Back to Journals » International Journal of Nanomedicine » Volume 11
TiO2 nanotube platforms for smart drug delivery: a review
Authors Wang Q, Huang J, Li H, Chen Z, Zhao AZ, Wang Y, Zhang K, Sun H, Al-Deyab SS, Lai Y
Received 19 March 2016
Accepted for publication 12 July 2016
Published 21 September 2016 Volume 2016:11 Pages 4819—4834
DOI https://doi.org/10.2147/IJN.S108847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Qun Wang,1,2,* Jian-Ying Huang,1,* Hua-Qiong Li,3 Zhong Chen,4 Allan Zi-Jian Zhao,3 Yi Wang,3 Ke-Qin Zhang,1 Hong-Tao Sun,2 Salem S Al-Deyab,5 Yue-Kun Lai1
1National Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou, 2College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, People’s Republic of China; 3Wenzhou Institute of Biomaterials and Engineering, Chinese Academy of Sciences, Wenzhou, People’s Republic of China; 4School of Materials Science and Engineering, Nanyang Technological University, Singapore, Singapore; 5Department of Chemistry, College of Science, King Saud University, Riyadh, Saudi Arabia
*These authors contributed equally to this work
Abstract: Titania nanotube (TNT) arrays are recognized as promising materials for localized drug delivery implants because of their excellent properties and facile preparation process. This review highlights the concept of localized drug delivery systems based on TNTs, considering their outstanding biocompatibility in a series of ex vivo and in vivo studies. Considering the safety of TNT implants in the host body, studies of the biocompatibility present significant importance for the clinical application of TNT implants. Toward smart TNT platforms for sustainable drug delivery, several advanced approaches were presented in this review, including controlled release triggered by temperature, light, radiofrequency magnetism, and ultrasonic stimulation. Moreover, TNT implants used in medical therapy have been demonstrated by various examples including dentistry, orthopedic implants, cardiovascular stents, and so on. Finally, a future perspective of TNTs for clinical applications is provided.
Keywords: TiO2 nanotubes, anodization, drug delivery, orthopedic implant
Introduction
In the conventional systemic drug delivery administration, drugs are typically delivered by oral, parenteral, and inhalation routes and so on, where drugs are distributed to the whole body and not to the specific site of interest.1 The inherent limitations of conventional therapies could be addressed on the basis of developing more efficient and rational drug delivery systems. In this regard, strong interdisciplinary research strategies combining the efforts of material scientists, engineers, medical scientists, biologists, and clinicians have recently demonstrated promising results.2 The localized drug delivery systems are recognized as the most promising methods for controlling drug release since drug-releasing implants possess persistent and controlled drug release.3 A recent review by Raliya et al provides a summary on the widespread use of nanostructured or nanocomposite materials for disease diagnostics, drug delivery and biomedical applications, as well as various nanoparticle synthesis routes, characterization, and functionalization methodologies for biomedical applications.4 The mesoporous silica prepared with a simple electrochemical process based on organic synthesis and porous silicon has been investigated extensively in recent years.5–9 Owing to their unique properties, much research interest has been focused on the electrochemical preparation of nanopores or nanotube materials from transition metal oxides.10–15 In particular, titania nanotubes (TNTs) and nanoporous anodic alumina are the most significant examples.16–20
Fabrication of TNTs is based on a low-cost, facile process, with controllable nanotube structures, due to self-ordering electrochemical anodization process. The TiO2 material possesses excellent biocompatibility and has demonstrated tunable drug-releasing performance. More importantly, it can be generated on the surface of the existing medical implants.21,22 The dimensions of the TNTs could be controlled by changing the electrochemical anodization parameters including anodized voltage, time, electrolyte, and so on.23–25 Compared with previously devised polymer based on implants, TNTs hold good stability to address problems such as implant swelling or disintegration. However, drug release will be uncontrollable when TNT implants are embedded into the living body.26–29 Thus, smart drug delivery strategies are urgently needed to control the drug release by tuning the diffusion mechanism.
To address the problems depicted earlier, this review aims at reporting the most recent advances on drug-releasing implants based on TNTs for localized drug delivery. The fabrication, properties, and biocompatibility of TNTs are briefly introduced. Some concepts for drug release controlled by externally triggered stimuli are also reviewed, and the advantages and inherent limitations of these systems are discussed in detail. In addition, the recent progress in the practical application of TNTs related to bone therapies, dentistry, cardiovascular stents, and brain tumors is described. Finally, the review is concluded with the prospective outlook on the TNTs implants used in clinical application.
Self-ordered nanotubular implants based on TNTs
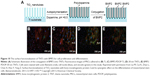
TNTs prepared by anodizing metallic titanium (Ti) in an electrolyte are recognized as one of the most outstanding drug-releasing implants in drug delivery systems.30,31 Some researchers have explored the optimization in anodization parameters to achieve a high degree of self-ordering in the grown TNTs.32–41 TNT arrays with controllable nanotube diameters and hexagonal arrangement fabricated by electrochemical anodization based on Ti surface with highly ordered nanotube structures are schematically shown in Figure 1.34,42,43 TNT fabrication is a unique electrochemical process termed self-assembling anodization,44 which is based on inexpensive materials and equipment. The dimension of TNTs could be adjusted by electrochemical anodization parameters including the anodization voltage, time, the composition of electrolyte, and so on.44–49 TNTs can be fabricated on various structures including three dimensional nonplanar and curved surfaces such as thin, long surgical wires and needles for bone fixture. Considering this versatility, TNTs are clinically used as implants or surgical supports in orthopedics because of their excellent malleability and biocompatibility in the host body.
  | Figure 1 The formation and structure of TNTs. |
As described earlier, TNTs fabricated by electrochemical anodization have attracted notable attention in the past few years due to various excellent properties of TNTs. In general, the typical electrochemical setup involves two electrodes comprising Ti (working electrode) and an alternate metal (counter electrode) that are immersed in electrolyte and are connected to an external voltage as shown in Figure 1A. The metal dissolution and then the oxide formation on the Ti substrate occur in the initial electrochemical process, and the dissolution of TiO2 layer is initiated with the formation of self-ordered and vertically aligned porous or tubular oxide nanostructures following the establishment of electrochemical equilibrium between these two reactions.
Biocompatibility of TNTs: in vitro and in vivo studies
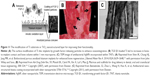
The application of TNTs is a promising alternative to develop various medical implants and devices because of their excellent biocompatibility, mechanical strength, and chemical resistivity; Ti and its alloys have been applied in orthopedic and dental implants for many years.49–54 Most biocompatibility studies of TNTs focused on their significant application in dentistry, orthopedics, and cardiovascular surgery; especially TNT implants presented a great affinity for bone cell adhesion and differentiation. In initial studies, it was demonstrated that the TNT surface could provide an excellent template for bone cell growth and osteoblast activity based on their better cell growth performance than Ti surface.55–58 Figure 2A and B reveals that bovine aortic endothelial cells (ECs) on Ti surfaces are more spread out and cover greater surface areas, whereas bovine aortic ECs on TNTs displayed elongated morphologies, which results in bovine aortic ECs on TNTs cover most of the average area occupied by the control cells.55 Popat et al demonstrated that TNT surface could be used as a favorable template for marrow stromal cell growth and differentiation and provided the evidence that the osteoblast activity can be greatly improved by controlling nanotopographies, as shown in Figure 2C and D.57
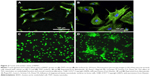  | Figure 2 F-actin and nuclear stains of BAEC. |
TNTs are recognized as promising implants for enhanced bone osseointegration, vascular stents with better control hemorrhage, and excellent blood-contacting devices, which results from TNTs possessing favorable surface for stem cell proliferation, hydroxyapatite formation, and the outstanding biochemistry inertness.59–61 Kim et al carried out the biocompatibility research on cellular responses of human osteosarcoma cells to TNTs,62 and Advincula et al investigated the cell responses of osteoblast-like MC3T3-E1 cells to TNTs.63 From these results, it is demonstrated that the cells on the TNTs proliferated more actively and that the alkaline phosphatase activity expressed to a higher degree compared with those on pure Ti. Other biocompatibility researches further demonstrated a higher alkaline phosphatase activity based on better cell growth on TNTs in vivo conditions.64–66 Oh et al reported a remarkable adhesion and reproduce of osteoblasts on TNTs, with the filopodia entering the nanotubes and forming an interlocked cell structure.67,68 Furthermore, TNTs also promote the development of drug-releasing implants for the treatment of bone-related diseases including cancer and post-implantation infections. Feschet-Chassot et al predicted the toxicity of TNT layer in biological systems using a protozoaic cell model and concluded that TNTs did not affect undefined esterases activity and cell growth rate.69
Human and rat mesenchymal stem cells (MSCs), their adhesion, propagation, and differentiation are directly related to the diameters of TNTs, which was described as an exceptional discovery in stem cell studies.70–72 Hu et al fabricated bone morphogenetic protein 2 (BMP2)-loaded TNTs and found that cells grew well on BMP2-loaded TNTs as schematically shown in Figure 3.71 MSCs adhered to bare TNTs array, which displayed round or narrow spreading morphologies, are shown in Figure 3A. By contrast, MSCs presented well-spreading morphologies when adhered to multilayer-coated TNTs as demonstrated in Figure 3C. Compared to the morphologies of MSCs cultured onto TNTs, MSCs adhered to BMP2-loaded TNTs have no obvious difference during the growth process as shown in Figure 3B. Figure 3E schematically illustrated the preparation of BMP2-loaded TNTs, the process of cell adhesion to multilayer-coated and BMP2-loaded TNTs and cellular responses. Bauer et al found that monolayers from octadecylphosphonic acid (OPDA) could significantly enhance the attachment of MSCs to TNT surfaces and demonstrated that the surface wettability was also an important parameter for cells adhesion.73 Furthermore, for the effects of TNT geometry on osteogenesis of MSCs, lots of studies have been carried out to investigate the effects of TNT geometry on osteogenesis of MSCs, such as crystal structure of TNTs, surface chemistry, and other differentiation approaches on the response of various stem cells.
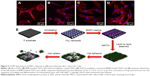  | Figure 3 CLSM observations of MSCs adhered to different substrates after culture for 1 day. |
For in vivo biocompatibility studies, von Wilmowsky et al investigated the effect of a TNT layer on bone formation by placing TNT implants into pigs. The immune-histological analysis was used to explore the bone implant.74 In this study, the effects of these implants were evaluated on the basis of the peri-implant bone formation, bone-implant contact, and immunohistochemistry, demonstrating that TNT coatings enhance osteoblast functions and resist shearing forces evoked by implant insertion, displaying positive outcome of bone formation characteristics of TNTs as compared to commercially available pure Ti implants. Another study performed by Park et al showed that the capability of improving osseointegration for protein-loaded TNT implants based on loading fibroblast growth factor (FGF) and human fibronectin fragment (hFNIII9-10) fusion protein inside TNTs on Ti implants inserted in rabbit tibia for 3 months.75 It is worthwhile stressing that these experiments were carried out over a period of 2–3 months, and more studies should be carried out to investigate longer healing periods of TNT implants for clinical therapies. Popat et al observed greatly increased chondrocyte adhesion on TNT surfaces compared with bare Ti and no existence of chronic inflammation or fibrosis in vivo biocompatibility study performed by implanting TNT surfaces subcutaneously in rats and performing histological analysis during 4 weeks.57 The results indicated that calcium and phosphorous concentrations were higher on the TNT surface, which suggested that matrix deposition was created on the nanotubular surface. In addition, Bjursten et al performed an in vivo study on TNT implants to verify that TNTs significantly enhanced bone bonding strength to the ambient tissues after 4 weeks of experimental implantation in rabbit tibias.76
All the earlier studies confirmed the safety of TNTs as biocompatible implants and thus demonstrated their safe usage in the in vivo and ex vivo contexts. However, more in vitro and in vivo studies are needed to resolve many other problems in the future, specifically in vivo studies are needed to explore the effect of the surface modification of TNTs on their biocompatibility and osseointegration properties.
External stimuli for responsive and on-demand drug delivery
It has been demonstrated that TNT structure is a suitable platform to develop drug-releasing implants due to its ability of loading different payloads of therapeutics, and TNT-based implants are recognized as one of the most excellent nanomaterials to address many disadvantages of traditional drug administration.3 It is well known that different drug release strategies need to be designed for different therapies, disease conditions, and specific parts of the host body. The drug release strategies may vary from short to long release, rapid on-demand release, or time-programmed release with single or multiple drug loadings. Therefore, TNT-based drug-releasing systems must be designed with flexible drug release capabilities and optimized parameters in order to fulfill the requirements of different therapies. Considering that, increasing studies are focused on exploring different strategies in TNT-based drug-releasing implants in order to design and advance their drug-releasing performances for specific drugs and therapies. Several typical concepts of stimulated drug release from TNTs with external triggers were described in this work.
pH- and temperature-sensitive drug delivery
TNTs have been demonstrated to be a promising platform for controlled local drug delivery, while the drug release from pure TNTs is very quick. Thus, pH-responsive polymers were applied to overcome the problem of too rapid drug release from TNTs. Jia et al reported that poly(lactic-co-glycolic acid) (PLGA) added into TNTs could improve the drug release profile.77 In their study, carprofen and lidocaine were used as the model drugs to investigate the drug release profile using PLGA/TNTs with different types of drugs. To investigate drug release from PLGA/TNTs under different pH conditions, lidocaine and carprofen releases were studied in sodium acetate buffer with pH 3.5, phosphate-buffered saline with pH 7.4, and phosphate buffer with pH 10.5, respectively, under the constant temperature of 37°C. The degree of TNT swelling was found to vary with pH values. The drug can easily diffuse into the medium through the swelling polymer during the critical time t1 as shown in Figure 4, followed the t2 time region, where the remaining drug completely releases as a result of polymer degradation.77 Furthermore, in order to reveal the mechanism and potential of lidocaine and carprofen release from TNTs, the drug release profiles were investigated on the basis of drug-loaded pure TNTs and drug-loaded PLGA/TNTs; the former was used as the control sample.77
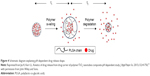  | Figure 4 Schematic diagram explaining pH-dependent drug release shape. |
For temperature-sensitive drug delivery based on TNTs, temperature-responsive polymers were explored to decorate TNT implants. Cai et al fabricated a temperature-sensitive drug delivery system based on TNTs by using vitamin B2 as a model drug and explored kinetics of controlled drug release from TNTs with temperature-responsive trigger.78 In their study, the hydrogel layer formed from poly(N-isopropylacrylamide) (PNIPAAm) and poly(acrylamide) (PAAm) was used as a cap that was coated on the surface of TNT layers for sealing the open nanotubes. The PNIPAAm/PAAm composite hydrogel presents a highly water swollen state that prevents the drug release from TNTs when the surrounding temperature is below its lower critical solution temperature, whereas the composite hydrogel is in a collapsing state to allow drug releasing from TNTs once the temperature is higher than the lower critical solution temperature of the composite hydrogel, as shown in Figure 5A. The drug release profile of pure TNTs at 25°C is higher than that of hydrogel-coated TNTs under the same condition, since there is no barrier to block the drug release at all. When the temperature increases to ~38°C due to the inflammatory reaction, hydrogel-coated TNTs show much higher release profiles than that of hydrogel-coated TNTs at 25°C as shown in Figure 5B. This is because 38°C is higher than the lower critical solution temperature of composite hydrogel; hence, the composite hydrogel was in a collapsing state to allow drug release from TNTs.
  | Figure 5 Schematic illustration of temperature-controlled stimulus to trigger drug release based on TNTs. |
Based on the existing research, temperature-sensitive drug delivery based on TNTs has promising potential for practical applications. However, it has to be cautioned that although interesting, the clinical relevance of such a device is still mainly hypothetical or that the composite hydrogel used needs to be adapted. In the same vein of thought, it is difficult to obtain in vivo pH control under normal conditions, and therefore, the actual value of a pH-sensitive drug delivery also seems quite hypothetical at the moment.
Light-sensitive drug delivery
For in-depth study of the controlled release of drugs or therapeutics using TNTs, light-sensitive release is also a promising strategy. Song et al reported that amphiphilic TNTs were used to provide a highly controllable drug release system based on a hydrophobic cap (monolayer of OPDA) on the top of TNTs, which can be removed by ultraviolet (UV)-induced chain scission. In this work, different drug-loading approaches were used to study hydrophilic drug release from the amphiphilic TNTs as outlined in Figure 6A.16 For reference, unmodified TNTs were used to load horseradish peroxidase (HRP) by simple immersion (Figure 6A, case I), which leads to physisorbed HRP molecules within TNTs. In the second case, TNTs capped with OPDA were immersed in HRP (Figure 6A, case II), which also leads to physisorbed drug molecules in the lower part of TNTs but remains trapped by OPDA after evaporation of the surfactant, dimethyl sulfoxide. TNTs capped without OPDA were used for HRP grafting by a 3-aminopropyltriethoxysilane/vitamin C monolayer linker covalently attached (Figure 6A, case III). TNTs capped with OPDA were attached to the HRP in the lower part of TNT wall as in case III (Figure 6A, case IV). For different loading approaches, the HRP release characteristics are presented in Figure 6B–E. From these results, it was demonstrated that UV light could make chain scission and then induce drug release from TNTs, thus opening potential perspectives for the drug delivery systems based on light control.
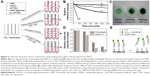  | Figure 6 Schematic illustration of four methods for drug loading and profiles of drug release from TNTs. |
In addition, chain scission-induced drug release from TNTs can also be triggered by suitable infrared (IR) laser irradiation. Moon et al carried out the study by immobilizing gold nanorods (GNRs) onto the surface of TNTs through a grafting technique and investigating the drug release from TNTs triggered by near-IR laser irradiation.15 The antimicrobial activity was detected to evaluate the effectiveness of the controlled drug release from GNR-grafted TNTs by IR laser irradiation. These results demonstrated that the photothermal activity of GNR could allow remote control of on–off drug elution by IR laser irradiation. GNRs are known to have much potential for mediating photodynamic and photothermal effects under near IR light.79–84 As such, GNR has been found to be as a photosensitizer for promising application in the field of cancer treatment. Since IR light can overcome the limitation of visible light that cannot penetrate deep into the skin, GNR-grafted TNTs can be expected to overcome the limitation of TiO2 showing photocatalytic activity within the UV to IR region, thereby facilitating the development of novel implantation materials.
Radiofrequency-sensitive drug delivery
Radiofrequency (RF)-responsive release is an excellent strategy for satisfying some therapies that require the use of noninvasive external stimuli. This concept has been introduced into the drug release based on TNT implants as shown in Figure 7A.85 In this drug delivery system, RF was used as an external stimuli to trigger the release of polymeric micelles and drugs from TNT deposited gold nanoparticles (AuNPs). Compared to release profiles under no-trigger conditions, the polymeric micelles displayed an abrupt in vitro release under RF triggering as shown in Figure 7B and C. From the results, it is demonstrated that near complete (90%–100%) release of all three samples was achieved within 1–3 hours after RF-induced heating of the AuNPs loaded inside TNTs. In addition, from the graphs, we also find that the drug release from AuNP-loaded TNTs is faster than drug-micelles release from AuNP-loaded TNTs, and drug-micelles without AuNPs achieved the lowest release. The release rate increases with extending the RF exposure time from 5 to 10 minutes, whereas there is no effect on drug-micelles release from TNTs without AuNP loading. Thus, it can be verified that AuNPs are good thermal transducers that transfer RF energy to trigger drug release from TNTs.
  | Figure 7 Schematic representation of a model drug release from TNTs implants for in vitro release studies with the RF trigger. |
In addition, single-walled carbon nanotubes, gold silica nanoshells, and water-soluble derivatives of C60 fullerenes were used as stimulants in the RF field to investigate the impact of RF.86–92 Raoof et al reported that noninvasive RF field-induced heating of metal nanoparticles owns outstanding properties over others in the treatment of hepatocellular cancer by summarizing recent approaches for delivering noninvasive RF field-mediated hyperthermia to malignant cells and its application to hepatocellular cancer.93,94 During the process of RF-induced drug release, the power of RF did not change or damage the micelle structure and TNTs implants. Therefore, RF-sensitive drug delivery possesses significant potential to ablate cancer cells at the implant location.
Magnetic-sensitive drug delivery
Magnetic-sensitive drug delivery is a new concept of drug encapsulated in nanomagnetic structures that possess excellent possibilities for magnetic field-triggered drug release. Aw et al designed drug-releasing implants assisted by external magnetic field based on magnetic nanoparticles (MNPs) loaded inside TNTs.95 In this study, TNTs were loaded with three types of amphiphilic micelles at the top acting as drug carriers and MNPs at the bottom of the nanotubes. Meanwhile, dopamine modification of iron oxide MNPs (DOPA-Fe3O4) with soft interfacial cushion were used to improve the biocompatibility of the MNPs, as shown in Figure 8. For the drug-release profiles, it was confirmed that immediate 100% release of all three drug carriers was achieved within 1–1.5 hours upon the application of the magnetic field. Although this strategy also presents some limitations such as uncontrolled release triggered by existing magnetic fields from the environment, it is still particularly valuable for drug-releasing implants in orthopedics and bone surgery where on-demand release is needed under emergency situation. Moreover, Shrestha et al used TNTs filled with MNPs to achieve magnetic- and photocatalytic-guided release of drugs.96 The release concept is based on the fact that the UV-induced hole generation in the valence band of the TiO2 will lead to chain-scission of a monolayer attached to TiO2.
  | Figure 8 Schematic representation of the magnetic stimuli-responsive drug release from TNTs which integrates polymer micelles as drug carriers incorporated with poorly soluble drugs and magnetic nanoparticles loaded at the bottom of the nanotubular structures. |
Ultrasound-sensitive drug delivery
The ultrasound-sensitive drug delivery based on applying the local ultrasonic field is expected to be a more reliable method compared with the magnetic-sensitive drug delivery that is uncontrollable in drug release when triggered by magnetic fields. Aw et al reported the application of local ultrasonic external field for triggering drug release from TNTs.97 In this study, ultrasonic waves were used as the trigger for stimulus-responsive local drug delivery system combining TNT implants as shown in Figure 9. The ultrasound-mediated drug-micelles release based on exerting oscillating pressure waves from a probe inserted in phosphate buffered saline (pH 7.2) close to the drug-micelle-loaded TNTs and a nonsteroidal anti-inflammatory drug (Indomethacin) was used as the model for water insoluble drug encapsulated in polymer micelles. With regard to the application of this concept, it can be applied for bone therapies, local drug delivery, and implantable drug delivery systems including stents and brain drug delivery. Of course, more studies of ex vivo or in vivo models using different drug-releasing implants and drugs are needed to achieve significant understandings based on the concept.
  | Figure 9 Schematic illustration of ultrasound-stimulated DD based on TNT implants and polymeric micelles as drug carriers. Reprinted from Int J Pharm, 443, Aw MS, Losic D, Ultrasound enhanced release of therapeutics from drug-releasing implants based on titania nanotube arrays, 154–162,97 Copyright (2013), with permission from Elsevier. |
These studies demonstrated that the drug release from TNT implants can be remotely triggered by external stimuli, thus it is expected to be applied in some medical therapeutics such as bone therapy, local chemotherapy, systemic chemotherapy, and so on. However, the development of these concepts are still at a preliminary stage, and further in vitro and in vivo studies are required to confirm their feasibility for practical applications in the living body.
TNT implants for clinical application
Although studies of TNT implants used for controlled drug delivery are still at their preliminary stage, they have demonstrated remarkable capabilities in terms of versatility in various clinical applications. Some typical examples of these are bone implants, dentistry, cardiovascular stents, and brain tumors, in which localized drug delivery devices based on TNTs are regarded as a promising alternative to overcome limitations of the conventional drug delivery systems.
Bone therapy and dentistry
Conventional therapies based on systemic administration of drugs for treating bone-related diseases present inherent limitations and associated side effects, whereas localized drug administration based on TNTs could offer outstanding potential advantages for treating bone-related diseases. Bone infections are associated with bone implants coming into contact with the living tissue. Considering the inflammatory response results from bone infections, Aninwene et al used penicillin/streptomycin- (anti-infection drugs) and dexamethasone- (an anti-inflammatory drug) loaded TNTs. The physical adsorption of the drugs could promote the anti-inflammatory properties of the TNTs, and the drug-eluting technique could be extended with enhanced osteoblast adhesion.98 Gulati and Aw et al reported water-insoluble indomethacin-loaded TNTs for anti-inflammation and anti-infection.99–101 In their works, in vitro studies were performed to demonstrate the potential applicability of TNTs for loading and releasing water-insoluble drugs using planar and wire implants, which provides the foundation for TNTs to be applied in delivering therapeutics to prevent inflammations in bone-related diseases. Moreover, Popat et al investigated the drug release kinetics of gentamicin-loaded TNTs and its effect on reducing bacterial adhesion on the surface.102
To enhance the osseointegration capability of orthopedic implants within the microenvironment surrounding the bone, the implant surface must promote the functions of different cell species, including osteoblasts and stem cells, as well as enhance bone healing.103 Lai et al reported BMP2 loaded inside TNTs could promote MSC proliferation and differentiation, extending the scope of stem cell engineering and cell-based therapies.104 To investigate the adhesion and proliferation of MSCs, the cytoskeleton morphology of MSCs was visualized with a triple staining of actin, vinculin, and nucleus by immunocytochemistry as shown in Figure 10. The result suggested that the conjugation of BMP2 onto TNTs promoted cell proliferation (Figure 10B–E). The diameters of TNTs were able to affect the adhesion, spreading, and differentiation of MSCs, and specifically, larger diameters could promote the protein adsorption.104–108 Therefore, the physical dimension of TNTs is an important factor for modulating biological functions in bone cells and tissue engineering.
  | Figure 10 The surface functionalization of TNTs with BMP2 for cell proliferation and differentiation. |
The implant surface is another important factor in terms of integration within the host body, given that it acts as an interface between artificial element and biological environment. Bovan et al highlighted the role of material surfaces in regulating cell response to implants, and the result demonstrated that the surface roughness and chemistry of bare orthopedic implants have a significant effect on osteoblasts and chondrocytes growth.109 Kunze et al reported that annealed TNT coatings with anatase phase are good precursors for the formation of calcium hydroxyapatite ceramic.110 In their study, more nuclei were formed on the TNT surface than on flat compact TiO2 in the initial stages of apatite growth, which demonstrated that TNTs are more beneficial to osseointegration than flat compact TiO2. Furthermore, Shim et al used Ti-anodized implants coated with FGF-2-loaded poly(lactide-co-glycolide) nanoparticles shown in Figure 11A for enhancing bone regeneration by an approach of the electrospray deposition, and the obtained results indicated that the factor-releasing particles fabricate stable and the releasing performance was extended over 2 weeks, thus enhancing the proliferation of bone tissues.111 It is known that some biologically active substances such as FGF, platelet-derived growth factor (PDGF), transforming growth factor β and insulin-like growth factor could break inflammatory cell release.112 Moioli et al demonstrated that incorporation of growth factor β (transforming growth factor β) in TNTs led to significantly enhanced bone-to-implant contact and increased bone volume within the 1 mm macropores as compared to placebo controls as shown in Figure 11B.113 TNTs loaded with Ag nanoparticles (AgNPs) possess a relatively long-term antibacterial ability and simultaneously promote cell functions, and AgNPs attached to the inner wall of the TNTs have a diameter of ~10–20 nm as shown in Figure 11C.114
  | Figure 11 The modification of Ti substrate or TiO2 nanostructured layer for improving their functionality. |
For dentistry diseases, typically for dental caries caused by bacteria, fluoride is a great effective agent in the therapeutic treatment and could prevent caries and enhance re-mineralization of enamel lesions.115–117 Therefore, some fluoride-releasing devices were designed to provide controlled fluoride release without accelerating the fluoride concentration in serum, thereby prevent caries formation. Ti and its alloys are well-accepted candidates for dental implants.21 The electrochemical stability of TNTs in artificial saliva was evaluated by Pirvu et al.118 Their study indicated a slight preference in terms of a human gingival fibroblast (HGF) survival and adhesion for TNTs with a more hydrophilic character, and the electrochemical date revealed that TNTs possess excellent stability in artificial saliva. HGFs were cultured on the Ag/FGF-2 immobilized TNTs for cytocompatibility determination.119 The result indicated that Ag/FGF-2-decorated TNTs has an excellent cytocompatibility compared to pure Ti, and the immobilized FGF-2 could enhance HGF cell attachment and proliferation. Furthermore, Bhattarai et al examined the feasibility of chitosan-gold nanoparticles conjugated with plasmid DNA/c-myb (Ch-GNPs/c-myb)-coated Ti implants and inserted it into rat mandibles to determine its in vivo effect.120 The obtained results support the view that c-myb can serve as a potent molecule in promoting tissue regeneration around dental implants. In short, these studies demonstrate that TNTs can provide more efficient and rational clinical treatments for dental diseases, with a great promising perspective for real clinical applications.
Cardiovascular stents
Coronary stents could improve immediate and late results of balloon angioplasty by tacking up dissections and preventing wall recoil,121 thus avoiding the heart occlusion in the treatment of coronary heart disease. However, traditional coronary stents present some inherent clinical complications such as restenosis, the need for further revascularization, and other various problems emerged after the cardiovascular surgery,122 which still remains as a crucial challenge in cardiology. To solve these problems, the concept of local drug delivery can be achieved by drug-eluting stents coated with polymer surfaces used for controlled drug release. However, several polymer coatings have shown an induction of inflammatory response and increased neointima formation, thus limited the clinical applicability of these alternative coronary stents. To avoid the side effect, new materials have been tested to overcome inherent drawbacks of drug-releasing cardiovascular stents coated with polymers.
In this regard, Wieneke et al investigated the effect of a new inorganic ceramic nanoporous aluminum oxide coating on neointima proliferation and its suitability as a carrier for the immunosuppressive drug tacrolimus.123 These cardiovascular stents were prepared as stainless steel stents coated with aluminum, which demonstrated the potential applicability of drug-releasing cardiovascular stents coated with nanoporous inorganic materials. The durability and biocompatibility of ceramic coatings are significantly important for drug-releasing coronary stents. Recent in vivo studies have demonstrated that the shedding of particle debris released from the nanoporous coatings produces a great increment of neointimal hyperplasia.124 Fine et al reported that drug-releasing coronary stents based on Ti promote better interactions with ECs.125 In this study, a new material called rosette nanotubes without drugs was coated on Ti stent surfaces, aiming to enhance the EC adhesion and proliferation. The obtained results indicated that rosette nanotube-coated Ti allows the growth of a uniform endothelium on their surface and prevents the stent from becoming loose and being dislodged after implantation and therefore prevent particle debris release from the implant surface. In addition, heart valves coated with ceramic TiO2 have been shown to exert profound antithrombogenic and break resistant properties.126,127
Although recent studies suggest that TNT arrays may be a well-accepted candidate for developing vascular implants, the effects of TNTs on vascular cells should be investigated carefully. With regard to this, Peng et al studied the response of primary human ECs and vascular smooth muscle cells (VSMCs) to TNT arrays through gene expression analysis.128 In their study, microarrays revealed that TNTs can enhance EC proliferation and motility, prevent VSMC proliferation, and decrease expression of molecules related to coagulation and inflammation in ECs and VSMCs. The work thus suggested that TNTs could be a promising candidate for the next-generation vascular device coating because of the divergent response of ECs and VSMCs. Nevertheless, it should be emphasized that more in vivo studies are required to evaluate their feasibility for the living body because the aforementioned drug-releasing stent experiments are far from real-life clinical conditions.
Brain tumors
For the treatment of brain tumors, systemic chemotherapy has some inherent drawbacks that significantly limit the clinical effectiveness. To address these limitations, a localized chemotherapy is desirable to treat malignant glioma. In this regard, drug-releasing implants based on nanotechnological approaches can provide alternative ways to satisfy the requirement. López et al prepared an implant based on nanostructured TiO2 loaded with an antiepileptic model drug to overcome the conventional systemic administration channel blocked by the blood–brain barrier.129 This study confirms that the device shows excellent biocompatibility to the brain tissue, which proves the viability of its safe implantation in the brain and its therapeutic effectiveness to treat epilepsy. Gulati et al reported a new alternative to treat brain-related diseases based on the drug-releasing implant prepared with anodizing Ti sheets to obtain TNTs on their surface for drug delivery application.130 The obtained in vitro results demonstrated that TNTs loaded with anticancer drugs can provide a controlled drug release to effectively destroy cancer cells and thus possess the outstanding capacity for localized treatment of brain-related diseases.
The biocompatibility of the as-prepared TNTs was studied by observing the growth of osteosarcoma (MG-63) cells on the TNT surface, and TNTs loaded with the antineoplastic drug were investigated by using MG-63 cells as targets and cisplatin as the model drug.131 Their results demonstrated that TNTs show excellent biocompatibility and can be used as a drug delivery vehicle, with efficient drug loading and releasing ability to significantly suppress the growth of MG-63 cells. Moreover, Kalbacova et al demonstrated that TNTs can be used as a photocatalyst to kill cancer cells.132 In this work, it was reported that a focused UV light or X-ray excitation to trigger a photocatalytic reaction on TiO2 administrated locally to a tumor. From these studies, it is suggested that TNTs have the potential to be used for localized cancer therapy, and more advanced systems are expected to be designed for exploring these concepts in the future.
Conclusion and future perspectives
This work reviewed the recent advances in the research of drug-releasing implants based on TNTs. It is demonstrated that the application of TNTs is a promising alternative to develop various localized drug delivery systems that possess the capability to overcome the limitations of systemic drug therapies. TNT layers can be used as orthopedic and stent implants without limitations in their shapes and forms based on the remarkable properties of TNTs such as excellent biocompatibility and mechanical and thermal stability, thereby promoting bone cell adhesion, differentiation and proliferation, hydroxyapatite formation, osseointegration, and hemocompatibility.
It is confirmed that several smart strategies including pH, thermal, light, RF, magnetic, and ultrasound were used as triggers for drug release from TNTs, which show outstanding features offering great perspectives and opportunities for TNT applications. Although still at initial stage, these external stimulus strategies can be described as promising triggers for controlling drug release from TNT implants. A series of studies involving ex vivo and in vivo animal models have been performed to prove the excellent biocompatibility of the TNT implants. These concepts may be applied for human clinical trials after long-term toxicity assay and tolerability studies carried out on animals to evaluate the safety. Therefore, more in vivo studies should be performed for the localized drug delivery systems applied in clinical trials.
Finally, the drug-releasing TNT implants are used for localized drug delivery in the field of medical applications, including bone therapies, dentistry, cardiovascular stents, treatment of localized cancer, and other therapies that required an implantable device. These multifaceted localized drug delivery systems based on TNTs are used to control the drug release with optimized concentration for a range of time scales, which is expected to facilitate their smoother translation into clinical practice in the future.
Acknowledgments
We thank the National Natural Science Foundation of People’s Republic of China (21501127; 51502185), Natural Science Foundation of Jiangsu Province of People’s Republic of China (BK20130313; BK20140400), and Natural Science Foundation of the Jiangsu Higher Education Institutions of People’s Republic of China (15KJB430025). We also acknowledge the funds from the project of the Priority Academic Program Development of Jiangsu Higher Education Institutions and King Saud University, Vice Deanship of Scientific Research Chairs.
Disclosure
The authors report no conflicts of interest in this work.
References
Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5:449–455. | ||
Hoffman AS. The origins and evolution of “controlled” drug delivery systems. J Control Release. 2008;132:153–163. | ||
Santos A, Aw MS, Bariana M, Kumeria T, Wang Y, Losic D. Drug-releasing implants: current progress, challenges and perspectives. J Mater Chem B. 2014;2:6157–6182. | ||
Raliya R, Singh Chadha T, Haddad K, Biswas P. Perspective on nanoparticle technology for biomedical use. Curr Pharm Design. 2016;22: 2481–2490. | ||
Lin CX, Qiao SZ, Yu CZ, Ismadji S, Lu GQ. Periodic mesoporous silica and organosilica with controlled morphologies as carriers for drug release. Micropor Mesopor Mat. 2009;117:213–219. | ||
Gary-Bobob M, Hocinea O, Breveta D, et al. Cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. Int J Pharm. 2012;423:509–515. | ||
Lina CX, Yuan P, Yua CZ, Qiao SZ, Lu GQ. Cooperative self-assembly of silica-based mesostructures templated by cationic fluorocarbon/hydrocarbon mixed-surfactants. Micropor Mesopor Mat. 2009;126:253–261. | ||
Anglina EJ, Cheng LY, Freemanb WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv Drug Delivery Rev. 2008;60:1266–1277. | ||
Salonen J, Kaukonen AM, Hirvonen J, Lehto V-P. Mesoporous silicon in drug delivery applications. J Pharm Sci. 2008;97:632–653. | ||
Martin CR, Kohli P. The emerging field of nanotube biotechnology. Nat Rev Drug Discov. 2003;2:29–37. | ||
Zhang Y, Jiang Z, Huang J, et al. Titanate and titania nanostructured materials for environmental and energy applications: a review. RSC Adv. 2015;5:79479–79510. | ||
Ge MZ, Cao CY, Huang JY, et al. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J Mater Chem A. 2016;4:6772–6801. | ||
Lai YK, Huang JY, Cui ZQ, et al. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small. 2016;12:2203–2224. | ||
Hamlekhan A, Sinha-Ray S, Takoudis C, et al. Fabrication of drug eluting implants: study of drug release mechanism from titanium dioxide nanotubes. J Phys D: Appl Phys. 2015;48:275401. | ||
Moon KS, Bae JM, Jin S, Oh S. Infrared-mediated drug elution activity of gold nanorod-grafted TiO2 nanotubes. J Nanomater. 2014;2014: 750813. | ||
Song YY, Schmidt-Stein F, Bauer S, Schmuki P. Amphiphilic TiO2 nanotube arrays: an actively controllable drug delivery system. J Am Chem Soc. 2009;131:4230–4232. | ||
Song YY, Roy P, Paramasivam I, Schmuki P. Voltage-induced payload release and wettability control on TiO2 and TiO2 nanotubes. Angew Chem Int Ed. 2010;49:351–354. | ||
Lai YK, Gao XF, Zhuang HF, Huang JY, Lin CJ, Jiang L. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv Mater. 2009;21:3799–3803. | ||
Ge M, Cao C, Li S, et al. Enhanced photocatalytic performances of n-TiO2 nanotubes by uniform creation of p-n heterojunctions with p-Bi2O3 quantum dots. Nanoscale. 2015;7:11552–11560. | ||
Paulose M, Shankar K, Yoriya S, et al. Anodic growth of highly ordered TiO2 nanotube arrays to 134 μm in length. J Phys Chem B. 2006;110:16179–16184. | ||
Losic D, Aw MS, Santos A, Gulati K, Bariana M. Titania nanotube arrays for local drug delivery: recent advances and perspectives. Expert Opin Drug Deliv. 2015;12:103–127. | ||
Roy P, Berger S, Schmuki P. TiO2 nanotubes: synthesis and applications. Angew Chem Int Ed. 2011;50:2904–2939. | ||
Bariana M, Aw MS, Moore E, Voelcker NH, Losic D. Radiofrequency-triggered release for on-demand delivery of therapeutics from titania nanotube drug-eluting implants. Nanomedicine. 2014;9:1263–1275. | ||
Lai Y, Pan F, Xu C, Fuchs H, Chi L. In situ surface-modification-induced superhydrophobic patterns with reversible wettability and adhesion. Adv Mater. 2013;25:1682–1686. | ||
Brancho JJ, Bartlett BM. Challenges in co-alloyed titanium oxynitrides, a promising class of photochemically active materials. Chem Mater. 2015;27:7207–7217. | ||
Jia H, Kerr LL. Sustained ibuprofen release using composite poly (lactic-co-glycolic acid)/yitanium dioxide nanotubes from Ti implant surface. J Pharm Sci. 2013;102:2341–2348. | ||
Brammer KS, Kim H, Noh K, et al. Highly bioactive 8 nm hydrothermal TiO2 nanotubes elicit enhanced bone cell response. Adv Eng Mater. 2011;13:B88–B94. | ||
Simchi A, Tamjid E, Pishbin F, Boccaccini AR. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomedicine. 2011;7:22–39. | ||
Lozano D, Hernandez-Lopez JM, Esbrit P, et al. Influence of the nanostructure of F-doped TiO2 films on osteoblast growth and function. J Biomed Mater Res A. 2015;103:1985–1990. | ||
Losic D, Simovic S. Self-ordered nanopore and nanotube platforms for drug delivery applications. Expert Opin Drug Deliv. 2009;6:1363–1381. | ||
Zwilling V, Darque-Ceretti E, Boutry-Forveille A, David D, Perrin MY, Aucouturier M. Structure and physicochemistry of anodic oxide films on titanium and TA6V alloy. Surf Interface Anal. 1999;27:629–637. | ||
Huang JY, Lai YK, Pan F, et al. Multifunctional superamphiphobic TiO2 nanostructure surfaces with facile wettability and adhesion engineering. Small. 2014;10:4865–4873. | ||
Ge MZ, Cao CY, Huang JY, et al. Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2. Nanotechnol Rev. 2016;5:75–122. | ||
Macak JM, Tsuchiya H, Ghicov A, et al. TiO2 nanotubes: self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci. 2007;11:3–18. | ||
Mor GK, Varghese OK, Paulose M, Shankar K, Grimes CA. A review on highly ordered, vertically oriented TiO2 nanotube arrays: fabrication, material properties, and solar energy applications. Sol Energ Mat Sol C. 2006;90:2011–2075. | ||
Macak JM, Schmuki P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim Acta. 2006;52:1258–1264. | ||
Prakasam HE, Shankar K, Paulose M, Varghese OK, Grimes CA. A new benchmark for TiO2 nanotube array growth by anodization. J Phys Chem C. 2007;111:7235–7241. | ||
Kant K, Losic D. A simple approach for synthesis of TiO2 nanotubes with through-hole morphology. Phys Status Solidi A. 2009;3:139–141. | ||
Wang LN, Jin M, Zheng YD, Guan YP, Lu X, Luo JL. Nanotubular surface modification of metallic implants via electrochemical anodization technique. Int J Nanomed. 2014;9:4421–4435. | ||
Macak JM, Albu SP, Schmuki P. Towards ideal hexagonal self-ordering of TiO2 nanotubes. Phys Status Solidi A. 2007;1:181–183. | ||
Paulose M, Peng L, Popat KC, et al. Fabrication of mechanically robust, large area, polycrystalline nanotubular/porous TiO2 membranes. J Memb Sci. 2008;319:199–205. | ||
Gulati K, Aw MS, Findlay D, Losic D. Local drug delivery to the bone by drug releasing implants: perspectives of nanoengineered titania nanotube arrays. Ther Deliv. 2012;3:857–873. | ||
Ge MZ, Cao CY, Li SH, et al. In situ plasmonic Ag nanoparticle anchored TiO2 nanotube arrays as visible-light-driven photocatalysts for enhanced water splitting. Nanoscale. 2016;8:5226–5234. | ||
Ghicov A, Schmuki P. Self-ordering electrochemistry: a review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem Commun. 2009;20:2791–2808. | ||
Losic D, Velleman L, Kant K, et al. Self-ordering electrochemistry: a simple approach for engineering nanopore and nanotube arrays for emerging applications. Aust J Chem. 2011;64:294–301. | ||
Grimes CA. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J Mater Chem. 2007;17:1451–1457. | ||
Smith BS, Popat KC. Titania nanotube arrays as interfaces for blood-contacting implantable devices: a study evaluating the nanotopography-associated activation and expression of blood plasma components. J Biomed Nanotechnol. 2010;8:642–658. | ||
Macak JM, Hildebrand H, Marten-Jahns U, Schmuki P. Mechanistic aspects and growth of large diameter self-organized TiO2 nanotubes. J Electroanal Chem. 2008;621:254–266. | ||
Tian A, Qin XF, Wu A, et al. Nanoscale TiO2 nanotubes govern the biological behavior of human glioma and osteosarcoma cells. Int J Nanomed. 2015;10:2423–2439. | ||
Ding XL, Zhou L, Wang JX, et al. The effects of hierarchical micro/nanosurfaces decorated with TiO2 nanotubes on the bioactivity of titanium implants in vitro and in vivo. Int J Nanomed. 2015;10:6955–6973. | ||
Lai YK, Huang YX, Wang H, Huang JY, Chen Z, Lin CJ. Selective formation of ordered arrays of octacalcium phosphate ribbons on TiO2 nanotube surface by template-assisted electrodeposition. Colloids Surf B. 2010;76:117–122. | ||
Yang Y, Lai Y, Zhang Q, et al. A novel electrochemical strategy for improving blood compatibility of titanium-based biomaterials. Colloids Surf B. 2010;79:309–313. | ||
Rafieerad AR, Bushroa AR, Zalnezhad E, et al. Microstructural development and corrosion behavior of self-organized TiO2 nanotubes coated on Ti-6Al-7Nb. Ceram Int. 2015;41:10844–10855. | ||
Chembath M, Balaraju JN, Sujata M. Surface characteristics, corrosion and bioactivity of chemically treated biomedical grade NiTi alloy. Mater Sci Eng C. 2015;56:417–425. | ||
Peng L, Eltgroth ML, LaTempa TJ, Grimes CA, Desai TA. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30:1268–1272. | ||
Li H, Lai Y, Huang J, et al. Multifunctional wettability patterns prepared by laser processing on superhydrophobic TiO2 nanostructured surfaces. J Mater Chem B. 2015;3:342–347. | ||
Popat KC, Leoni L, Grimes CA, Desai TA. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials. 2007;28:3188–3197. | ||
Rahman ZU, Haider W, Pompa L, Deen KM. Electrochemical & osteoblast adhesion study of engineered TiO2 nanotubular surfaces on titanium alloys. Mater Sci Eng C. 2016;58:160–168. | ||
Li Z, Yang X, Guo H, Yang X, Sun L, Dong S. Hydroxyapatite additive influenced the bioactivity of bioactive nano-titania ceramics and new bone-forming capacity. J Nanopart Res. 2012;14:1145–1153. | ||
Fan X, Feng B, Weng J, Wang J, Lu X. Processing and properties of porous titanium with high porosity coated by bioactive titania nanotubes. Mater Lett. 2011;65:2899–2901. | ||
Mohan CC, Chennazhi KP, Menon D. In vitro hemocompatibility and vascular endothelial cell functionality on titania nanostructures under static and dynamic conditions for improved coronary stenting applications. Acta Biomater. 2013;9:9568–9577. | ||
Kim HW, Koh YH, Li LH, Lee S, Kim HE. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol-gel method. Biomaterials. 2004;25:2533–2538. | ||
Advincula MC, Rahemtulla FG, Advincula RC, Ada ET, Lemons JE, Bellis SL. Osteoblast adhesion and matrix mineralization on sol-gel-derived titanium oxide. Biomaterials. 2006;27:2201–2212. | ||
Lai YK, Lin LX, Pan F, et al. Bioinspired patterning with extreme wettability contrast on TiO2 nanotube array surface: a versatile platform for biomedical applications. Small. 2013;9:2945–2953. | ||
Brammer KS, Oh S, Gallagher JO, Jin S. Enhanced cellular mobility guided by TiO2 nanotube surfaces. Nano Lett. 2008;8:786–793. | ||
Wang H, Lai YK, Zheng RY, Bian Y, Zhang KQ, Lin CJ. Tuning the surface microstructure of titanate coating on titanium implants for enhanced bioactivity of implants. Int J Nanomed. 2015;10:3887–3896. | ||
Oh S, Daraio C, Chen LH, Pisanic TR, Fiñones RR, Jin S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J Biomed Mater Res A. 2006;78A:97–103. | ||
Oh S, Jin S. Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater Sci Eng C. 2006;26C:1301–1306. | ||
Feschet-Chassot E, Raspal V, Sibaud Y, et al. Tunable functionality and toxicity studies of titanium dioxide nanotube layers. Thin Solid Films. 2011;519:2564–2568. | ||
Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7:1686–1691. | ||
Hu Y, Cai K, Luo Z, et al. TiO2 nanotubes as drug nanoreservoirs for the regulation of mobility and differentiation of mesenchymal stem cells. Acta Biomater. 2012;8:439–448. | ||
Park J, Bauer S, Schlegel KA, Neukam FW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15 nm-an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009;5:666–671. | ||
Bauer S, Park J, von der Mark K, Schmuki P. Improved attachment of mesenchymal stem cells on super-hydrophobic TiO2 nanotubes. Acta Biomater. 2008;4:1576–1582. | ||
von Wilmowsky C, Bauer S, Lutz R, et al. In vivo evaluation of anodic TiO2 nanotubes: an experimental study in the pig. J Biomed Mater Res B. 2009;89B:165–171. | ||
Park JM, Koak JY, Jang JH, Han CH, Kim SK, Heo SJ. Osseointegration of anodized titanium implants coated with fibroblast growth factor-fibronectin (FGF-FN) fusion protein. Int J Oral Maxillofac Implants. 2006;21:859–866. | ||
Bjursten LM, Rasmusson L, Oh S, Smith GC, Brammer KS, Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J Biomed Mater Res A. 2010;92:1218–1224. | ||
Jia H, Kerr LL. Kinetics of drug release from drug carrier of polymer/TiO2 nanotubes composite-pH dependent study. J Appl Polym Sci. 2015;132:41750. | ||
Cai K, Jiang F, Luo Z, Chen X. Temperature-responsive controlled drug delivery system based on titanium nanotubes. Adv Eng Mater. 2010;12:B565–B570. | ||
Guo R, Zhang L, Qian H, Li R, Jiang X, Liu B. Multifunctional nanocarriers for cell imaging, drug delivery, and near-IR photothermal therapy. Langmuir. 2010;26:5428–5434. | ||
Sada T, Fujigaya T, Niidome Y, Nakazawa K, Nakashima N. Near-IR laser-triggered target cell collection using a carbon nanotube-based cell-cultured substrate. ACS Nano. 2011;5:4414–4421. | ||
Choi J, Yang J, Bang D, et al. Targetable gold nanorods for epithelial cancer therapy guided by near-IR absorption imaging. Small. 2012;8:746–753. | ||
Chen R, Wang X, Yao X, Zheng X, Wang J, Jiang X. Near-IR-triggered photothermal/photodynamic dual modality therapy system via chitosan hybrid nanospheres. Biomaterials. 2013;34:8314–8322. | ||
Joshi PP, Yoon SJ, Chen YS, Emelianov S, Sokolov KV. Development and optimization of near-IR contrast agents for immune cell tracking. Biomed Opt Express. 2013;4:2609–2618. | ||
Robinson JT, Welsher K, Tabakman SM, et al. High performance in vivo near-IR (>1 μm) imaging and photothermal cancer therapy with carbon nanotubes. Nano Res. 2010;3:779–793. | ||
Aw MS, Kurian M, Losic D. Non-eroding drug-releasing implants with ordered nanoporous and nanotubular structures: concepts for controlling drug release. Biomater Sci. 2014;2:10–34. | ||
Glazer ES, Curley SA. Radiofrequency field-induced thermal cytotoxicity in cancer cells treated with fluorescent nanoparticles. Cancer. 2010;116:3285–3293. | ||
Curley SA, Cherukuri P, Briggsetal K. Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. J Pharmacol Exp Ther. 2008;7:313–326. | ||
Glazer ES, Massey KL, Zhu C, Curley SA. Pancreatic carcinoma cells are susceptible to noninvasive radio frequency fields after treatment with targeted gold nanoparticles. Surgery. 2010;148:319–324. | ||
Cherukuri P, Curley SA. Use of nanoparticles for targeted, noninvasive thermal destruction of malignant cells. Methods Mol Biol. 2010;624:359–373. | ||
Cherukuri P, Glazer ES, Curley SA. Targeted hyperthermia using metal nanoparticles. Adv Drug Deliv Rev. 2010;62:339–345. | ||
Gannon CJ, Cherukuri P, Yakobson BI, et al. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–2665. | ||
Gannon CJ, Patra CR, Bhattacharya R, Mukherjee P, Curley SA. Intracellular gold nanoparticles enhance non-invasive radiofrequency thermal destruction of human gastrointestinal cancer cells. J Nanobiotechnol. 2008;6:1–9. | ||
Raoof M, Curley SA. Non-invasive radiofrequency-induced targeted hyperthermia for the treatment of hepatocellular carcinoma. Int J Hepatol. 2011;2011:676957. | ||
Cardinal J, Klune JR, Chory E, et al. Noninvasive radiofrequency ablation of cancer targeted by gold nanoparticles. Surgery. 2008;144:125–132. | ||
Aw MS, Addai-Mensah J, Losic D. Magnetic-responsive delivery of drugcarriers using titania nanotube arrays. J Mater Chem. 2012;22:6561–6563. | ||
Shrestha NK, Macak JM, Schmidt-Stein F, et al. Magnetically guided titania nanotubes for site-selective photocatalysis and drug release. Angew Chem Int Ed. 2009;48:969–972. | ||
Aw MS, Losic D. Ultrasound enhanced release of therapeutics from drug-releasing implants based on titania nanotube arrays. Int J Pharm. 2013;443:154–162. | ||
Aninwene GE, Yao C, Webster TJ. Enhanced osteoblast adhesion to drug-coated anodized nanotubular titanium surfaces. Int J Nanomed. 2008;3:257–264. | ||
Gulati K, Kant K, Findlay D, Losic D. Periodically tailored titania nanotubes for enhanced drug loading and releasing performances. J Mater Chem B. 2015;3:2553–2559. | ||
Shokuhfar T, Sinha-Ray S, Sukotjo C, Yarin AL. Intercalation of anti-inflammatory drug molecules within TiO2 nanotubes. RSC Adv. 2013;3:17380–17386. | ||
Aw MS, Gulati K, Losic D. Controlling drug release from titania nanotube arrays using polymer nanocarriers and biopolymer coating. Biomater Nanobiotech. 2011;2:477–484. | ||
Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials. 2007;28:4880–4888. | ||
Rungsiyakull C, Li Q, Sun GY, Li W, Swain MV. Surface morphology optimization for osseointegration of coated implants. Biomaterials. 2010;31:7196–7204. | ||
Lai M, Cai K, Zhao L, Chen X, Hou Y, Yang Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules. 2011;12:1097–1105. | ||
Park J, Bauer S, Schmuki P, von der Mark K. Narrow window in nanoscale dependent activation of endothelial cell growth and differentiation on TiO2 nanotube surfaces. Nano Lett. 2009;9:3157–3164. | ||
Yang W, Xi X, Ran Q, Liu P, Hu Y, Cai K. Influence of the titania nanotubes dimensions on adsorption of collagen: an experimental and computational study. Mater Sci Eng C. 2014;34:410–416. | ||
Xu D, Yang W, Hu Y, et al. Surface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responses. Colloids Surf B. 2013;110:225–235. | ||
Oha S, Brammera KS, Li YSJ, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci U S A. 2009;106:2130–2135. | ||
Bovan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137–146. | ||
Kunze J, Müller L, Macak JM, Greil P, Schmuki P, Müller FA. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim Acta. 2008;53:6995–7003. | ||
Shim IK, Chung HJ, Jung MR, et al. Biofunctional porous anodized titanium implants for enhanced bone regeneration. J Biomed Mater Res A. 2014;102A:3639–3648. | ||
Probst A, Spiegel HU. Cellular mechanisms of bone repair. J Invest Surg. 1997;10:77–86. | ||
Moioli EK, Clark PA, Xin X, Lal S, Mao JJ. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv Drug Deliv Rev. 2007;59:308–324. | ||
Zhao L, Wang H, Huo K, et al. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011;32:5706–5716. | ||
Gawri S, Shukla P, Chandrakar A. A survey of micro flora present in dental caries and it’s relation to enviornmental factors. Recent Res Sci Technol. 2012;4:9–12. | ||
Biesbrock A, Faller R, Bartizek R, McClanahan S. Reversal of incipient and radiographic caries through the use of sodium and stannous fluoride dentifrices in a clinical trial. J Clin Dent. 1998;9:5–10. | ||
Linton JL. Quantitative measurements of remineralization of incipient caries. Am J Orthod Dentofacial Orthop. 1996;110:590–597. | ||
Demetrescu I, Pirvu C, Mitran V. Effect of nano-topographical features of Ti/TiO2 electrode surface on cell response and electrochemical stability in artificial saliva. Bioelectrochemistry. 2010;79:122–129. | ||
Ma Q, Mei S, Ji K, Zhang Y, Chu PK. Immobilization of Ag nanoparticles/FGF-2 on a modified titanium implant surface and improved human gingival fibroblasts behavior. J Biomed Mater Res A. 2011;98:274–286. | ||
Bhattarai G, Lee Y, Lee M, Yi H. Gene delivery of c-myb increases bone formation surrounding oral implants. J Dent Res. 2013;92:840–845. | ||
Erbel R, Mario CD, Bartunek J, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369:1869–1875. | ||
Serruys PW, de Jaegere P, Kiemeneij F, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N Engl J Med. 1994;331:489–495. | ||
Wieneke H, Dirsch O, Sawitowski T, et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheter Cardiovasc Interv. 2003;60:399–407. | ||
Kollum M, Farb A, Schreiber R, et al. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimuseluting stents in a porcine model of restenosis. Catheter Cardiovasc Interv. 2005;64:85–90. | ||
Fine E, Zhang L, Fenniri H, Webster TJ. Enhanced endothelial cell functions on rosette nanotube-coated titanium vascular stents. Int J Nanomed. 2009;4:91–97. | ||
Changrong L, Zhihong Z, Fuming Z, et al. TiO2 films prepared by ion beam assisted deposition. Nucl Instr Med Phys Res. 2000;169:21–25. | ||
Wang X, Zhang F, Li C, et al. Improvement of blood compatibility of artificial heart valves via titanium oxide film coated on low temperature isotropic carbon. Surf Coat Tech. 2000;128:36–42. | ||
Peng L, Barczak AJ, Barbeau RA, et al. Whole genome expression analysis reveals differential effects of TiO2 nanotubes on vascular cells. Nano Lett. 2010;10:143–148. | ||
López T, Ortiz E, Quintana P, González RD. A nanostructured titania bioceramic implantable device capable of drug delivery to the temporal lobe of the brain. Colloids Surf A. 2007;300:3–10. | ||
Gulati K, Sinn Aw M, Losic D. Nanoengineered drug-releasing Ti wires as an alternative for local delivery of chemotherapeutics in the brain. Int J Nanomed. 2012;7:2069–2076. | ||
Xiao X, Yang L, Guo M, Pan C, Cai Q, Yao S. Biocompatibility and in vitro antineoplastic drug-loaded trial of titania nanotubes prepared by anodic oxidation of a pure titanium. Sci China B Chem. 2009;52:2161–2165. | ||
Kalbacova M, Macak JM, Schmidt-Stein F, Mierke CT, Schmuki P. TiO2 nanotubes: photocatalyst for cancer cell killing. Phys Status Solidi RRL. 2008;2:194–196. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
