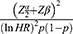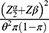Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 12
Time-to-Recovery from Severe Pneumonia and Its Determinants Among Children Under-Five Admitted to University of Gondar Comprehensive Specialized Hospital in Ethiopia: A Retrospective Follow-Up Study; 2015–2020
Authors Assfaw T , Yenew C , Alemu K , Sisay W, Geletaw T
Received 10 February 2021
Accepted for publication 29 March 2021
Published 21 April 2021 Volume 2021:12 Pages 189—196
DOI https://doi.org/10.2147/PHMT.S305383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Tigabnesh Assfaw,1 Chalachew Yenew,1 Kassahun Alemu,2 Wullo Sisay,2 Teshome Geletaw3
1Public Health, College of Medicine and Health Science, Debre Tabor University, Debre Tabor, Ethiopia; 2Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 3Pediatrics and Child Health Nursing, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Tigabnesh Assfaw
Public Health, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia
Tel +251 989763993
Email [email protected]
Background: Pneumonia, which is an infection and inflammation of an air-space in the lungs due to an impurity. Child mortality due to pheumonia is estimated at 921,000 children under 5 years (U5) in 2015.
Objective: To determine the TTR and factors of severe pneumonia among U5 children admitted at UOGCSH, Northwest Ethiopia.with
Methods: A facility-based retrospective follow-up study was conducted on children U5 severe pneumonia from 2015 to 2020. The data were collected using pre-test and structured questionnaires. Statistical analysis was performed using Stata version 14.1.
Result: The average TTR was 3 days IQR (3– 6). TTR from severe pneumonia was 13.5 (95% CI: 13.54– 17.15) per 100-persons. The cumulative time for children at risk was 1112 days, with a TTR of 29.7 per 100 children per day. Severity, signs and symptoms of pneumonia (AHR, 3.88 (95% CI =3.12– 5.57)); mode of infancy feeding (cows milk feeding) (AHR, 2.4, (95% CI: 2.22– 6.6)), and formula feeding (AHR, 0.68, (95% CI 0.58– 1.25)) as compared to breastfeeding; nutritional status (underweight) (AHR, 2.2, (95% CI: (2.1– 3.76)) as compared to normal, age (2– 3-years) (AHR, 1.4, (95% CI: 1.31– 2.22)), and ≥ 4-years (AHR, 1.32, (95% CI: 1.3– 2.32)) as compared to age of ≤ 1 year were important factors of TTR.
Conclusion: The overall TTR was 3 days IQR (2– 6). This study identifies severity, signs, and symptoms of pneumonia, Mode of infancy feeding (cows milk feeding, formula feeding), nutritional status, and age were main determinants of TTR.
Keywords: predictors, severe pneumonia, TTR, Gondar, Northwest Ethiopia
Background
Pneumonia is an important cause of morbidity and mortality, which caused 896,000 deaths from a total of 5.6 million deaths in 2016 alone.1 Among childhood diseases, ARIs are the greatest public diseases in children under 5 years (U5) throughout the globe, and it is the primary cause of mortality in LICs.2 Many measures have been taken to decrease child mortality. WHO guidelines provide health workers with the diagnostic methods for pneumonia so that children treated with simple antibiotics, and severe and complex cases can be managed through referral to higher levels of care. But many children have died because of failures in the health system, inequity, international cooperation, and failures of the public–private partnership for affordable vaccine.3
The WHO estimated that 10,000,000 U5 children died in 1998, 99% from LICs, additionally the number of losses was 920,000 or 16% in 2015.4
UNICEF assessed that pediatric pneumonia deaths are 3,000,000 children across the globe each year. Close to 150,000,000 cases of pneumonia occur yearly around the globe. Pneumonia is increasingly common in South Asia and sub-Saharan Africa, accounting for approximately 10,000,000 to 20,000,000 hospitalizations, the highest incidence of pneumonia cases among U5 children.5
In a mixed-methods study in Peruvian Amazon most children U5 in HICs and LICs, 4 to 6 times per year were affected to ARIs.6
The study of therapeutic and bacteriology reasons for death would be useful to assist public health efforts.7 The results of this study would help to reduce the extended time of hospitalization and its actual TTR; and would also fill the existing information gap related to poor treatment outcomes of pediatrics patients. There is one study on TTR from severe pneumonia among children in Ethiopia but it was done on under 15 years of age on mean TTR, our current study is unique in that it focused on U5 children TTR.
Methods
Study Site
The study was carried out in the UOGCSH which is a referral and teaching hospital, serving around 5,000,000 people. It is located 730 kilometers northwest of the capital of Ethiopia, Addis Ababa.
Study Design and Period
A facility-based retrospective follow-up study was conducted from 2015 to 2020.
Population
Source Population
Entire U5 children with severe pneumonia were admitted to UOGCSH.
Study Population
Records of eligible U5 children with unembellished pneumonia admitted to UOGCSH from 2015 to 2020.
Exclusion and Inclusion Criteria
Inclusion Criteria
Children aged 1–59 months who were admitted to pediatric wards; children with one or more of the following signs, oxygen ability 90% or central cyanosis or extreme respiratory pain or inability to drink or lactate or heaving anything, rehabilitated consciousness, and shaking were all included (WHO, 2013).
Exclusion Criteria
Children with incomplete records concerning variables of interest, children whose treatment outcome was not recorded, and those whose admission date and discharge dates were not recorded were eliminated from the study.
Sample Size Estimation
In order to have a representative sample of U5 severe pneumonia children, sample size was determined by using the Log rank test, the Freedman method.
Ho: S1 (t) = S2 (t) two-sided significance level (α = 5%), Za/2 = Z value at 95% confidence interval = 1.96, power 80% and p = 50% cumulative recovery rate, hazard ratio = 0.5. Accordingly, a total of 330 severe pneumonia children records were recruited.8
Zα/2: Z value at 95% confidence interval = 1.96
Zβ: the power of the study.
HR: hazard ratio.
θ = ln (HR), i.e HR = exp (θ) and then θ = ln (HR)
The total sample size is calculated as n=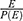
E=number of events that interested.
Sampling Techniques
A simple, random sampling technique was applied. To take a sample randomly from the sampling amount, Open-Epi software version 3 was used to generate random numbers. Unique severe pneumonia number was retrieved from the patient registry from smallest to the highest entered into the software to choose a trial of 330 records.
Study Variables
Dependent Variable
TTR from severe pneumonia.
Predictors
Socio-demographic factors: place of residence, age of children, sex of children.
Clinical factors: nutritional status, vaccination status of children, breastfeeding history, history of illness and drug regimen, chest x-ray finding, presence of anemia, and presence of rickets.
Comorbidity status: HIV-infection, heart-failure, congenital heart diseases, asthma, Down syndrome, DM, and hyperactive airway illness.
Data Gathering Tool and Technique
Data was gathered using pre-test and structured questionnaires. These questionnaires assessed demographic characteristics and clinical characteristics. Two BSc nurses, one supervisor, supervised the data collection, one postgraduate student took part. Two professional data collectors and one administrator were engaged, who were competent and trained in severe pneumonia supervision. Moreover, an additional one day of training was provided for data collectors to apprise on the data gathering process.
Data Processing and Analysis
Epi data version 4.3.2 was used to extract, code, tabulate, and enter data, which was then entered into Stata 14.1 for analysis. Based on the essence of the distribution, basic descriptive dissection was performed in terms of central tendency and dispersion value for continuous data and frequency distribution for categorical data.
Days serve as a time scale for calculating TTR. The Log rank test and Kaplan–Meier investigation were used to estimate the survival curve and the existence of a difference in survival among explanatory variables.
Multicollinearity and model assumptions for each covariate were tested before running the Cox proportional hazard regression model. The model assumption was tested applying Schoenfeld residual tests, graphically adopting a log-log plot of survival, and a time-dependent test using (TVC) command. Additionally, the model was confirmed using the goodness-of-fit test by Schoenfeld residual, and variables having P > 0.05 were considered as fulfilling the assumption. Bivariable Cox regression was used, and those independent variables that matched the bivariable regression with a level of significance less than or equal to 0.25 were included in the multivariable analysis. Furthermore, a stepwise backward variable selection method was used to begin further variable selection. As the final model, a multivariable Cox proportional hazard regression model was used.
To assess the net effect of each explanatory variable on TTR of serious pneumonia, researchers used multivariable Cox proportional hazard regression with a 0.05 degree of significance. In the multivariable Cox proportional hazard regression study, a P-value of less than 0.05 was considered statistically important. The results of these models were expressed as 95% modified hazard ratios.
Data Quality Control
Supervisors and data collectors were instructed about how and what information they could obtain from the targeted data source to maintain data quality. Before collecting data, data extraction types are double-checked. The supervisor and the principal investigator reviewed the completeness and accuracy of the collected data regularly during data collection and provided adequate feedback.
Results
Socio-Demographic Characteristics
Among 330 study participants, the male to female ratio was=1.024 and 57.6% came from rural areas. The average age of the study participants was 2.86 years, with 168 (50.9%) of them being between the ages of two and three. Among modes of infancy feeding, the highest was breastfeeding which accounts for 274 (83%). Exclusive breast-feeding for the first six months applied to most children (240), at the time at risk of 767 with an incidence rate of 0. 213. IQR 3(2, 6) (Table 1).
 |
Table 1 Socio-Demographic Characteristics Among U5 Severe Pneumonia Children Admitted at the UOGCSH, Northwest Ethiopia, 2015–2020 |
Risk Factors and Clinical Characteristics of TTR
Underweight, stunting and wasting were found in 53 (16%), 38 (11.5%), and 47 (14.24%) of the study participants, respectively. 273 (or 82.8%) of the study participants were vaccinated, while 57 (17.2%) were not. 116 (35.2) of the children admitted with serious pneumonia had asthma. More than half of all children admitted as inpatients were given ceftriaxone, and 49 (14.8%) were given ampicillin with gentamicin (Table 2).
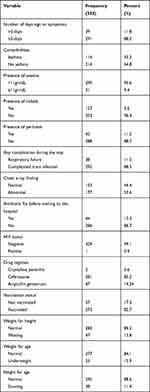 |
Table 2 Risk Factor and Baseline Clinical Characteristics Among U5 Severe Pneumonia Children Admitted at the UOGCSH, Northwest Ethiopia, 2015 to 2020 |
Treatment Outcome of TTR
Regarding the treatment implication of severe pneumonia 136 (41.21%) children were censored, 74 (22.42%) children had died, while 120 (36.36%) recovered (Figure 1). The global test value was (0.5622) shows p-value >0.05 this indicates, the proportional hazard assumption is not violated.
 |
Figure 1 Treatment outcome among U5 severe pneumonia children admitted at the UOGCSH, Northwest Ethiopia from 2015 to 2020. |
Over the Kaplan–Meier Survival Curve
The overall median survival TTR from severe pneumonia among U5 children was found to be 3-days (95% CI: 5–7). The chance of survival was higher on the first day after a serious pneumonia diagnosis, but it decreased as follow-up time increased (Figure 2).
 |
Figure 2 Overall Kaplan–Meier estimation of survival function from admission to recovery among U5 severe pneumonia children admitted at the UOGCSH, Northwest Ethiopia from 2015 to 2020. |
Evaluation of Survival Time
The survival time between groups of different predictors was measured using the Log rank test. Survival time was significantly different among various groups of predictors, such as nutritional status (WFA, WFH) and other baseline clinical characteristics, based on this examination, at a 5% level of significance (Table 3).
 |
Table 3 Multivariable Cox Proportional Regression Analysis for Independent Predictors of Recovery Among U5 Severe Pneumonia Children Admitted at the UOGCSH, Northwest Ethiopia from 2015 to 2020 |
Predictors of TTR from Severe Pneumonia
In the multivariable Cox proportional hazard regression model age, mode of breastfeeding, weight for age, and severity, sign and symptom of pneumonia were significant determinants (P< 0.05). As multivariable Cox-proportional hazard regression analysis showed that age was one of the social and demographic characteristic variables. For a unit increment in age above 4 years of U5 severe pneumonia patients, the TTR recent from severe pneumonia was increased by 1.32 times as compared to age less than or equal to one year (AHR, 1.32, (95% CI: 1.3–2.32)). For a unit increment in age (2–3years), the TTR recent from severe pneumonia increased by 1.35 times as compared to less than or equal to one year (AHR, 1.35, (95% CI: 1.31–2.22)).
This indicates that the pneumonic children for less than or equal to one year were shorter TTR than 2–3 years of age. The TTR of cows milk fed children from severe pneumonia was increased by 2.4 times as compared to breastfeeding (AHR, (2.4, 95% CI: 2.22–6.6)). This indicates that breastfed children had a shorter recovery time than cows milk fed children. The TTR of formula fed children from severe pneumonia was decreased by 0.32 times as compared to breastfed children (AHR, 0.68, (95% CI 0.58–1.25)). This indicates that the TTR was reduced by 32%. The TTR of wasting children from severe pneumonia was decreased by 0.11 times as compared to normal weight (AHR, 0.99, (95% CI:0.88–1.02)). This indicates that the TTR was reduced by 11%.
The TTR of underweight children from severe pneumonia was increased by 2.2 times as compared to normal weight (AHR, 2.2, (95% CI: 2.1–3.76)). This indicates that normal weight was a shorter TTR than under-weight. The TTR of children who were non-admitted with severity, sign, and symptoms of severe pneumonia was increased by 3.88 as compared to admitted with severity, signs, and symptoms (AHR, 3.88, (95% CI: 3.12–5.57)). This indicates that children admitted with severity, sign, and symptoms were shorter TTR than non-admitted severity, signs, and symptoms (Table 3).
Discussion
The median TTR from Severe pneumonia of the current study was 3 days. This is smaller than a survey from the year before at Mulago hospital Uganda and Debre Markose Hospital Ethiopia, the duration of hospital stays from admission to death were 7 days and 17.4 days, respectively.9
This study outcome is higher than the previous study done in Nepal for 2 days.10 This variation might be due to socio-economic differences in the study areas, time variation in which the studies were conducted. As the study was conducted from 2015 to 2020, different situations such as treatment protocol, a small number of cases affected by it which could reduce the hospitalization period have been done after it. An additional possible reason for this discrepancy might be related to differences in treatment, care practices, and health care settings where the study was conducted. Another explanation for the discrepancy may be due to differences in baseline health conditions and treatment protocols between study areas.
The age of children was an important socio-demographic predictor in this research, and it had a major impact on the improvement of extreme pneumonia patients, which is consistent with a study in Nepalese hospitalized young children.11
Breastfeeding was found to be a significant determinant of the need for antibiotic changes and a longer hospital stay in serious pneumonia, according to a case-control study conducted in Kenya.12
The research looked at the nutritional condition of children who were admitted to the hospital due to serious pneumonia. One of the significant predictors of TTR from extreme pneumonia is being underweight. When compared to average children, underweight children take longer to recover. This means that a patient who is of average weight is more likely to survive than one who is underweight, since nutrition was an integral part of the body’s overall metabolic process and recovery from illness.
This research matches that of a Ugandan report.13
Another significant clinical indicator of TTR from serious pneumonia was the occurrence of pneumonia incidence, signs, and symptoms. This suggests that children admitted with severity, signs, and symptoms recovered more quickly than children who were not admitted with severity, signs, and symptoms. This result is in line with the findings of a follow-up study conducted in Kenya.14
In this study, hyperactive airway disease (HAAD), pertussis, tuberculosis, HIV infection, DM, and other diseases were considered as comorbid diseases with severe pneumonia. Children who were admitted to the hospital without comorbidity lower recovery as compared to children who were admitted with comorbidity.
This finding is consistent with other studies.15
Since the research was conducted retrospectively by analyzing patient charts, all variables, including socio-demographic, socio-economic, and environmental characteristics that could be possible predictors of the outcome variable.
Conclusions
This research assesses the length of time it takes to recover from serious pneumonia, as well as possible predictors. The TTR from serious pneumonia was 3 days on average. Age, mode of breastfeeding, weight for age, and severity, and signs and symptoms of pneumonia were statistically relevant measures of TTR from the diseases in the last multivariable Cox-proportional hazard regression model, However, there was no statistical correlation between drug regimen, weight for height, history of breastfeeding for the first six months, and pertussis and TTR. Steps would be taken to and the time it takes to heal from diseases.
Abbreviations
TTR, Time-To-Recovery; AHR, Adjusted Hazard Ratio; HFA, Height for Age; HICs, High-income countries; LICs, low-income countries; U5, Under-five; UOGCSH, University of Gondar Comprehensive Specialized Hospital; UNICEF, United Nation Children’s Fund; WFA, Weight for Age; WFH, Weight for Height; WHO, World Health Organization.
Data Sharing Statement
All data generated and analyzed during this study are included in the published article.
Ethics Approval and Consent to Participe
Ethical clearance was obtained from the Institutional Review Board of the UOGCSH and an official letter was submitted to the concerned bodies. The concerned bodies were informed to get the assurance of the study and confidentiality was maintained at all levels of the study. Informed consent was obtained from children's parents or legal guardians. The Institutional Review Board University of Gondar approved it with Ethical Approval of Research protocol letter with reference number IRB837/2020.
Acknowledgments
The authors want to acknowledge the University of Gondar and the pediatric staff who helped to do this study. Our acknowledgment also extends to Mr. Chalachew Yenew, Dr. Teshome Geletaw, Mr. Derso Sisay, and Mr. Berihun lake for extensive and critical review and editing of the manuscript.
Author Contributions
KA, WS and TG were actively involved in the conception and design of the research issues, execution and acquisition of the data, analysis and interpretation and critical review the manuscript. CY actively worked on conception and design of the research issues, drafting the article and critically reviewing and revising the manuscript, and TA was a major contributor in conception and design of the research issues, execution and acquisition of the data, data coding and entry, data analysis, writing a research report and the final manuscript. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
No funds were obtained for this particular study.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Dembele BP, Kamigaki T, Dapat C, et al. Aetiology and risks factors associated with the fatal outcomes of childhood pneumonia among hospitalised children in the Philippines from 2008 to 2016: a case series study. BMJ Open. 2019;9(3):e026895.
2. Ramezani M, Aemmi SZ, Emami Moghadam Z. Factors Affecting the Rate of Pediatric Pneumonia in Developing Countries: a Review and Literature Study. Int J Pediatr. 2014;3(6.2):1173–1181.
3. Le Roux DM, Zar HJ. Community-acquired pneumonia in children—a changing spectrum of disease. Pediatr Radiol. 2017;47(11):1392–1398. doi:10.1007/s00247-017-3827-8
4. Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82(12):895–903.
5. Bennett NJ. What is the Global Prevalence of Pneumonia: Medscape; 2018. Available from: https://www.medscape.com/answers/961169-171992/what-is-the-global-prevalence-of-bacteremia.
6. Pajuelo MJ, Anticona Huaynate C, Correa M, et al. Delays in seeking and receiving health care services for pneumonia in children under five in the Peruvian Amazon: a mixed-methods study on caregivers’ perceptions. BMC Health Serv Res. 2018;18(1):149. doi:10.1186/s12913-018-2950-z
7. Benet T, Picot VS, Awasthi S, et al, Severity of Pneumonia in Under 5-Year-Old Children from Developing Countries: a Multicenter, Prospective, Observational Study. Am J Tropical Med Hygiene. 2017;97(1):68–76.
8. Boschi-Pinto C, Dilip TR, Costello A. Association between community management of pneumonia and diarrhoea in high-burden countries and the decline in under-five mortality rates: an ecological analysis. BMJ Open. 2017;7(2):e012639. doi:10.1136/bmjopen-2016-012639
9. Das RR, Singh M. Treatment of severe community-acquired pneumonia with oral amoxicillin in under-five children in developing country: a systematic review. PLoS One. 2013;8(6):e66232. doi:10.1371/journal.pone.0066232
10. Champatiray J, Satapathy J, Kashyap B, Mondal D. Clinico-aetiological study of severe and very severe pneumonia in two months to five years children in a tertiary health care centre in Odisha, India. J Clin Diagnostic Res. 2017;11(9):SC06–SC10. doi:10.7860/JCDR/2017/26027.10595
11. Basnet S, Sharma A, Mathisen M, et al. Predictors of Duration and Treatment Failure of Severe Pneumonia in Hospitalized Young Nepalese Children. PLos One. 2008;10(3):e0122052. doi:10.1371/journal.pone.0122052
12. Fonseca Lima EJ, Mello MJ, Albuquerque MF, et al. Risk factors for community-acquired pneumonia in children under five years of age in the post-pneumococcal conjugate vaccine era in Brazil: a case control study. BMC Pediatr. 2016;16(1):157. doi:10.1186/s12887-016-0695-6
13. Onyango D, Kikuvi G, Amukoye E, et al. Risk factors of severe pneumonia among children aged 2–59months in western Kenya: a case control study. Pan African Med J. 2012;13(1).
14. Chisti MJ, Ahmed T, Ashraf H, et al. Clinical predictors and outcome of metabolic acidosis in under-five children admitted to an urban hospital in Bangladesh with diarrhea and pneumonia. PLoS One. 2012;7(6):e39164. doi:10.1371/journal.pone.0039164
15. Gordon KA, Biedenbach DJ, Jones RN. Comparison of Streptococcus pneumoniae and Haemophilus influenzae susceptibilities from community-acquired respiratory tract infections and hospitalized patients with pneumonia: five-year results for the SENTRY Antimicrobial Surveillance Program. Diagn Microbiol Infect Dis. 2003;46(4):285–289. doi:10.1016/S0732-8893(03)00087-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.