Back to Journals » Clinical Ophthalmology » Volume 17
Tissue Adhesives for the Management of Corneal Perforations and Challenging Corneal Conditions
Authors Sharma A , Sharma N , Basu S, Sharma R, Aggarwal S, Gupta PC, Ram J, Nirankari VS
Received 22 October 2022
Accepted for publication 22 December 2022
Published 15 January 2023 Volume 2023:17 Pages 209—223
DOI https://doi.org/10.2147/OPTH.S394454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ashok Sharma,1 Namrata Sharma,2 Sayan Basu,3 Rajan Sharma,1 Shruti Aggarwal,4 Parul Chawla Gupta,5 Jagat Ram,6 Verinder S Nirankari7
1Dr Ashok Sharma Cornea Centre, Chandigarh, UT, India; 2Cornea Service, Dr. R. P. Centre, AIIMS, New Delhi, India; 3LVPEI Banjara Hills, Hyderabad, Telangana, India; 4Anterior Segment Cataract Surgery, Katzen Eye Group, Baltimore, MD, USA; 5Advanced Eye Centre, PGIMER, Chandigarh, UT, India; 6Advanced Eye Centre PGIMER, Chandigarh, UT, India; 7University of Maryland, Baltimore, MD, USA
Correspondence: Ashok Sharma, SCO 2463-2464, Sector 22C, Cornea Service, Dr Ashok Sharma’s Cornea Centre, Chandigarh, 160022, India, Email [email protected]
Abstract: Corneal perforations are ophthalmological emergencies which can have serious and detrimental consequences, if not managed timely and appropriately. These are a significant cause of ocular morbidity and can result in decreased vision, blindness, and even loss of the eye. Corneal perforations can be managed using a range of treatment approaches, including temporary solutions such as the application of corneal glue and bandage contact lens, as well as definitive treatment such as corneal transplantation. Tissue glues/adhesives were developed as substitutes for sutures in ophthalmic surgery. Unlike sutures, these glues are associated with shorter overall surgical times and reduced inflammation, thus improving postoperative comfort without compromising wound strength. The available tissue adhesives can be broadly classified into two types: synthetic (eg, cyanoacrylate derivatives) and biological (eg, fibrin glue). Cyanoacrylate glue is chiefly used as a corneal patch to manage acute corneal perforations and improve visual outcomes. Fibrin glue can be used instead of cyanoacrylate glue in many conditions with the benefits of reduced conjunctival and corneal inflammation and reaction. Apart from this, each type of adhesive is distinct in terms of its benefits as well as limitations and is accordingly used for different indications. The present review focuses on the two main types of tissue adhesives, their applications in the management of corneal perforations, the associated complications, safety and efficacy data related to their use available in the literature and the need for newer adhesives in this field.
Keywords: corneal perforation, tissue adhesives, cyanoacrylate, fibrin glue, corneal patch
Introduction
Corneal perforations are ocular emergencies that may cause ocular morbidity and profound visual loss.1,2 Corneal perforations may arise from numerous infectious, inflammatory, trauma, surgical, ocular surface related xerosis, exposure, neurotropic, degeneration/ectasia and toxic/keratolytic causes.1
Most infectious corneal ulcers occur secondary to a breach in the corneal epithelium; however, microorganisms such as Corynebacterium diphtheriae, Haemophilus aegyptius, Neisseria gonorrhoeae, etc. can penetrate an intact epithelium. Corneal melting and subsequent perforation are typically seen in corneal ulcers that are refractory to medical treatment. Apart from infections, alterations in the basement membrane of the epithelial cells and corneal neurotrophic conditions can lead to persistent epithelial defects. Alterations in stromal collagen in autoimmune diseases such as rheumatoid arthritis and Mooren’s ulcer also facilitate corneal melting.2 Trauma is also a common cause of corneal perforations.
The main clinical symptoms of corneal perforations include a sudden decrease in visual acuity, pain, and increased tearing. The most common signs include a shallow or flat anterior chamber, positive Seidel’s test, uveal tissue prolapse, and hypotony (Figure 1).1
 |
Figure 1 Demonstrates positive Seidel’s test. |
Immediate treatment aimed at the closure of corneal perforation is of paramount importance to preserve corneal integrity and prevent complications like secondary glaucoma or endophthalmitis. Several management approaches are available for corneal perforations, including non-surgical measures such as the application of glue and bandage contact lens or more surgical definitive treatments such as conjunctival pedicle flaps, corneal patch grafts, and penetrating keratoplasty.3 The appropriate treatment approach is generally selected considering the size and location of the perforation as well as the etiology.3,4
Tissue Adhesives
Tissue adhesives are synthetic or naturally occurring compounds that aid in the reconstruction of natural as well as intraoperative or postoperative wounds.4 Their use in ophthalmologic procedures dates back to the 19th century. Since then, they have gained popularity as adjuvant materials for surgical wound closure in ophthalmology. The need for the development of tissue adhesives arose from the disadvantages associated with using sutures, such as long surgical and healing times and the risk of infection and scarring. Tissue adhesives offer the advantage of shorter surgical times and lesser inflammation, thereby reducing postoperative discomfort without compromising wound strength. They also may be a more economic option than suturing.4,5
An ideal tissue adhesive is cost-effective, transparent, easy to apply, biodegradable, and biocompatible. It should set rapidly, have high tensile strength (by creating a strong bridge between wound margins), and offer postoperative comfort. Currently, two main classes of tissue adhesives are in use: synthetic (eg, cyanoacrylate and acrylic-based polymers) and biological (eg, fibrin glue, bio-dendrimers, and riboflavin–fibrinogen compounds).5 In this review, we will discuss the properties and applications of these two classes of tissue adhesives and review the available literature on their use.
Synthetic Tissue Adhesives: Cyanoacrylate Derivatives
Cyanoacrylate derivatives are the oldest and most commonly used glues in ophthalmic surgeries. Cyanoacrylates are esters of cyanoacrylic acid, and are produced by the condensation of cyanoacetate with formaldehyde in the presence of a chemical catalyst.5 This type of synthetic glue polymerizes rapidly on coming in contact with a wet surface.4
Indications
Cyanoacrylates have been used in various ocular conditions, as reviewed below. However, it should be noted that while successful, the use of cyanoacrylate glue is not FDA approved and is considered off-label use in the United States.
The use of cyanoacrylates is indicated in various ocular conditions, some of which are listed below:
Corneal Perforations
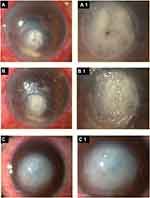
Application of cyanoacrylate glue (along with topical antibiotics or a bandage contact lens to avoid microbial infections) over small corneal perforations, less than 3mm, helps seal the cornea, allowing the formation of the anterior chamber and healing of the perforated cornea underneath the glue. In cases of impending perforations due to microbial infections (Figures 2 and 3), neurotrophic keratitis, chemical keratitis, trauma or non-infectious corneal melts, applying glue over the thin area may prevent perforation.3,5
- Glaucoma surgery and bleb leaks: Cyanoacrylate adhesives have been used for sealing leaking blebs after glaucoma surgery. Their use for sealing conjunctival buttonholing during glaucoma surgery and for the repair of conjunctival buttonholing during glaucoma drainage surgery has also been reported.5
- Sealing corneal cataract wounds: Cyanoacrylate glues have proven to be a safe and effective alternative to sutures in small-incision cataract surgery.5
- Closure of scleral fistula: Cyanoacrylate tissue adhesive has been used to close the scleral fistula in retinochoroidal coloboma successfully.6
- Retinal detachment surgery: Cyanoacrylate glue can be used to seal retinal breaks in pediatric vitreoretinal surgery for retinal detachment. It can either be applied on the wound edges or used as an adhesive plug for retinal breaks.5
- Macular hole surgery: The risk of macular hole progression can be reduced by transvitreal application of cyanoacrylate adhesives directly onto the macular hole. This reduces the need for vitreous surgery through gas tamponade or macular buckling.5
- Other ophthalmic applications: Cyanoacrylate adhesives have been used for healing limbal ulceration as an adjunct to tenonplasty, securing hard/rigid contact lenses in severe ocular surface disease (eg, chemical burns), as well as in the treatment of amblyopia (as a method of occlusion therapy), removal of non-magnetic deep corneal foreign bodies, and temporary tarsorrhaphy in corneal exposure.7,8
Application Techniques
After obtaining informed consent, the procedure can be performed in the office, minor procedure room or the operating room in a sterile setting, depending on the indication. For corneal perforations, most ophthalmologists perform the procedure in the office after reclining the patient back to prevent aqueous humor from leaking. The surface is dried as the glue polymerizes and solidifies instantaneously when it touches anything liquid. A squeezable vial can be used for the direct application of cyanoacrylate-based adhesives. Alternatively, an applicator, which can be a simple syringe attached to the vial, a 23-gauge catheter, or a plastic micropipette, can be used for the same. The corneal patch technique is a useful technique that involves the preparation of tissue adhesive on a corneal plastic dressing or patch. This patch is then directly attached to the perforated site. This technique is beneficial as it provides a uniform surface. Instead of a plastic dressing, a polyethylene disk can also be used as a patch. Topical antibiotics should be applied both before and after this procedure in order to avoid infections. Given that cyanoacrylate glue expands in volume during polymerization, the quantity used during surgery should be selected with caution. Even a minute amount of glue can be enough to seal small corneal perforations 1–2 mm in size.3,7
The adherence of glue is restricted by the size of the perforation and the condition of the surrounding tissue to which the glue is bonding. Reapplication may be necessary in cases of persistent stromal melting or wound leaks. Larger defects can be treated with the application of multiple overlapping patches, if required.7 After the cyanoacrylate polymerizes and solidifies, the surface is often rough and can cause significant discomfort and foreign body sensation with blinking. A bandage contact lens is applied over the treated area to prevent the glue from dislodging and also to promote patient comfort.
Efficacy of Cyanoacrylate Adhesives
Cyanoacrylate tissue adhesives are considered the standard of care for impending or manifest corneal perforations. They have manifold benefits, including ease of application and antibacterial activity, as well as their ability to offer tectonic support and arrest the progression of keratolysis.9 Various types of cyanoacrylate adhesives have been developed for clinical use. The early derivatives have short side chains (methyl, ethyl, etc.), while newer, medical-grade derivatives have longer side chains (butyl and octyl cyanoacrylate).10
Several studies have demonstrated definitive benefits of the early use of cyanoacrylate glue.2 Hirst et al performed a retrospective review of 104 nontraumatic corneal perforations or descemetoceles treated with tissue adhesives or other techniques between 1960 and 1980. They found that eyes treated with cyanoacrylate tissue adhesives had a lower enucleation rate than those treated with other methods (6% vs 19%), and a higher rate of achieving visual acuity of 20/200 or better (29% vs 19%).11
The use of cyanoacrylate glue has been found to be effective in obviating the need for other surgical treatment in cases with frank as well as impending perforations. Weiss et al treated 80 eyes with frank or manifest corneal perforations using isobutyl or N-butyl cyanoacrylate glue and found that 44% of the eyes healed with the use of the glue alone.12 Setlik et al also reported isobutyl cyanoacrylate tissue adhesive to be effective in treating small corneal perforations (≤3 mm), with 40.9% of the eyes healing with the use of the adhesive alone and 77.8% showing improvement in visual acuity.9 Kasetsuwan et al used a short-chain cyanoacrylate tissue adhesive, ethyl-2-cyanoacrylate, to treat small-sized (<3 mm) frank and impending corneal perforations with both infectious and non-infectious etiologies. The success rate of the procedure (defined as the rate of scarring of the cornea, which maintained the integrity of the eye) was reported to be 91%.10 A few cases of successful use of 2-octyl cyanoacrylate for the treatment of corneal perforations have also been reported.13,14
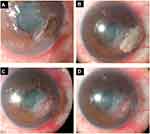
The management of larger (>3 mm) perforations with cyanoacrylate glue is more challenging because of the risk of entry of the glue into the anterior chamber, which might lead to the development of cataracts, glaucoma, or granulomatous keratitis. Sharma et al proposed a new technique of scleral patch graft-augmented cyanoacrylate tissue adhesive application for treating non-infectious corneal perforations measuring 3.5–4.5 mm. In this procedure, a partial-thickness scleral patch equal to the size of the corneal perforation is placed at the perforation site, followed by the application of the cyanoacrylate glue at the interface of the host cornea and scleral patch (Figure 4). The scleral patch provides a scaffold for the growth of fibrous tissue and corneal epithelium and also offers tectonic support. In their series of 16 eyes treated with this procedure, the authors found that 14 (87.5%) eyes healed within 5–9 weeks; only 2 (12.5%) eyes were refractory to the treatment and had to be treated with tectonic penetrating keratoplasty.15
Patients having a corneal perforation in association with rheumatoid arthritis may have continued collagenolysis after cyanoacrylate tissue adhesive application. Sharma has described an innovative technique of intracorneal scleral patch (ICSP) supported cyanoacrylate tissue adhesive application in corneal perforations greater than 3.0 mm secondary to rheumatoid arthritis. ICSP-supported CTA application successfully healed all the corneal perforations and avoided emergency PKP in corneal perforations sized 3.5 to 4.5 mm with associated rheumatoid arthritis. Long-term systemic immunosuppressive treatment needs to be continued.16
In some other studies, however, the rate of healing of perforations with the use of cyanoacrylate glue alone was lower, and further intervention was required in a majority of the eyes. Leahy et al used N-butyl cyanoacrylate for eyes with several ocular conditions such as corneal perforation, descemetoceles, stromal thinning, wound leaks etc, and found that only 33% (14/44) of the eyes healed without the need for any further intervention. The rest of the eyes required interventions such as enucleation, penetrating keratoplasty, scleral patch graft, conjunctival transplant, etc. Moreover, many eyes required more than one application of the tissue adhesive (mean number of applications, 1.6 per eye; range, 1–6).17 In another study conducted by Moorthy et al, the application of N-butyl cyanoacrylate tissue adhesive (along with a bandage contact lens) was successful in healing corneal perforations in only 37% of the eyes. A total of 31% of eyes needed multiple applications of the tissue adhesive, while 57% of the eyes required therapeutic keratoplasty due to failure of the glue. The overall findings of this study indicated that glue application alone might not be sufficient to heal corneal perforations associated with herpetic keratitis, and corneal transplantation surgery may be required to maintain the structural integrity of the eye.18
Similar results were obtained in a study by Lekskul et al, in which 53% of nontraumatic corneal perforations treated with histoacryl glue required regluing for recurrent leaks or glue dislodgment within several days of application of the adhesive, and 7% needed a penetrating keratoplasty for refractory leaking. It is important to note, however, that after accounting for the different levels of baseline visual acuity, the final visual outcome with tissue adhesive use was similar to those with the use of other techniques (penetrating keratoplasty and medical treatment).19 In a recent study by Yin et al, the application of cyanoacrylate tissue adhesive was found to be moderately effective in stabilizing corneal perforation/thinning in the very short term. The success rate of glue application (defined as an intact globe without the need for surgical intervention) was found to be 72%, 61%, and 46% at 10, 30, and 90 days, respectively, after glue application. The larger size of the perforation/thinning, perforation (vs thinning), and single glue application (vs multiple) were correlated with a higher failure rate. Multiple applications of the glue were frequently required, maintenance of globe integrity decreased with time, and the need for surgical intervention was high.20
Limitations of Cyanoacrylate Adhesives
The major concern with the use of cyanoacrylate adhesives for corneal applications is its potential toxicity to the corneal endothelium and lens.7 The toxicity of these adhesives depends on the length of the side chains. Shorter-chain derivatives (methyl, ethyl) are, in general, more toxic, either directly or in the form of products formed after degradation.5 They may cause acute or chronic inflammation of the eye, which can lead to intense discomfort and may potentially worsen the underlying condition as well.4,10 In contrast, longer-chain derivatives (butyl, octyl, etc.) are relatively less toxic.5 Carlson et al reported giant papillary conjunctivitis as a complication after using cyanoacrylate glue for a corneal perforation secondary to microbial keratitis.21 In corneal perforations secondary to microbial keratitis, after application of cyanoacrylate tissue adhesive bandage contact lens is avoided. Sharma et al have advocated a technique that uses a patch of sterile drape to apply cyanoacrylate tissue adhesive. A sterile drape piece provides a smooth surface and eliminates the need for bandage contact lens application.22 In the study by Leahy et al, scarring of conjunctival filtering bleb (5%, 2/44 eyes) and symblepharon formation (2%, 1/44 eyes) were among the commonly reported complications.17 Inadvertent instillation of a histoacryl adhesive in the anterior chamber has been reported to cause polymerization of the glue on the corneal endothelial surface, with iridocorneal and iridolenticular adhesion.23 Cyanoacrylates used for punctal occlusion may glide into the lacrimal sac and cause obstructive dacryocystitis and reactive xanthogranuloma. When used with a hard contact lens as an artificial epithelium, this glue has been observed to induce substantial stromal and endothelial cell loss of the cornea.24
The tolerance of ocular tissue to cyanoacrylates also varies with the quantity of glue used. Small quantities of the glue cause minimal inflammatory reaction and vascularization, but larger quantities covering up to a quarter of the cornea usually yield poor results.25 However, using too small quantities of the glue can leave some areas leaking, which makes the application of the glue cumbersome.5 Gandhewar et al proposed a technique to avoid inflammation in patients treated with cyanoacrylate glue for corneal perforations complicated with iris prolapse. In their technique, two 2- and 3-mm diameter discs are cut from a sterile plastic surgical drape. Rather than applying the glue directly to the iris, the smaller disc is first placed on the corneal perforation. The larger disc is glued over it and then covered with a therapeutic contact lens, thereby sealing the perforation while avoiding the risk of uveitis.26 In a study by Dean and Krenzelok, corneal abrasion requiring treatment was found to be a major complication (observed in 44% of the cases) associated with ocular contamination involving cyanoacrylates. Immediate (within 15 minutes after contamination) irrigation with water lowered the chances of corneal abrasion, while delayed irrigation increased the risk of mechanical injury.27
Infectious complications have also been reported after the use of cyanoacrylate glue for sealing corneal perforations. Sharma et al reported infectious complications in 3 of the 22 (13.6%) eyes treated with N-butyl cyanoacrylate.28 Weiss et al reported corneal infiltrates in 7 of 80 eyes after treatment with cyanoacrylate glue, and 5 of these infiltrates were bacterial in nature.12 Leahy et al also reported recurrent corneal ulcers in 2 of the 44 (5%) eyes and endophthalmitis in 1 of the 44 (2%) eyes treated with N-butyl cyanoacrylate.17 Monitoring for the presence of corneal infiltrate/infection is thus essential after the application of cyanoacrylate adhesives, particularly when they have been in place for more than six weeks.5 Other reported complications of cyanoacrylate glue include glaucoma28,29 or an increase in intraocular pressure15 and hypotony.30
Synthetic Tissue Adhesives: Polyethylene glycol (PEG) based hydrogel sealant.
Synthetic polyethylene glycol-based hydrogel (ReSure sealant, Optix therapeutics) was approved by the FDA in 2014 to seal clear corneal incisions in cataract removal and IOL implantation surgeries.
Indications
In addition to the FDA-approved indication of using hydrogel sealant for sealing clear corneal incisions in cataract extraction and intraocular implant surgery, it has also been used off-label in other ocular conditions since its approval.
- Wound closure in pars plana vitrectomy (PPV) surgery.
A recent study reported successful closure of a surgical sclerotomy site that was found to be leaking at the end of a 23G PPV, membrane peel, and air-fluid exchange for a 70-year-old patient with a history of epiretinal membrane and branch retinal vein occlusion.31
- Pterygium surgery.
Hydrogel sealant has been used in pterygium excision surgery to secure amniotic membrane graft to the conjunctival edges. Nine eyes were treated and showed successful adherence without any reported dislodgement of AMT or recurrence of pterygium.32
- Flap edge closure for epithelial ingrowth after refractive surgery.
Hydrogel sealant has been successfully used to prevent recurrent epithelial ingrowth in the setting of a LASIK flap buttonhole. Presumably, the adhesive seals the flap and any fistula, thus preventing entry of epithelial cells underneath the LASIK flap.33,34 Like in LASIK surgery, hydrogel sealant was used successively to treat recalcitrant epithelial ingrowth after small incision lenticule extraction (SMILE) procedure, which had previously recurred despite two flap lifts with epithelial debridement and flap edge re-suturing.35 The use of hydrogel sealant to seal refractive surgery flaps has been suggested in cases with diabetes which have a propensity to develop epithelial ingrowth.
Application Technique
The application is performed in a sterile setting. The sealant kit (commercially available as ReSure sealant, Optix therapeutics) contains a tray with mixing wells with two separate lyophilized reactants – polyethylene glycol solution and a tri-lysine amine solution, a plastic dropper filled with diluent and two foam tip applicators. The surgeon mixes together the diluent with the two reactants for about 5 seconds and applies it to the incision using a foam-tipped applicator. The surface is dried completely, and the solution is applied over the entire length of the incision, covering the margins. Reapplication may be required if the incision site is not completely covered. In clinical trials, reapplication was required in 40% of the subjects. The gel polymerizes within 30 seconds and forms a soft temporary sealant over the wound to prevent fluid egress. It lasts between 1 and 3 days until re-epithelialization occurs and then sloughs off in the patient’s tears. In clinical trials done for FDA approval, ReSure Sealant was reported to be in place in 76.1%, 31.3%, 2.6% and 0% of the eyes at postoperative days 1, 3 7 and 14, respectively.
It has been suggested to delay administration of any drops to the surface for at least 30 seconds after application of sealant to prevent dilution and dislodgement of the sealant before complete adherence. A recent study evaluated gel formation times of ReSure sealant when mixed with topical medications at the time of mixing and administration; and found that the addition of non-steroidal drop bromfenac slightly reduced mean gel time while all other topical non-steroidals, steroids and antibiotics, including moxifloxacin, gatifloxacin, ofloxacin, ciprofloxacin, loteprednol etabonate, prednisolone acetate, dexamethasone, ketorolac, and nepafenac, unacceptably increased mean gel time.36
Efficacy
In a large clinical trial, a prospective, controlled, multicenter study was undertaken. This study randomized patients with spontaneous leakage at the corneal incision at the time of cataract surgery into two groups based on the method for wound closure, ie, ReSure sealant (295 eyes) or single suture placement (176 eyes). With a 4.1% rate of wound leakage within the first postoperative week in the ReSure group compared to 34.1% of the suture group, superiority of hydrogel sealant was demonstrated. Regarding safety, it was found that overall adverse events were lower for ReSure sealant (22.7%) versus suture (45.4%).37
Another study found no significant effect of using sealant on surgical duration and day 1 postoperative corneal edema and foreign body sensation in patients who underwent bilateral cataract surgery, where only one eye received hydrogel sealant.38
Limitations
Hydrogel sealant requires the incision site to be completely dry before application. This sealant cannot be effectively used when there is a brisk leak or where a temporary dry surface cannot be achieved. Additionally, wounds that are at a higher risk for a postoperative leak or require more extended mechanical support at the incision (typically more than three days), suture placement is considered a safer option. ReSure sealant cannot be used in patients allergic to FD&C Blue #1. Further, prophylactic use in incisions without active leaking has not been evaluated.
Biological Adhesives: Fibrin Glue
Fibrin glue is the most frequently used biological tissue adhesive. Purified fibrin was used to treat parenchymal bleeding as early as the 19th century, and the combination of fibrin and thrombin has been used for skin graft adhesion. In the field of ophthalmology, fibrin glue was used for the first time in 1946 by Katzin to attach corneal grafts in rabbit eyes. Since then, its use has become exceedingly common in research and clinical practice for various sutureless ophthalmic procedures.5
Fibrin glue is absorbable and easy to use; it can be stored at room temperature or in a refrigerator.28,39 Fibrinogen and thrombin are the two major components of fibrin glue. In addition, it also contains two coagulating factors, aprotinin (fibrinolysis inhibitor) and calcium chloride.5 Fibrin glue mimics the blood coagulation cascade, which involves the activation of human Fibrinogen by thrombin. The polymerization of clot is completed within 2 to 3 minutes after application of fibrin glue. The fibrin clot once formed seals the corneal perforation and subsequently undergoes physiological degradation in few weeks to months.39
Indications
Fibrin glue has been used for several ophthalmic indications, which include the following:
- Conjunctival surgery: Fibrin glue has been shown to be as effective as sutures for conjunctival wound closure and transplantation. Its additional benefits include reduced operative time, postoperative discomfort, and inflammation.5
- Pterygium surgery: Fibrin glue has often been used for attaching conjunctival autografts in pterygium surgery. Its use is associated with lower recurrence rates than those associated with suturing; it also reduces the operating time, postoperative discomfort, and inflammation.5
- Strabismus surgery: The use of fibrin sealant is preferred over suturing for conjunctival wound closure in infants and children, considering that suture removal may not be possible in these patients. As in other cases, the lack of postoperative discomfort is the primary advantage of using a fibrin sealant.5,40
- Corneal surgery: Fibrin glue is mainly used for plugging ulcer-induced perforations. Fibrin glue-assisted corneal patch or amniotic membrane grafting (AMG) has been shown to be effective for treating corneal defects ≥3 mm in size.5,41 Also, in simple limbal epithelial transplant surgery (SLET), AMG is secured over the recipient cornea and limbus, and pieces of limbal biopsies to the AMG with the help of fibrin glue.42
- Glaucoma surgery: Fibrin glue is used for wound closure after trabeculectomy, the most common glaucoma surgery. It has also been shown to be effective in treating bleb dysfunction/leaking, securing conjunctival flaps, and securing glaucoma drainage devices.5
- Lens surgery: Several studies have used fibrin glue for sealing cataract wounds. It has also shown good visual acuity results without any postoperative complications when used in posterior chamber intraocular lens implantation in eyes with capsular support deficiency.5
- Vitreoretinal surgery: It has been shown that the application of autologous fibrin glue mixture on the macular holes and retinal tears of patients causes the tear edges to seal earlier than those of tears that have not been treated with fibrin glue.5 Glue-assisted retinopexy for rhegmatogenous retinal detachments (GuARD) helps the surgeon to avoid the use of gas or oil tamponade, and may aid in early visual recovery.43
Preparation
Several techniques are used to prepare fibrin glue using either homologous or autologous plasma. The risk of viral transmission can be avoided with the use of an autologous source. Homologous fibrin glue is prepared from donors who are first screened (as in the preparation of other blood products), with subsequent inactivation of viruses by solvent/detergent treatment.28,39
Centrifugation of plasma yields a precipitate that contains fibrinogen and a supernatant that contains thrombin. The precipitate, re-suspended in a small volume of the supernatant, is used as the fibrinogen component of fibrin glue. The supernatant is then treated by clotting in order to convert the residual fibrinogen to fibrin, which is subsequently removed by filtration. The serum thus obtained (after removal of the fibrin) is used as the thrombin component of fibrin glue.28,39
The safety of fibrin glue prepared from donors is comparable to that of other tested blood products; solvent/detergent treatment can successfully inactivate most, though not all, viruses. A useful alternative approach to obtain virus-free fibrin glue is to prepare it from homologous fresh frozen plasma from donors who test negative for viral markers for at least six months after the donation. This precludes the possibility of the donors having been in the “window period” at the time of donation of blood or plasma. Sterilization with gamma irradiation is an additional measure to ensure the safety of the prepared fibrin glue.28,39
Application Techniques
Based on the surgeon’s preference, the two components of fibrin glue (purified fibrinogen [a protein] and purified thrombin [an enzyme]) can either be applied simultaneously or sequentially.28,39
- Simultaneous application: Both the components are injected via two syringes whose tips form a common port (Duploject syringe). They meet in equal volumes at the point of delivery, whereupon the enzymatic action of thrombin converts the fibrinogen to fibrin at a rate determined by the concentration of thrombin. The clot’s fibrin monomers are crosslinked and stabilized by Factor XIII present in the fibrinogen component of the glue. Aprotinin enhances clot stability by inhibiting fibrinolytic enzymes.28,39
- Sequential application: This method involves the application of thrombin to the area of interest, followed by a thin layer of fibrinogen. The process of coagulation starts within a minute or two and clot polymerization is complete within 2–3 minutes after the application of the glue.28,39
- In cases where apposition between opposing surfaces is needed, the thrombin solution can be applied to one and fibrinogen to the other surface.28,39
In all of these cases, it is important to ensure that the surgical field is completely dry before the glue is applied. After the application of the glue, the tissue should be pressed gently over the glue for 3 minutes to ensure a firm adhesion. Finally, a pad and bandage should be applied after the instillation of antibiotic drops.28,39
Efficacy of Fibrin Glue
Fibrin glue, like cyanoacrylate glue, has been used successfully to treat impending as well as frank corneal perforations.2,23 This biocompatible and completely biodegradable tissue adhesive is associated with minimal stromal inflammation and foreign body reaction; it also does not cause tissue necrosis. Studies that used fibrin glue for sealing corneal perforations suggested that it is efficient and well tolerated.44 Lagoutte et al successfully used a fibrin sealant to close perforated and pre-perforated corneal ulcers in 9 eyes of 8 patients. This obviated the need for keratoplasty in these patients, 4 of whom had severe sicca syndrome, which indicated a poor prognosis with any graft even after complete healing of the perforation. No complications related to infection of the treated ulcer or residual inflammation of the anterior chamber were observed. According to the authors, an additional benefit of the use of fibrin glues for treating corneal perforations is that even if the procedure fails, it does not hinder keratoplasty and may even improve the prognosis.25
Siatiri et al evaluated the efficacy of fibrin glue (along with soft contact lens) in the treatment of corneal perforations up to 3 mm in diameter in 18 eyes. At three months after the procedure, 15 (83.3%) eyes exhibited successful healing of the perforation. However, the success rate was significantly lower for perforations with a diameter >2 mm, suggesting that the use of fibrin glue is effective in the closure of corneal perforations up to 2 mm in diameter.42 Dong et al used fibrin glue-assisted lamellar keratoplasty to successfully close corneal perforations of up to 2.5 mm diameter in 6 eyes using glycerin-cryopreserved corneal tissue. The best-corrected visual acuity was found to have increased after the procedure in all cases, and no major complications such as graft failure or graft dislocation were noted.45 Kumar et al also used fibrin glue in a modified technique of lamellar penetrating sclerokeratoplasty to treat a total corneal ulcer with scleral extension and impending perforation. In this technique, the partial-thickness scleral and full-thickness corneal bed was prepared. Next, fibrin glue was used to fix a donor lenticule with the partial-thickness sclera and full-thickness cornea on this bed. The technique was found to be less painful and less time-consuming, induced less postoperative inflammation, and appeared to be a safe and effective option for managing cases of total corneal ulcers with limbus-to-limbus involvement.46
Fibrin glue has also been used to seal perforations developing during deep anterior lamellar keratoplasty (DALK). In one such report by Anwar et al, fibrin glue was used successfully to close micro-perforations in the Descemet membrane in 2 patients undergoing DALK for keratoconus. No opacification, increased inflammation, or vascularization of the corneal stroma was observed after the procedure.47 Jacob et al have also reported fibrin glue-assisted closure of a macro-perforation using a donor lenticule from small incision lenticule extraction in a patient who had undergone predescemetic DALK.48 As mentioned previously, large corneal perforations (≥2 mm in diameter) are difficult to treat using tissue adhesives. Amniotic membrane transplants have been used for sealing perforations and have been reported to facilitate recovery and stabilize the ocular architecture.49 There have been clinical reports of the use of amniotic membrane transplants along with fibrin glue for the successful closure of large corneal perforations.49,50 Hick et al used amniotic membrane transplant and fibrin glue for treating 14 corneal ulcers with perforations measuring up to 3 mm. The success rate (re-epithelialization and stable corneal surface at two months) was 92.9% (13/14). The authors suggested that this technique can be used as an initial procedure before performing keratoplasty or conjunctival flap. It can also be used as a temporary measure to delay penetrating keratoplasty till ocular inflammation has decreased and the corneal surface has re-epithelialized.51 Kim et al proposed a new technique, referred to as “fibrin glue-assisted augmented amniotic membrane transplantation”, to treat corneal perforations measuring ≥2 mm in diameter. In this technique, a thick, 5- or 7-ply “augmented amniotic membrane” was prepared by applying fibrin glue to each sheet of amniotic membrane. This augmented amniotic membrane was transplanted onto the perforation site with the help of nylon sutures. In a retrospective case series of 10 patients with corneal perforations measuring ≥2 mm that were treated with this technique, the authors reported a very high success rate (90%, 9/10 eyes; success was defined as complete epithelialization over the amniotic membrane). No cases of infection or recurrent corneal melting were observed during the follow-up.52 Gupta et al reported the use of fibrin glue for treating 2 patients of paracentral corneal melting, 3–5 mm in diameter. In their technique, a full-thickness corneal patch graft was punched using a dermatological trephine and secured with fibrin-aprotinin biological tissue adhesive, thereby obviating the need for sutures. The procedure was successful, and a best-corrected visual acuity of 6/24 was achieved in both cases, which circumvented the need for an emergency full-thickness penetrating keratoplasty. This appears to be one of the rare reports in which corneal defects larger than 3 mm in diameter were sealed using fibrin glue.53 Fibrin glue has also been used successfully for sealing trophic corneal perforations (measuring 2 mm) developing after cataract surgery.54 Sharma et al reported successful management of 31 cases of sterile corneal perforation up to 5-mm size using tuck-in Tenon’s patch graft (TPG). The authors harvested the tenon graft, tucked it into the stromal pocket, and secured it either by applying cyanoacrylate glue or 10–0 monofilament sutures. The advantages of the tenon patch included the autologous nature of the graft, cost-effectiveness, and easy availability.54,55 Chaudhary et al described the long-term successful outcome of Tenon’s patch graft in a case of corneal perforation secondary to Herpes Zoster Ophthalmicus. The authors performed subsequent cataract surgery with gratifying results.56
Limitations of Fibrin Glue
The major risk associated with the use of fibrin glue is the risk of viral transmission with the use of formulations containing fibrinogen that has been extracted from multiple donors. Although several measures have been developed to minimize this risk, such as solvent/detergent treatment and a combination of gamma irradiation, cryoprecipitation, adsorption, vapour heating, pasteurization, and nanofiltration, the safest approach is to prepare fibrin glue from the patient’s own blood.4 The use of thrombin derived from animal (usually bovine) sources carries a high risk of adverse immunological reactions and transmission of prion/viral diseases.2,5 Sharma et al reported three cases of postoperative infectious complications (including one case of giant papillary conjunctivitis) among 19 eyes treated with fibrin glue. However, in general, the use of fibrin glue for sealing corneal perforations appears to be largely safe as most of the reports do not mention any major postoperative infectious complications.44–57
The biological action of fibrinogen itself may be a source of complications. Fibrinogen is known to be involved in the acute inflammatory response phase. It activates neutrophil markers that increase phagocytosis and cellular cytotoxicity as well as delay apoptosis. Therefore, it is recommended to use small amounts of fibrinogen for surgical procedures to prevent complications related to inflammation.5 Byun et al reported a case of a patient who experienced worsening bacterial keratitis and development of a secondary fungal infection after receiving treatment with fibrin glue for managing an impending perforation. Thus, it is recommended that the use of fibrin glue be avoided in cases of infectious keratitis as it masks the underlying lesion and also interferes with proper penetration of the drug into the lesion.58
Efficacy and Safety of Cyanoacrylate and Fibrin Adhesives: A Comparison
Both types of tissue adhesives, cyanoacrylate derivatives and fibrin glue, have their own strengths and limitations. Cyanoacrylate derivatives are non-biodegradable and hence may induce inflammation, corneal neovascularization, foreign body reaction, and tissue necrosis. In contrast, fibrin glue, being biologic in nature, is biocompatible and completely biodegradable. It induces minimal stromal inflammation and foreign body reaction and does not cause tissue necrosis.28 In addition, fibrin glue solidifies quickly, is easy to apply, and causes minor discomfort.2 However, it starts to degrade much faster than cyanoacrylate, is expensive, has low adhesive strength, and carries the risk of transmission of viral/prion diseases.5 It has no bacteriostatic effects (unlike cyanoacrylate adhesives), which is a major disadvantage because some corneal melting/perforations requiring tissue adhesive treatments are in fact caused by infectious keratitis.5,59
Few studies comparing cyanoacrylate and fibrin glues have been reported to date. A comparative study between fibrin glue and two commercially available cyanoacrylate derivatives—N-butyl cyanoacrylate and methoxypropyl cyanoacrylate—Chen et al showed that the cyanoacrylates had a bacteriostatic effect against a number of bacterial species, but fibrin glue had no such effect against either Gram-negative or Gram-positive bacteria. Fibrin glue had lower cytotoxicity than the cyanoacrylates, but its ability to seal corneal incisions was also lower.59 In another study, Sharma et al compared the efficacy of fibrin glue and N-butyl-2-cyanoacrylate in sealing corneal perforations up to 3 mm in diameter in 41 eyes (19 eyes treated with fibrin glue and 22 with N-butyl-2-cyanoacrylate). Both the adhesives were found to be equally effective (success rates of 79% and 86% with fibrin glue and N-butyl-2-cyanoacrylate, respectively; p > 0.05). Fibrin glue offered the advantage of faster healing and significantly less corneal vascularization than cyanoacrylate glue; however, it required a considerably longer time for adhesive plug formation.28 A comparison of the different properties of cyanoacrylate and fibrin tissue adhesives is provided in Table 1.
 |
Table 1 Comparison of Cyanoacrylate and Fibrin Tissue adhesives |
Photocrosslinkable Adhesives
This class of tissue adhesives includes bovine serum albumin-based bioadhesives, riboflavin-based bioadhesives, and hyaluronic acid-based photocatalytic glue. Bovine serum albumin-based bioadhesive is a photoactivated tissue adhesive.4 It has been used in strabismus surgery for muscle-to-muscle, sclera-to-sclera, and muscle-to-sclera adhesions. Riboflavin-based bioadhesive causes collagen cross-linking in the cornea in the presence of ultraviolet A radiation, thus increasing the biomechanical strength of the stroma.4 It is used to treat corneal pathologies such as keratoconus, post-LASIK ectasia, and postinfectious corneal melts. Hyaluronic acid-based photocatalytic glue is applied on lacerated corneal wounds.4 Upon activation with an argon LASER beam, it polymerizes and closes the wound. These newer bioadhesives show better biocompatibility, faster sealing, and increased binding forces compared to the existing bioadhesives, but are still under research.4
Conclusion
Tissue bioadhesives are fast gaining popularity as an alternative or adjuvant to surgical wound closure in ophthalmology. The newer techniques of applications of tissue adhesives using tissue scaffolds have enhanced their use in various clinical conditions.14,15,54–56,60 The different properties of cyanoacrylate derivatives and fibrin adhesives make them suitable for various indications. However, there is a growing need for new bioadhesives with better biocompatibility and greater bonding strength, which also provide rapid sealing. A number of newer adhesives are currently under research,61,62 and long-term clinical trials are required to establish their efficacy in the management of corneal perforations.
Ethical Approval
This manuscript is a review article, compiling information on already published studies, hence this study is exempted from ethical approval.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stamate AC, Tătaru CP, Zemba M. Emergency penetrating keratoplasty in corneal perforations. Rom J Ophthalmol. 2018;62:253–259.
2. Saini JS, Sharma A, Grewal SP. Chronic corneal perforations. Ophthalmic Surg. 1992;23(6):399–402.
3. Jhanji V, Young AL, Mehta JS, Sharma N, Agarwal T, Vajpayee RB. Management of corneal perforation. Surv Ophthalmol. 2011;56:522–538. doi:10.1016/j.survophthal.2011.06.003
4. Vyas S, Kamdar S, Vyas P. Tissue adhesives in ophthalmology. J Clin Ophthalmol Res. 2013;1:107–112. doi:10.4103/2320-3897.112179
5. Park HC, Champakalakshmi R, Panengad P, Raghunath M, Mehta JS. Tissue adhesives in ocular surgery. Expert Rev Ophthalmol. 2011;6:631–655. doi:10.1586/eop.11.64
6. Gupta A, Narang S, Gupta V, Sharma A, Pandav SS, Singh P. Successful closure of spontaneous scleral fistula in retinochoroidal coloboma. Arch Ophthalmol. 2001;119(8):1220–1221. doi:10.1001/archopht.119.8.1220
7. Vote BJ, Elder MJ. Cyanoacrylate glue for corneal perforations: a description of a surgical technique and a review of the literature. Clin Exp Ophthalmol. 2000;28:437–442. doi:10.1046/j.1442-9071.2000.00351.x
8. Sharma A, Pandey S, Sharma R, Mohan K, Gupta A. Cyanoacrylate tissue adhesive augmented tenoplasty: a new surgical procedure for bilateral severe chemical eye burns. Cornea. 1999;18(3):366–369. doi:10.1097/00003226-199905000-00020
9. Setlik DE, Seldomridge DL, Adelman RA, Semchyshyn TM, Afshari NA. The effectiveness of isobutyl cyanoacrylate tissue adhesive for the treatment of corneal perforations. Am J Ophthalmol. 2005;140:920–921. doi:10.1016/j.ajo.2005.04.062
10. Kasetsuwan N, Sukharoch P, Meesoupong P, Reinprayoom S, Puangsricharern V, Pariyakanok P. Efficacy and safety of ethyl-2-cyanoacrylate adhesives for corneal gluing. Asian Biomedi. 2013;7:437–441.
11. Hirst LW, Smiddy WE, Stark WJ. Corneal perforations. Changing methods of treatment, 1960–1980. Ophthalmology. 1982;89:630–635. doi:10.1016/S0161-6420(82)34742-9
12. Weiss JL, Williams P, Lindstrom RL, Doughman DJ. The use of tissue adhesive in corneal perforations. Ophthalmology. 1983;90:610–615. doi:10.1016/S0161-6420(83)34508-5
13. Okabe M, Kitagawa K, Yoshida T, et al. Application of 2-octyl-cyanoacrylate for corneal perforation and glaucoma filtering bleb leak. Clin Ophthalmol. 2013;7:649–653. doi:10.2147/OPTH.S43106
14. Taravella MJ, Chang CD. 2-Octyl cyanoacrylate medical adhesive in treatment of a corneal perforation. Cornea. 2001;20:220–221. doi:10.1097/00003226-200103000-00024
15. Sharma A, Mohan K, Sharma R, Nirankari VS. Scleral patch graft augmented cyanoacrylate tissue adhesive for treatment of moderate-sized non-infectious corneal perforations (3.5–4.5 mm). Cornea. 2013;32:1326–1330. doi:10.1097/ICO.0b013e31829cb625
16. Sharma A, Sharma R, Nirankari VS. Intracorneal scleral patch supported cyanoacrylate application for corneal perforations secondary to rheumatoid arthritis. Indian J Ophthalmol. 2021;69(1):69–73. doi:10.4103/ijo.IJO_2258_19
17. Leahey AB, Gottsch JD, Stark WJ. Clinical experience with N-butyl cyanoacrylate (Nexacryl) tissue adhesive. Ophthalmology. 1993;100:173–180. doi:10.1016/S0161-6420(93)31674-X
18. Moorthy S, Jhanji V, Constantinou M, Beltz J, Graue-Hernandez EO, Vajpayee RB. Clinical experience with N-butyl cyanoacrylate tissue adhesive in corneal perforations secondary to herpetic keratitis. Cornea. 2010;29:971–975. doi:10.1097/ICO.0b013e3181cbfa13
19. Lekskul M, Fracht HU, Cohen EJ, Rapuano CJ, Laibson PR. Nontraumatic corneal perforation. Cornea. 2000;19:313–319. doi:10.1097/00003226-200005000-00011
20. Yin J, Singh RB, Al Karmi R, Yung A, Yu M, Dana R. Outcomes of cyanoacrylate tissue adhesive application in corneal thinning and perforation. Cornea. 2019;38:668–673. doi:10.1097/ICO.0000000000001919
21. Carlson AN, Wilhelmus KR. Giant papillary conjunctivitis associated with cyanoacrylate glue. Am J Ophthalmol. 1987;104:437–438. doi:10.1016/0002-9394(87)90249-2
22. Sharma A, Mohan K, Nirankari VS. Management of nontraumatic corneal perforation with tectonic drape patch and cyanoacrylate glue. Cornea. 2012;31(4):465–6;author reply 466. PMID: 21857507. doi:10.1097/ICO.0b013e31821de358
23. Markowitz GD, Orlin SE, Frayer WC, Andrews AP, Prince RB. Corneal endothelial polymerization of histoacryl adhesive: a report of a new intraocular complication. Ophthalmic Surg. 1995;26:256–258.
24. Strempel I. Complications by liquid plastics in ophthalmic surgery. Histopathologic Study Dev Ophthalmol. 1987;13:137–146.
25. Lagoutte FM, Gauthier L, Comte PR. A fibrin sealant for perforated and preperforated corneal ulcers. Br J Ophthalmol. 1989;73:757–761. doi:10.1136/bjo.73.9.757
26. Gandhewar J, Savant V, Prydal J, Dua H. Double drape tectonic patch with cyanoacrylate glue in the management of corneal perforation with iris incarceration. Cornea. 2013;32:e137–e138. doi:10.1097/ICO.0b013e3182801809
27. Dean BS, Krenzelok EP. Cyanoacrylates and corneal abrasion. J Toxicol Clin Toxicol. 1989;27:169–172. doi:10.3109/15563658909038580
28. Sharma A, Kaur R, Kumar S, et al. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology. 2003;110:291–298. doi:10.1016/S0161-6420(02)01558-0
29. Vasquez-Perez A, Matarazzo F, Mandal N, Tuft S. Acute glaucoma following cyanoacrylate glue patch for corneal perforation. J Glaucoma. 2018;27:e148–e150. doi:10.1097/IJG.0000000000001009
30. Moschos M, Droutsas D, Boussalis P, Tsioulias G. Clinical experience with cyanoacrylate tissue adhesive. Doc Ophthalmol. 1996;93:237–245. doi:10.1007/BF02569064
31. Ho VY, Shah GK, Liu EM. ReSure sealant for pars plana vitrectomy wound closure. Ophthalmic Surg Lasers Imaging Retina. 2015;46(10):1042–1044. doi:10.3928/23258160-20151027-09
32. Bondalapati S, Ambati B. Minimally invasive pterygium surgery: sutureless excision with amniotic membrane and hydrogel sealant. Case Rep Ophthalmol. 2016;7(1):79–84. doi:10.1159/000444076
33. Hirabayashi KE, Manche EE. Hydrogel sealant to prevent recurrent epithelial ingrowth in the setting of a LASIK flap buttonhole. Am J Ophthalmol Case Rep. 2019;15:100518. doi:10.1016/j.ajoc.2019.100518
34. Ramsook SS, Hersh PS. Use of a hydrogel sealant in epithelial ingrowth removal after laser in situ keratomileusis. J Cataract Refract Surg. 2015;41(12):2768–2771. doi:10.1016/j.jcrs.2015.11.024
35. Thulasi P, Kim SW, Shetty R, Randleman JB. Recalcitrant epithelial ingrowth after SMILE treated with a hydrogel ocular sealant. J Refract Surg. 2015;31(12):847–850. doi:10.3928/1081597X-20151111-09
36. Mah FS. Effect on gel formation time of adding topical ophthalmic medications to resure sealant, an in situ hydrogel. J Ocul Pharmacol Ther. 2016;32(6):396–399. doi:10.1089/jop.2015.0112
37. Masket S, Hovanesian JA, Levenson J, et al. Hydrogel sealant versus sutures to prevent fluid egress after cataract surgery. J Cataract Refract Surg. 2014;40(12):2057–2066. doi:10.1016/j.jcrs.2014.03.034
38. Nallasamy N, Grove KE, Legault GL, Daluvoy MB, Kim T. Hydrogel ocular sealant for clear corneal incisions in cataract surgery. J Cataract Refract Surg. 2017;43(8):1010–1014. doi:10.1016/j.jcrs.2017.05.035
39. Panda A, Kumar S, Kumar A, Bansal R, Bhartiya S. Fibrin glue in ophthalmology. Indian J Ophthalmol. 2009;57:371–379. doi:10.4103/0301-4738.55079
40. Mohan K, Malhi RK, Sharma A, Kumar S. Fibrin glue for conjunctival closure in strabismus surgery. J Pediatr Ophthalmol Strabismus. 2003;40(3):158–160. doi:10.3928/0191-3913-20030501-10
41. Sinha R, Kumar C, Sharma N. Use of tissue adhesives in ophthalmology. Indian J Ophthalmol. 2009;57(5):409–413. doi:10.4103/0301-4738.55059
42. Shanbhag SS, Patel CN, Goyal R, Donthineni PR, Singh V, Basu S. Simple limbal epithelial transplantation (SLET): review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J Ophthalmol. 2019;67:1265–1277. doi:10.4103/ijo.IJO_117_19
43. Tyagi M, Basu S. Glue-assisted retinopexy for rhegmatogenous retinal detachments (GuARD): a novel surgical technique for closing retinal breaks. Indian J Ophthalmol. 2019;67(5):677–680. PMID: 31007238; PMCID: PMC6498943. doi:10.4103/ijo.IJO_1943_18
44. Siatiri H, Moghimi S, Pourabdollah E, Rahimi F, Fallah M, Siatiri N. The efficacy of fibrin glue in corneal perforations. Iran J Ophthalmol. 2008;20:10–14.
45. Dong N, Li C, Chen WS, Qin WJ, Xue YH, Wu HP. Fibrin glue-assisted for the treatment of corneal perforations using glycerin-cryopreserved corneal tissue. Int J Ophthalmol. 2014;7:62–65. doi:10.3980/j.issn.2222-3959.2014.01.11
46. Kumar S, Panda A. Fibrin glue aided sutureless sclerokeratoplasty with maintenance of chamber angle. Cornea. 2011;30:588–590. doi:10.1097/ICO.0b013e3182000fbb
47. Anwar HM, El-Danasoury A, Hashem AN. The use of fibrin glue to seal Descemet membrane microperforations occurring during deep anterior lamellar keratoplasty. Cornea. 2012;31:1193–1196. doi:10.1097/ICO.0b013e318242fd94
48. Jacob S, Dhawan P, Tsatsos M, Agarwal A, Narasimhan S, Kumar A. Fibrin glue-assisted closure of macroperforation in predescemetic deep anterior lamellar keratoplasty with a donor obtained from small incision lenticule extraction. Cornea. 2019;38:775–779. doi:10.1097/ICO.0000000000001918
49. Grau AE, Durán JA. Treatment of a large corneal perforation with a multilayer of amniotic membrane and TachoSil. Cornea. 2012;31:98–100. doi:10.1097/ICO.0b013e31821f28a2
50. Duchesne B, Tahi H, Galand A. Use of human fibrin glue and amniotic membrane transplant in corneal perforation. Cornea. 2001;20:230–232. doi:10.1097/00003226-200103000-00027
51. Hick S, Demers PE, Brunette I, La C, Mabon M, Duchesne B. Amniotic membrane transplantation and fibrin glue in the management of corneal ulcers and perforations: a review of 33 cases. Cornea. 2005;24:369–377. doi:10.1097/01.ico.0000151547.08113.d1
52. Kim HK, Park HS. Fibrin glue-assisted augmented amniotic membrane transplantation for the treatment of large non-infectious corneal perforations. Cornea. 2009;28:170–176. doi:10.1097/ICO.0b013e3181861c54
53. Gupta N, Sachdev R, Tandon R. Sutureless patch graft for sterile corneal melts. Cornea. 2010;29:921–923. doi:10.1097/ICO.0b013e3181ca3684
54. Sharma N, Singhal D, Maharana PK, Vajpayee RB. Tuck-in tenon patch graft in corneal perforation. Cornea. 2019;38(8):951–954. doi:10.1097/ICO.0000000000001955)
55. Kate A, Vyas S, Bafna RK, Sharma N, Basu S. Tenon’s patch graft: a review of indications, surgical technique, outcomes and complications. Semin Ophthalmol. 2022;37(4):462–470. doi:10.1080/08820538.2021.2017470
56. Chaudhary S, Basu S, Donthineni PR. Long term outcome of Tenon’s patch graft in corneal perforation secondary to neurotrophic keratitis: a case report on a 4-year anatomical functional outcome. Int J Surg Case Rep. 2021;83:106046. doi:10.1016/j.ijscr.2021.106046
57. Röpke AK, Plange N. Einsatz von humanem Fibrinkleber bei perforiertem trophischem Hornhautulkus [Use of human fibrin glue for perforated trophic retinal ulcer]. Ophthalmologe. 2014;111:158–160. German. doi:10.1007/s00347-013-2873-3
58. Byun YS, Kim MS. Superimposed fungal ulcer after fibrin glue sealant in infectious corneal ulcer. Korean J Ophthalmol. 2011;25:447–450. doi:10.3341/kjo.2011.25.6.447
59. Chen WL, Lin CT, Hsieh CY, Tu IH, Chen WY, Hu FR. Comparison of the bacteriostatic effects, corneal cytotoxicity, and the ability to seal corneal incisions among three different tissue adhesives. Cornea. 2007;26:1228–1234. doi:10.1097/ICO.0b013e3181506129
60. Bafna RK, Agarwal R, Beniwal A, Bhandari A, Sharma N, Titiyal JS. Emulsion polymer isocyanate-gluing: autologous epithelial transplant with cyanoacrylate glue application for small corneal perforations. Indian J Ophthalmol. 2020;68(8):1636–1639. doi:10.4103/ijo.IJO_69_20
61. Chandru A, Agrawal P, Ojha SK, et al. Human cadaveric donor cornea derived extra cellular matrix microparticles for minimally invasive healing/regeneration of corneal wounds. Biomolecules. 2021;11(4):532. doi:10.3390/biom11040532
62. Chameettachal S, Prasad D, Parekh Y, et al. Prevention of corneal myofibroblastic differentiation in vitro using a biomimetic ECM hydrogel for corneal tissue regeneration. ACS Appl Bio Mater. 2021;4(1):533–544.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.