All data are presented as the mean ± standard error of 3–4 independent experiments. Statistical analysis was determined by one-way analysis of variance and Tukey’s post-hoc test, and P-values <0.05 were considered statistically significant. All statistical analyses were performed using GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA, USA). Pearson correlation analysis was conducted to investigate the relationship between SIR3 and MnSOD, and SIR3 and O2•−.
Results and discussion
In the present study, we studied the effects of TiO2NPs on the viability, metabolism, and oxidative stress of hFOB 1.19 cells. As far as we are aware, it is the first report to show the link between TiO2NP-induced osteoblast toxicity and impairment of the antioxidant system, including changes of SIR3 at protein levels in hFOB 1.19 cells.
Characterization of nanoparticles
We used commercially available TiO2NPs with a size of 5–15 nm, according to the supplier. The predominant size of the TiO2NPs measured in culture media ranged from 10 to 15 nm (Figure 1) and the NPs had a zeta potential value of −28.91±1.25 mV, reflecting good stability.21
Titanium dioxide-nanoparticle exposure decreases viability of hFOB 1.19 cells
The TiO2NP-mediated cytotoxicity was evaluated using WST-1 assay, which is indicative of mitochondrial damage. This test is based on water-soluble dye and it does not interfere with TiO2NP measurement, compared with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.15,16
The TiO2NPs (5, 25, 50, 100 μg/mL), when incubated with cells for 24 hours, did not cause statistically significant changes in mitochondrial activity. In contrast, the exposure of cells to TiO2NPs (25, 50, and 100 μg/mL) for 48 hours resulted in a significant decrease in osteoblast cell viability (Figure S2). Our results are consistent with those of others, who have demonstrated the cytotoxic effect of TiO2NPs in different cell lines and in vivo.4,22–24
Based on all these data, 48 hours was chosen as the incubation time for further biochemical study.
Uptake of titanium dioxide nanoparticles by hFOB 1.19 cells and ultrastructural changes in cells
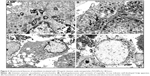
We also evaluated TiO2NP toxicity using transmission electron microscopy. Images of ultrathin sections provided information on cell death, survival, and metabolic activities.25 TEM analysis revealed that TiO2NPs were engulfed and internalized within the osteoblast cytoplasm (Figure 2).
The invaginations in the cell membrane before NP uptake and vacuolization are indicative of nonspecified internalization.26 On the other hand, Zhang et al5 indicated that the uptake of TiO2NPs by murine MC3T3-E1 pro-osteoblast cells occurred via receptor-mediated endocytosis. In osteoblasts, TiO2NPs were deposited over 24 and 48 hours inside multiple vacuoles, sometimes forming aggregates. The TEM analysis indicates that the incubation of TiO2NPs with osteoblasts for 48 hours led to alterations in mitochondria organization, known as “condensed” configurations (Figures 2C and 3A).
These configurations were identified as dark, condensed matrices and expanded, translucent cristae. Intensive vacuolization of mitochondria was also noted (Figure 2C). Such morphological changes observed in mitochondria in TiO2NP-treated osteoblast cultures were accompanied by biochemical changes (WST-1 assay) described here. The TEM examination revealed also single-membrane vacuoles called “autophagolysosomes”. These vacuoles contain remnants of organelles, especially mitochondria. Autophagolysosomes are able to degrade sequestered autophagosomal components (Figure 3B). It is likely that this autophagy process constitutes a stress adaptation pathway that promotes cell survival during osteoblast exposure to TiO2NPs.27 We also observed features of necrotic lysis, such as advanced swelling of intracellular organelles associated with cell-membrane disruption and intensive vacuolization (Figure 2B). Such changes are typical for necrotic cell death without signs of massive cell death or apoptosis.
Titanium dioxide nanoparticles induce loss of hFOB 1.19 cell-membrane integrity
TiO2NP-induced cell-membrane disruption was further confirmed by the measurement of LDH, a stable cytosolic enzyme that is released early in necrosis, and only in late stage apoptosis.28 Incubation of osteoblast cells with TiO2NPs for 48 hours led to increased release of LDH (Figure S3).
Our results are in keeping with data from Zhang et al5 who indicated that TiO2NPs of 5 and 32 nm diameters increased LDH release in a size-, concentration-, and time-dependent manner in pro-osteoblast cells (MC3T3-E1). Interestingly, Lai et al29 indicated that apoptosis, necrosis, as well as possibly apoptosis-like and necrosis-like were the cell-death mechanisms underlying the effects of TiO2 microparticles and NPs on human astrocytoma U87 (astrocyte-like) cells.
Titanium dioxide nanoparticles decrease metabolism of hFOB 1.19 cells
As expected, TiO2NP-induced reduction of osteoblast survival led to decreased function. Indeed, incubation of hFOB 1.19 cells with 25, 50, and 100 μg/mL TiO2NPs for 48 hours decreased the activity of ALP (Figure S4), an enzyme participating in bone-tissue mineralization and commonly used as a marker of osteoblastic differentiation in in vitro studies.30,31 These results are consistent with those of Hou et al32 who showed that ALP activity was decreased in mesenchymal stem cells incubated with TiO2NPs.
Titanium dioxide nanoparticles induce generation of the superoxide anion in hFOB 1.19 cells
Recently, it has been proposed that the cytotoxicity of TiO2NPs is related to the induction of oxidative damage.33–35 However, the exact molecular mechanism by which TiO2NPs affect oxidative metabolism, especially in the context of the cellular antioxidant defense system in osteoblast cells, is unknown. Therefore, we investigated the effects of incubating osteoblasts with TiO2NPs at concentrations of 5, 25, 50 and 100 μg/mL for 48 hours on O2•– generation, TAC, and SOD1, SOD2, and SIR3 levels.
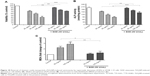
It is known that ROS play physiological and pathological roles in bone metabolism.36 They modulate bone-cell function and they are also implicated in the pathophysiology of skeletal tissues. ROS represent a family of molecules and free radicals. They are generated during mitochondrial oxidative metabolism as well as in cellular response to cytokines, bacterial invasion, environmental factors, and different xenobiotics, including NPs.34,36 The reduction of oxygen by one electron at a time produces relatively stable intermediates. In our study, we focused on the role of O2•− in TiO2NP-induced cytotoxicity in osteoblast cells. O2 is the product of a one-electron reduction of oxygen, the precursor of potent oxidizing species – such as H2O2, the hydroxyl radical (•OH), peroxynitrite (ONOO•−) – and a mediator in oxidative chain reactions.37 We observed a significant increase in O2•− levels in cells incubated with 25, 50, and 100 μg/mL TiO2NPs for 48 hours (Figure 4).
Wang et al38 noticed that TiO2NPs induce oxidative stress in a rat synovial cell line (RSC-364) by increasing the generation of free radicals. It has been previously demonstrated that metal, half-metal, and metal-oxide NPs induce production of ROS in different cell lines. We have shown an elevation of ROS levels in human gingival fibroblast cells exposed to silver NPs and human lung submucosal cells exposed to silicon dioxide NPs.17,39 Moreover, it has been reported that nanoscale TiO2 could be phagocytized by neurons and microglia, which then release ROS: OH and O2•−.40–42
Titanium dioxide-nanoparticle exposure impairs the antioxidant defense system in hFOB 1.19 cells
It is likely that during evolution the constant exposure of cells to free radicals resulted in the development of complex defense mechanisms involving enzymatic and nonenzymatic antioxidants. Major enzymatic antioxidants are SOD, catalase, and glutathione peroxidase. Therefore, we studied the effects of TiO2NPs on the activity and protein level of SOD in hFOB 1.19 cells. SODs are a family of enzymes that function to efficiently catalyze the dismutation of O2•−. SOD1, or Cu/ZnSOD, is a Cu- and Zn-containing homodimer localized in intracellular cytoplasmic spaces. SOD2, or MnSOD, is an Mn-containing tetramer of 22 kDa subunits, and is one of the primary mitochondrial antioxidants. The extracellular SOD is expressed in significant amounts in lung, kidney, and fat tissue and is regulated mainly by cytokines.43 Nojiri et al44 indicated that intracellular redox imbalance caused by SOD1 deficiency plays an important role in the development and progression of bone fragility. They also noticed that oxidative stress induced cell death and reduced proliferation in osteoblast but not in osteoclast cells. We have found that the incubation of osteoblast cells with TiO2NPs leads to significant reduction in activity of total – that is, cytosolic and mitochondrial – SOD (Figure 5) and it is associated with decreased protein levels of SOD1 and SOD2 (Figure 6A and B, respectively). To the best of our knowledge, this is the first report to have characterized changes in SOD activity and protein levels in osteoblast hFOB 1.19 cells.
Recently, the sirtuin enzyme family has attracted increasing attention due to its involvement in many cellular processes.12,13 Sirtuins importantly protect cells against stress and control a number of metabolic pathways. In our study, we focused on SIR3, which deacetylates and enhances the activity of various proteins that play a key role in the antioxidative defense system of mitochondria such as MnSOD.12,13 Interestingly, we observed that TiO2NPs significantly reduce the levels of SIR3 protein in osteoblast cells (Figure 7).
Furthermore, we found a significant positive correlation between SIR3 and MnSOD at the protein level and a significant negative correlation between decreased SIR3 protein level and increased O2•− level (Table 1).
Our findings indicate that SIR3 in conjunction with SOD2 may play an important role in protecting osteoblasts against TiO2NP-induced damage. The protective role of SIR3 has been also shown in different cells and systems. Indeed, Zeng et al45 demonstrated that SIR3 plays a crucial role in age-related dysfunction of the central auditory system. Tseng et al46 demonstrated that SIR3 stabilizes FOXO3 via deacetylation thus enhancing the mitochondrial antioxidant defense system in endothelial cells during hypoxia. Finally, Han and Someya47 observed that during calorie restriction, SIR3 can delay the progression of age-related hearing loss by enhancing the glutathione-mediated mitochondrial antioxidant defense system.
It is important to emphasize that some antioxidants can interact with each other, regenerating their properties, and this mechanism is often referred to as the “antioxidant network”.48 Therefore, the measurement of a panel of antioxidants as well as the TAC may provide more relevant biological information than the measurement of a single factor of the antioxidant system.49 Interestingly, we found a decrease in TAC in osteoblasts after 48 hours’ exposure to TiO2NPs (Figure 8).
In addition to SOD and SIR3, TAC levels were significantly decreased in a RSC-364 after exposure to nano-TiO2.42 Thus, TiO2NPs exert very strong inhibitory effects on cellular defense systems.
Of particular significance is the fact that TiO2NPs induce oxidizing stress not only in mammalian cells but also in lower animals. Hao et al33 found a significant decrease in SOD and catalase activities in liver, gill, and brain tissues of carp (Cyprinus carpio) during subacute exposure to 100 and 200 mg/L TiO2NPs. However, Zhu et al35 observed that the activity of SOD significantly increased in mature marine abalone (Haliotis diversicolor supertexta) exposed to 1.0 mg/L TiO2NPs. This increase could result from the mobilization of antioxidant enzyme activities during the rapid increase of ROS generation in cells exposed to high concentrations of xenobiotic. Indeed, in the same experiment, a decrease of reduced glutathione was observed. Therefore, the inadvertent presence of TiO2NPs in an ecosystem is likely to have profound environmental repercussions.
Titanium dioxide nanoparticles induce lipid peroxidation in hFOB 1.19 cells
When antioxidant defenses fail to restore the redox equilibrium, an elevated level of oxidative stress could lead to cellular damage and death.50,51 In the next step of our study, we demonstrated that TiO2NPs induced the overproduction of O2•− and an impairment of antioxidant defense system was accompanied by enhanced lipid peroxidation. The cell-membrane peroxidation and damage was reflected by the elevated MDA levels (Figure 9) and was also observed using TEM (Figure 3B).
Lipid peroxidation reactions can alter the structure and function of membrane lipids, thus result in cell injury and cell death.51 Indeed, oxidized lipids stimulated bone resorption by increasing recruitment and differentiation of osteoclast precursors and decreasing osteoblast differentiation.50 Carré et al52 also suggested that TiO2NPs lead to excessive generation of O2•−, resulting in enhanced lipid peroxidation and oxidative stress. Moreover, Ma et al34 showed that when TiO2NPs were injected into the abdominal cavities of mice every day for 14 days, lipid peroxidation increased in their brains. Significantly, the impact of TiO2NPs has also been shown in invertebrates. After 3 days’ exposure in an invertebrate model organism (Porcellio scaber) fed with food containing TiO2NPs, Valant et al53 found destabilized cell membranes, but lipid peroxidation was not detected. However, the levels of lipid peroxidation increased in the liver, gill, and brain tissues of carp exposed to TiO2NPs for up to 8 days.33
Pre-incubation of hFOB 1.19 cells with superoxide dismutase attenuates titanium dioxide nanoparticle-induced toxicity
Finally, to provide conclusive evidence for the involvement of O2•− in TiO2NP-induced osteoblast toxicity, cells were pre-incubated with purified SOD before adding NPs. Following incubation, osteoblast viability, ALP activity, and MDA levels were measured. Figure 10A–C shows that the treatment with SOD significantly attenuated the cytotoxic effect of TiO2NPs, showing that O2•− radicals participate in TiO2NP-induced cell death, decrease osteoblast metabolism, and increase lipid peroxidation.
Conclusion
We have demonstrated that excessive production of O2•− and impairment of the antioxidant system resulting in oxidative damage are likely to constitute a major mechanism implicated in TiO2NP-induced toxicity in osteoblast cells. Moreover, our study highlights, for the first time as far as we are aware, that TiO2NPs cause the impairment of SIR3 protein and SOD2 protein, leading to increased O2•− levels. These findings indicate that that regulation of SIR3 expression might provide a new approach to reduce TiO2NP-induced oxidative damage in osteoblast cells.
Acknowledgment
This research was supported by the Grant St-46 from the Medical University of Gdansk.
Disclosure
The authors declare no conflicts of interest in this work.
References
Kubota S, Johkura K, Asanuma K, et al. Titanium oxide nanotubes for bone regeneration. J Mater Sci Mater Med. 2004;15(9):1031–1035. | ||
Jain S, Jain AP, Jain S, Gupta ON, Vaidya A. Nanotechnology: An emerging area in the field of dentistry. J Dent Sci. 2013. Epub December 5. | ||
Tautzenberger A, Kovtun A, Ignatius A. Nanoparticles and their potential for application in bone. Int J Nanomedicine. 2012;7:4545–4557. | ||
Chen Z, Wang Y, Ba T, et al. Genotoxic evaluation of titanium dioxide nanoparticles in vivo and in vitro. Toxicol Lett. 2014;226(3):314–319. | ||
Zhang Y, Yu W, Jiang X, Lv K, Sun S, Zhang F. Analysis of the cytotoxicity of differentially sized titanium dioxide nanoparticles in murine MC3T3-E1 preosteoblasts. J Mater Sci Mater Med. 2011;22(8):1933–1945. | ||
Wang JX, Fan YB, Gao Y, Hu QH, Wang TC. TiO2 nanoparticles translocation and potential toxicological effect in rats after intraarticular injection. Biomaterials. 2009;30(27):4590–4600. | ||
Du H, Zhu X, Fan C, Xu S, Wang Y, Zhou Y. Oxidative damage and OGG1 expression induced by a combined effect of titanium dioxide nanoparticles and lead acetate in human hepatocytes. Environ Toxicol. 2012;27(10):590–597. | ||
Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol Lett. 2008;180(3):222–229. | ||
Saquib Q, Al-Khedhairy AA, Siddiqui MA, Abou-Tarboush FM, Azam A, Musarrat J. Titanium dioxide nanoparticles induced cytotoxicity, oxidative stress and DNA damage in human amnion epithelial (WISH) cells. Toxicol In Vitro. 2012;26(2):351–361. | ||
Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523–1527. | ||
Bhattacharya K, Alink GM, Dopp E. Oxidative stress and changed gene expression profiles in fiber-/particle-induced carcinogenesis. Int J Hum Genet. 2007;7(1):1–21. | ||
Merksamer PI , Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY). 2013;5(3):144–150. | ||
Nogueiras R, Habegger KM, Chaudhary N, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev. 2012;92(3):1479–1514. | ||
Subramaniam M, Jalal SM, Rickard DJ, Harris SA, Bolander ME, Spelsberg TC. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ER alpha cells: bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J Cell Biochem. 2002;87(1):9–15. | ||
Pujalté I, Passagne I, Brouillaud B, et al. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part Fibre Toxicol. 2011;3;8:10. | ||
Wörle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6(6):1261–1268. | ||
Inkielewicz-Stepniak I, Santos-Martinez MJ, Medina C, Radomski MW. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int J Nanomedicine. 2014;9:1677–1687. | ||
Xia T, Hamilton RF, Bonner JC, et al. Interlaboratory evaluation of in vitro cytotoxicity and inflammatory responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect. 2013;121(6):683–690. | ||
Mészáros G1, Szalay B, Toldi G, Kaposi A, Vásárhelyi B, Treszl A. Kinetic measurements using flow cytometry: new methods for monitoring intracellular processes. Assay Drug Dev Technol. 2012;10(1):97–104. | ||
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. | ||
Jiang J, Oberdörster, G, Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2009;11(1):77–89. | ||
Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–983. | ||
Liu R, Yin L, Pu Y, et al. Pulmonary toxicity induced by three forms of titanium dioxide nanoparticles via intra-tracheal instillation in rats. Prog Nat Sci. 2009;19(5):573–579. | ||
Liu H, Ma L, Zhao J, et al. Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res. 2009;129(1–2):170–180. | ||
Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–4352. | ||
Yurchenko OV, Todor IN, Khayetsky IK, Tregubova NA, Lukianova NY, Chekhun VF. Ultrastructural and some functional changes in tumor cells treated with stabilized iron oxide nanoparticles. Exp Oncol. 2010;32(4):237–242. | ||
Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. | ||
Do TN, Rosal RV, Drew L, Raffo AJ, et al. Preferential induction of necrosis in human breast cancer cells by a p53 peptide derived from the MDM2 binding site. Oncogene. 2003;22(10):1431–1444. | ||
Lai JC, Lai MB, Jandhyam S, et al. Exposure to titanium dioxide and other metallic oxide nanoparticles induces cytotoxicity on human neural cells and fibroblasts. Int J Nanomedicine. 2008;3(4):533–545. | ||
Gotoh Y, Hiraiwa K, Nagayama M. In vitro mineralization of osteoblastic cells derived from human bone. Bone Miner. 1990;8(3):239–250. | ||
Tilgar V, Kilgas P, Viitak A, Reynolds SJ. The rate of bone mineralization in birds is directly related to alkaline phosphatase activity. Physiol Biochem Zool. 2008;81(1):106–111. | ||
Hou Y, Cai K, Li J, Chen X, et al. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int J Nanomedicine. 2013;8:3619–3630. | ||
Hao L, Wang Z, Xing B. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio). J Environ Sci (China). 2009;21(10):1459–1466. | ||
Ma L, Liu J, Li N, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31(1):99–105. | ||
Zhu X, Zhou J, Cai Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar Pollut Bull. 2011;63(5–12):334–338. | ||
Sontakke AN, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002;318(1–2):145–148. | ||
Raja B, Pugalendi KV. Evaluation of antioxidant activity of Melothria maderaspatana in vitro. Cent Eur J Biol. 2010;5(2):224–230. | ||
Wang J, Ma J, Dong L, et al. Effect of anatase TiO2 nanoparticles on the growth of RSC-364 rat synovial cell. J Nanosci Nanotechnol. 2013;13(6):3874–3879. | ||
McCarthy J, Inkielewicz-Stępniak I, Corbalan JJ, Radomski MW. Mechanisms of toxicity of amorphous silica nanoparticles on human lung submucosal cells in vitro: protective effects of fisetin. Chem Res Toxicol. 2012;25(10):2227–2235. | ||
Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418(1–2):87–90. | ||
Long TC, Saleh N, Tilton RD, Lovry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–4352. | ||
Wang JX, Liu Y, Jiao F, et al. Time-dependent translocation and potential impairment on central nervous system by intranasally instilled TiO(2) nanoparticles. Toxicology. 2008;254(1–2):82–90. | ||
Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2(2):219–236. | ||
Nojiri H, Saita Y, Morikawa D, et al. Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res. 2011;26(11):2682–2694. | ||
Zeng L, Yang Y, Hu Y, et al. Age-related decrease in the mitochondrial sirtuin deacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model. PLoS One. 2014;4;9(2):e88019. | ||
Tseng AH, Wu LH, Shieh SS, Wang DL. SIRT3 interactions with FOXO3 acetylation, phosphorylation and ubiquitinylation mediate endothelial cell responses to hypoxia. Biochem J. 2014;464(1):157–168. | ||
Han C, Someya S. Maintaining good hearing: calorie restriction, Sirt3, and glutathione. Exp Gerontol. 2013;48(10):1091–1095. | ||
Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135(5):969–672. | ||
Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37(4):277–285. | ||
Abd Manan N, Mohamed N, Shuid AN. Effects of Low-Dose versus High-Dose γ-Tocotrienol on the Bone Cells Exposed to the Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis. Evid Based Complement Alternat Med. 2012;2012(2012):Article ID 680834. | ||
Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77(6):817–827. | ||
Carré G, Hamon E, Ennahar S, et al. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl Environ Microbiol. 2014;80(8):2573–2581. | ||
Valant J, Drobne D, Novak S. Effect of ingested titanium dioxide nanoparticles on the digestive gland cell membrane of terrestrial isopods. Chemosphere. 2012;87(1):19–25. |
Supplementary materials
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.