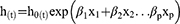Back to Journals » Open Access Surgery » Volume 17
Time to Recovery and Its Predictors in Patients with Traumatic Brain Injury Who Underwent Urgent Neurosurgical Intervention at ALERT Trauma Center, Ethiopia
Authors Woldesenbet EB , Belachew FK, Gezae KE , Meles GG , Ali FY, Firissa YB, Kyaruzi VM
Received 19 September 2023
Accepted for publication 30 January 2024
Published 9 February 2024 Volume 2024:17 Pages 21—33
DOI https://doi.org/10.2147/OAS.S440836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Luigi Bonavina
Ermiyas Belay Woldesenbet,1,2 Fitsum Kifle Belachew,2,3 Kebede Embaye Gezae,4 Gebrekiros Gebremichael Meles,4 Fedila Yassin Ali,1 Yared Boru Firissa,5 Victor Meza Kyaruzi6,7
1Department of Public Health, College of Health Science, Wolkite University, Wolkite, Ethiopia; 2Debre Berhan University Asrat Woldyes Health Sciences Campus, Network for Perioperative and Critical Care, Addis Ababa, Ethiopia; 3Division of Global Surgery, University of Cape Town, Cape Town, South Africa; 4Department of Biostatistics, School of Public Health, College of Health Sciences, Mekelle University, Mekelle, Tigray, Ethiopia; 5Emergency Medicine and Critical Care Department, ALERT Hospital, Addis Ababa, Ethiopia; 6Research Department, Winners Foundation, Yaounde, Cameroon; 7Department of Surgery, School of Medicine, Muhimbili University of Health Sciences, Dar Es Salaam, Tanzania
Correspondence: Ermiyas Belay Woldesenbet, Email [email protected]
Purpose: This study aimed to assess and compare the in-hospital recovery times between two groups: those exposed to early intervention and those with late intervention in a cohort of Traumatic Brain Injury (TBI) patients requiring urgent neurosurgical intervention in ALERT Trauma Center in Addis Ababa, Ethiopia.
Methods: The study was conducted over seven consecutive months, from March 14, 2020, to October 13, 2020. Patients were consecutively recruited from the emergency department until the final sample size was fulfilled. The recovery time between the early and late surgery groups was compared using the Log rank test. The Cox proportional hazard model was used to analyze the event data, with the assumption of proportional hazards being checked. The measure of effect was reported using the adjusted hazard ratio, and a stepwise approach was used to build the final model.
Results: A total of 117 TBI patients undergoing urgent neurosurgical intervention were observed and the median survival time for the early surgery group was 4.1 days, and for the late surgery group, it was 6.4 days, with no statistically significant difference (CHR: 0.73; 95% CI; 0.47– 1.11). On the other hand, severe TBI grade emerged as a significant independent predictor, indicating an 86% lower rate of recovery compared to mild TBI cases. Additionally, higher diastolic blood pressure within the range of 50 to 100 was associated with a 24% increased rate of recovery.
Conclusion: This study identified factors influencing recovery outcomes and predictors of prolonged recovery, specifically severe TBI grade and lower diastolic blood pressure. The results emphasize the importance of timely intervention and provide specific considerations for optimizing patient outcomes in TBI cases and guiding further research in the area.
Keywords: Glasgow outcome score, severe, TBI, neurosurgery
Introduction
Traumatic Brain Injury (TBI) is a major cause of death and disability in Africa, with an incidence in Sub-Saharan Africa of 150–170/100 000 compared with a global average of 106/100 000 and associated with high mortality.1,2 Despite the growing incidence of TBI in Sub-Saharan Africa, there is a lack of comprehensive studies that map the current burden of the disease on the continent and investigate patients’ outcomes and access to timely surgery.2 This study aimed to address this gap by assessing how the time between hospital admission and surgery impacts the recovery and outcome of TBI patients in one of the trauma centers in Ethiopia and by investigating how this time interval relates to patient factors like age, injury severity, and pre-existing medical conditions.
Traumatic Brain Injury (TBI) is a type of brain damage caused by an external mechanical force, such as a forceful blow, bump, or jolt to the head or body, or by an object that penetrates the skull and enters the brain,3 this injury can result in temporary or permanent impairment of brain function.3 In clinical practice and epidemiologic studies traumatic brain injury is graded into three to measure its severity. This is done by a widely used clinical scoring system called the Glasgow Coma Score (GCS). It ranges from 3 to 15 and the lower the scale the higher the severity. Based on this, TBI is classified as mild injury (GCS 13–15), moderate injury (GCS 9–12), and severe injury (GCS 3–8).4,5
Traumatic Brain Injury (TBI) is a commonest reason for emergency visits, requiring prompt care and a collaborative team approach. It is a leading cause of disability and mortality worldwide, particularly affecting the younger population.6,7 In Low- and Middle-Income Countries (LMICs), trauma-related deaths account for 89% of total deaths, with head injuries being the primary cause, resulting in long-term health, psychosocial, and economic consequences for injured individuals and their families.8,9
Mechanisms of recovery from TBI are poorly understood and there is much variability in the patterns of recovery. People with mild TBIs are expected to recover more quickly. Nonetheless, some might have psychological consequences which will require assessment and management. Most people with mild TBI recover fully within a few days to months, but a small percentage (1–20%) of them continue to experience symptoms 3 months after the injury.10 Recovery from moderate or severe TBI shows a negatively accelerating curve, which was seen to be most rapid in the first 3–6 months.11
Traumatic Brain Injury (TBI) is considered a neurosurgical emergency when clinical signs of brain herniation or suggestive radiographic findings are present on a CT scan.12 Managing this emergency requires a consistent and uninterrupted chain of care for the trauma patient, starting from the scene of the incident to the hospital, with prompt neurosurgical intervention crucial for preventing irreversible brain damage.13 However, the impact of time intervals, specifically from the incident site to hospital admission and from hospital admission to surgery, on the recovery process of TBI patients, particularly in low-resource settings, remains largely unknown. By providing information on the clinical profiles, the cause of injury, the severity, and the importance of timely intervals for TBI patients, this study is expected to contribute to the limited amount of evidence available regarding TBI management, as well as guide future healthcare policy and intervention in these settings.
Methods
Study Design, Study Period and Sampled Population
This study is undertaken in accordance with the principles stated in the declaration of Helsinki. Prior to the conduct of the study ethical approval was obtained from the Ethical Review Committee of College of Health Sciences, Mekelle University (reference number ERC 1534/2020). The committee has reviewed and approved all aspects of the study protocol including the conduct of the study with an informed verbal consent. Permission letter was also sought from ALERT Trauma Center. This study takes place in ALERT Trauma Center from March 14, 2020, to October 13, 2020. The hospital was initially established in 1934 as Princess Zenebework Memorial Hospital and was renamed as ALERT (All African Leprosy Rehabilitation and Training Center) on December 11, 1965, G.C. Currently it is also serving as one of the Trauma Center located in the city of Addis Ababa. We used a cohort study design to investigate how the timing of surgical intervention, either early or late, affects the recovery time and hospital discharge among traumatic brain injury patients. We prospectively selected 120 patients who had undergone urgent neurosurgical intervention, three were excluded from analysis due to failure to ascertain exposure group. Patients who had undergone minor surgery were excluded from the study, and no other exclusion criteria were applied.
The study employs an accrual period of six months and two weeks (ending on September 29), followed by a two-week follow-up period. We used Epi info software to determine the final sample size with an 80% power and 5% lost to follow-up. The total sample size was 114 and with a 5% non-respondent rate the final sample size was estimated as 120. To measure the time to recovery, we recorded the number of days from the date of surgery (Day 0) until the patient achieved a recovery score of 7 or 8 and was discharged from the hospital. Overall, this study aimed to provide evidence-based guidelines for optimal patient care and improving patient outcomes in this population.
Primary Outcome
This study investigate time to recovery as a primary outcome which is defined as the length of time (in days) it took for a patient to be discharged from the hospital with an Extended Glasgow Outcome score (EGOS) of 7 or 8, which represent lower and upper good recovery outcomes respectively. The EGOS score ranges from 1 to 8, with higher scores indicating better outcomes. The assessment of outcomes varies along the range of scores, with the worst outcomes being death (score 1) and vegetative status (score 2), while scores 7 and 8 represent different levels of good recovery.14
Definitions of Operational Terms
Failure: The occurrence of the event (recovery) as defined by a EGOS score of 7 or 8.
Censured: Nonoccurrence of the event during the study period. This happens when the exact timing of recovery as defined by the EGOS score could not be known during the follow-up. This includes patients who were discharged with a EGOS score of less than or equal to 6, or end of study (at October 13, 2020, this one is also known as administrative censoring) before patient gets discharged.
Minor surgery: any procedure that can be safely performed in an outpatient setting, without the use of general anesthesia or the need for cardiac or respiratory assistance.
In-hospital mortality: defined as death of a patient during the follow-up period before discharge.
Early group: Patients were classified into the early group if surgery was started within four hours starting from admission to the hospital.
Late group: Patients were classified into late group if surgery was done after four hours starting from admission to the hospital.
Prehospital time: An estimate of the number of hours starting from the onset of injury until patient reach to the hospital (arrival to the triage or Emergency department in the hospital).
Data Collection, Management, and Analysis
Data were collected using a standardized data abstraction checklist which was prepared after reviewing different previous literature and customized based on the local practice and the objectives of the study. The checklist was prepared in English language with three forms including admission form, intraoperative form, and discharge form. Prehospital information in admission form were translated to local language (Amharic language). The forms involve sociodemographic factors, TBI event-related factors, clinical factors, neurologic and perioperative factors and outcome status.
Data was collected from emergency department of ALERT Trauma Center, medical records and trauma registry. Before enrollment to the study verbal informed consent was obtained from patients if they are capable to decide or via substitute decision maker or kin. Patients were followed until discharge with recovery, death or until censuring. To assure the quality of data, close supervision was done by the study investigators. Any ambiguities during the data collection process were resolved by discussion with data collectors, principal investigator, and the duty clinical staff and in return feedback was given on daily bases.
We use Stata version 15 and RStudio statistical software for data cleaning and statistical computations. Descriptive summary measures were done using tables, graphs and statistical summary measures. For numeric variables, median with interquartile range was used and for the categorical variables frequency with percentage was used. Survival curves were plotted using the Kaplan Meier’s estimates.
We used four hours as a cut point for grouping patients into early and late time which is near to the median time to surgery. A similar cut off point has also been used in previous studies to classify patients timing of surgery.15,16
Time to recovery was measured from the day of surgery until the last day of hospital admission (discharge day) or censoring. The overall cumulative incidence rate of recovery was computed. The recovery rate was also determined for each group. The outcome status of patients was tabulated with the observed frequency and percentage in each category. The cumulative survival rate after surgical intervention was estimated by the Kaplan-Meier survival analysis method at each distinct ordered failure times. The Log rank test was used to compare differences in survival time between the early and late surgery groups.
To assess the association between baseline variables and recovery, two strategies were used. First, for each baseline variable Cox Proportional Hazards (CPH) regression model done. Second, a multivariable CPH model was fitted using a stepwise approach. The cox model has many advantages that makes it more popular, here the cox model was preferred over the other because; It will yield estimates which are comparable with the parametric models without specifying the baseline hazard. For example, if the appropriate model was the Weibull model it will yield estimates which are comparable with the Weibull model. In addition to this, the CPH model, unlike parametric models, will not be biased with misspecification of the baseline hazard parameters.17
The assumptions of proportional hazards were checked by the following procedures: Log (-log (St) plots (for categorical variable) and Global test. When the proportional hazards (PH) assumptions do not seem to keep graphically, the goodness of fit test followed by inclusion of interaction with time was assessed. Since the results from the graphs are too subjective,17 the latter two methods were done following the graphical method for final decision. In this way, all variables appear to satisfy the proportional hazard assumption. For model building, a non-automatic stepwise approach was done as follows.
Step 1: Fitting a model that contain each of the significant variables one at a time.
Step 2: The variables that show important change in −2 log L from Step 1 are then fitted together.
Step 3: Adding Variables that were not important in step 1 one at a time.
Step: A final check to ensure that no term in the model can be omitted.
In step 1 all the significant variables in the univariate cox model were compared individually against the null model and the change in the partial log likelihood was assessed at a p-value of 0.15 using the likelihood ratio test for the nested models. In addition, those significant variables in step 1 were used in step 2. Those significant variables from step one together fitted a model and then backward elimination was applied, variables were omitted from the model one at a time when their removal does not make a significant change in −2 log L at a significant p-value of 0.15. In Step 3 forward selection was applied and six variables which were not significant by themselves became significant when added to the model in step 2. In step 4 backward elimination was applied to the full model from step 3. The full model was reduced with elimination of two variables. A p-value of 0.15 was applied throughout the stepwise procedure and a p-value of 0.05 was used for the final model. All possible interaction terms were also assessed one at a time and there was no significant interaction term found. The model adequacy for the final model was checked using the goodness of fit test through martingale’s residuals and mathematically the final model can be denoted as follows:
Whereas,
h (t) = The hazard at time t
h0 (t) = The baseline hazard at time t
β1 = The coefficient for the variable x1
β2 = The coefficient for the variable x2
x1 = Variable one in the model
x2 = variable two in the model
βp = The coefficient for the pth variable
xp = The pth variable included in the model
Results
We analyzed the survival data of 117 patients who underwent urgent neurosurgical interventions for traumatic brain injury at ALERT Trauma Center, in Addis Ababa from March 14, 2020, to September 29, 2020. The patients were divided into two groups, early and late surgery, with 58 subjects in the early group and 59 in the late group. Three patients who had incomplete follow-up or unknown exposure status were excluded from the analysis, resulting in a 2.5% attrition rate from the estimated sample size. The median age of the participants was 30, with an interquartile range (IQR) of 25–48. The age distribution was comparable between the two groups, with median ages of 35 and 30 in the early and late groups, respectively.
Of the total 117 patients, 93 (79.4%) were male. The distribution of sex also had little difference between the early and late surgery groups, with 75.8% versus 83.0% male and 24.1% versus 16.9% female in each group, respectively. Regarding marital status, 37 (33.0%) were married, 35 (31.2%) were single, and the remaining 40 (35.7%) fell into other categories. A higher proportion of participants, 48 (41.7%), had an elementary or lower academic status (Table 1).
 |
Table 1 Sociodemographic Characteristics of Surgically Managed TBI Patients at ALERT Trauma Center, Addis Ababa from March 14, 2020, to September 29, 2020 |
Prehospital Conditions
Regarding mode of arrival 67.3% in the early surgery group and 62.2% in the late surgery group arrived by ambulance. Private care was used by 11.5% in the early surgery group and 20.7% in the late surgery group, while taxi and other modes constituted smaller percentages. It was also observed that 48.2% were referred from another facility in the early surgery group, whereas 64.4% were referred in the late surgery group while the remaining patients have made a direct visit to the hospital.
As for the cause of injury, falls accounted for 35.0%, assaults for 34.2%, road traffic accidents (RTC) for 28.0%, and other causes for 2.6%. In the early surgery group, falls affected 31.5%, assaults 35.0%, and RTC 29.8%. Out of 104 patients for whom the type of injury was confirmed, the majority (89.4%) had blunt injuries and the remaining had penetrating injuries. In the early surgery group, blunt injuries account for 92.5%, similarly it accounts for 86.0% of injuries in the late surgery group.
On the other hand, the distance from the scene to the hospital was within a 10 km radius for 37.8% of the overall study participants, whereas 39.7% were over 40 km away. In the early surgery group, 42.8% were within 10 km, while 37.5% were within 40 km. In contrary to this, majority of patients in the late surgery group came from far (41.8% from over 40km versus 32.7% from within 10km). 41.5% of the overall injuries occurred during daytime on weekdays, while 48.5% occurred during the nighttime on weekdays, and 9.9% occurred on weekends (Table 2).
 |
Table 2 TBI Event-Related Characteristics of Surgically Managed TBI Patients at ALERT Trauma Center, Addis Ababa from March 14, 2020, to September 29, 2020 |
Clinical and in Hospital Findings
The median time it took for patients to undergo surgery was 4.8 hours, with a range of 0.26 hours to 51.9 hours. The data was skewed to the right, indicating that majority of patients had shorter wait times. The early group had a median time to surgery of 1.7 hours, while the late group had a median time of 14.8 hours.
The time it took for the first CT scan to be performed was measured in minutes. The median time was 64 minutes, with a range of 30 to 228 minutes. The data was skewed to the right, indicating that there were some longer waiting times. Seven observations were identified as potential outliers or data entry errors and were excluded from the analysis. When these outliers were removed, the median time for the CT scan was 60 minutes. The median time from the first CT scan to surgery was 293 minutes, with a range of 30 to 228 minutes.
The median duration of surgery was 180 minutes. There was no difference in duration between the early and late surgery groups; both had a median duration of 180 minutes. The length of stay for patients ranged from less than a day to 35 days, with a median of 4.6 days. The early group had a median length of stay of 3.4 days, while the late group had a median of 5.4 days. The overall median in-hospital recovery time was 4.2 days, with an interquartile range of 2.1 to 6.7 days. Furthermore, the time components are summarized in Table 3 including time to CT scan, time from CT scan to surgery, time to surgery, and duration of surgery, for the total number of observations, as well as separately for the early and late surgery groups.
 |
Table 3 Summary of Different Time Components During Service Delivery in TBI Patients at ALERT Trauma Center, Addis Ababa from March 14, 2020, to September 29, 2020 |
Pre-Operative Vital Signs
The median systolic blood pressure for all patients was 120 mmHg, with an interquartile range (IQR) of 110–126 mmHg. The early surgery group had a slightly lower median systolic blood pressure of 120 mmHg (IQR: 110–125 mmHg), while the late surgery group had a slightly higher median of 125 mmHg (IQR: 115–130 mmHg). The median diastolic blood pressure for all patients was 70 mmHg, with an IQR of 65–75 mmHg. Both the early and late surgery groups had similar median diastolic blood pressure values of 70 mmHg (IQR: 65–73 mmHg for the early group and IQR: 60–75 mmHg for the late group).
The median respiratory rate for all patients was 18 breaths per minute, with an IQR of 16–20 breaths per minute. Both the early and late surgery groups had a median respiratory rate of 18 breaths per minute (IQR: 16–20 breaths per minute). The median oxygen saturation level for all patients was 96%, with an IQR of 93–98%. The early surgery group had a slightly higher median oxygen saturation level of 97% (IQR: 94.2–98%), while the late surgery group had a median saturation level of 96% (IQR: 93.0–97.0%).
The median temperature for all patients was 36.3°C, with an IQR of 35.8–36.6°C. The early surgery group had a lower median temperature of 35.8°C (IQR: 34.0–36.5°C), while the late surgery group had a slightly higher median temperature of 36.5°C (IQR: 36.2–36.7°C).
The data for the vital signs were found to be right-skewed, indicating that there were more patients with values on the lower end of the range. Furthermore, this information is presented in Table 4, which shows the clinical parameters, and median values for the overall patient group, as well as separate values for the early and late surgery groups.
 |
Table 4 Preoperative Clinical Finding in Surgically Managed TBI Patients at ALERT Trauma Center from March 14, 2020, to September 29, 2020 |
Neurologic and Intraoperative Findings
According to the Glasgow coma score, 52 patients (44.4%) had mild TBI, 51 patients (43.5%) had moderate TBI, and 14 patients (11.9%) had severe TBI. Among these, 46.5% of the mild TBI cases were in the early group and 42.3% were in the late group. Similarly, 39.6% of the moderate TBI cases were in the early group and 47.4% were in the late group. Additionally, 13.7% of all TBI cases were from the early group and 10.1% were from the late group.
In terms of surgical procedures, 53 patients (45.2%) received a craniotomy, 34 patients (29.0%) underwent elevation procedures, and 30 patients (25.6%) had treated with bur hole procedures. Among the patients who received craniotomy, 64.1% were in the early group and 35.8% were in the late group. For bur hole procedures, 70.0% were in the late group and 30.0% were in the early group. The difference in the number of elevation procedures between the two groups was only 11.7%, with 44.1% in the early group versus 55.8% in the late group.
The CT scan findings showed that acute subdural hematoma was the most common type of TBI, with higher prevalence in the early group compared to the late group. In contrast, chronic subdural hematoma was more common in the late group. The occurrence of epidural hematoma was relatively comparable between the two groups as shown in Table 5.
 |
Table 5 CT Scan Findings on the Type of Traumatic Brain Injury in Surgically Managed TBI Patients at ALERT Trauma Center from March 14, 2020, to September 29, 2020 |
In Hospital Outcome
The most common outcome observed among the participants was recovery. Overall, 78.6% of the patients experienced in-hospital recovery. In the early surgery group, 81.0% of the patients achieved recovery, while in the late surgery group, the proportion was 76.2% (Table 6). The overall incidence rate was found to be 141 per 1000 persons days of observations, with a 95% confidence interval between 115 up to 173 per 1000 persons days. In the early surgery group, the incidence rate was 152 per 1000 persons days of follow-up, and in the late surgery group, it was 131 per 1000 persons days of follow-up.
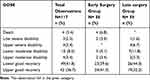 |
Table 6 Outcome Assessment of Surgically Managed TBI Patients Using GOSE at ALERT Trauma Center from March 14, 2020, to September 29, 2020 |
Effect of Time to Surgery on Recovery Time
The median survival time was found to be 4.1 days for the early surgery group and 6.4 days for the late surgery group. However, this observed difference was not statistically significant at a p-value of 0.1, according to the log rank test. This indicates that the timing of surgery did not have a significant impact on the time to recovery.
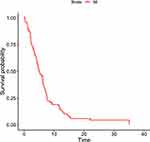
The time to surgery was also analyzed as a continuous measure in hours but was not found to be statistically significant as a predictor of time to recovery, with a p-value of 0.06. This suggests that the exact timing of surgery, measured in hours, did not significantly affect the recovery time. The incidence rate ratio of recovery for the exposed group (early surgery) compared to the non-exposed group (late surgery) was 0.86. However, this finding was statistically insignificant. Figure 1 shows a survival curve estimated by the Kaplan-Meier method, indicating the overall survival to EGOS 7/8 at discharge. The median survival time for all patients included in the study was 5.1 days. Figure 2 displays the adjusted survival curves for the early and late surgery groups. Although there appears to be a difference in survival to EGOS 7/8 at discharge between the two groups, indicating that patients in the early group are more likely to be discharged earlier in the first few days, this difference was not statistically significant. Overall, there is no adequate information to measure a noticeable impact of early surgical interventions on the recovery time of the studied population.
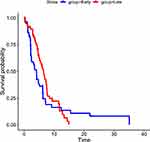 |
Figure 2 Kaplan Meier estimates based on the time to surgery duration among TBI patients who underwent urgent surgical interventions at ALERT Trauma Center from March 14, 2020, to October 13, 2020. |
Predictors of Time to Recovery
The multivariable cox proportional hazards model (Table 7) showed that TBI grade (severe) and diastolic blood pressure were found to be independent predictors of time to recovery. A severe TBI grade was associated with a significantly reduced hazard of recovery (AHR: 0.14, 95% CI: 0.04–0.50), indicating that patients with severe head injury had an 86% lower rate of recovery compared to those with mild head injury. Additionally, for every five-unit increase in diastolic blood pressure within the range of 50 to 100, the rate of recovery at discharge increases by 24% (AHR: 1.24, 95% CI: 1.072–1.45). These findings suggest that the severity of TBI and blood pressure levels play a paramount role in determining the time it takes for patients to recover.
 |
Table 7 Bivariable and Multivariable Cox Regression Result for Predictors of Time to Recovery in Surgically Managed TBI Patients at ALERT Trauma Center from March 14, 2020, to October 13, 2020 |
Discussion
This study analyzed the in-hospital survival data of 117 patients who underwent urgent neurosurgical interventions for traumatic brain injury at ALERT Trauma Center in Addis Ababa from March to September 2020 and provided insights into factors influencing surgical outcomes for traumatic brain injury patients. Patients were categorized into early (n=58) and late (n=59) surgery groups. Socio-demographic characteristics, prehospital conditions, clinical parameters, neurologic findings, intraoperative details, and in-hospital outcomes were assessed. While there were differences in mode of arrival, cause of injury, and distance from the trauma site between the groups, factors such as age, sex, and marital status showed minimal variation. The median time to surgery, CT scan findings, and vital signs were evaluated, and most patients experienced recovery as an outcome. However, the timing of surgery did not significantly impact recovery time, and predictors of recovery included TBI grade and diastolic blood pressure.
The median age of the TBI patients in this study (30 years) is slightly lower than the reports in other studies (ranging from 31.5 to 34.3 years).18,19 These findings suggest that the age distribution of TBI patients in this study is comparable to that of previous studies conducted at TASH.20 However, it is important to note that the slight difference in age could be attributed to various factors, such as sample size or the selection criteria. On the other hand, males are more commonly affected by TBIs compared to females, which is consistent with previous studies conducted across the country and at TASH. This could be due to various factors, such as differences in driving patterns, a higher incidence of assault and risk-taking behavior in males, and occupational exposure, as these are also shown in different studies.21,22 Cultural and societal norms may also contribute to the higher incidence of TBIs among males.23 These norms can influence the types of activities and occupations that males engage in, increasing their risk of experiencing a traumatic brain injury.
In this study, only 43.3% of the patients were diagnosed with mild head injury whereas from other studies on the epidemiology of head injury, it was known that 70 to 90% of head injuries are mild (25). This huge difference could be because in this study the inclusion of patients who require neurosurgical interventions may have skewed the percentage of mild head injuries reported compared to other epidemiological studies that encompass a broader range of cases that may have been treated with non-surgical interventions. There is a notable difference of 2% in the recovery rate between the early and late surgery groups. This difference could potentially stem from two main factors. Firstly, the higher recovery rate within the early group might be attributed to prompt treatment initiation compared to the late group. Alternatively, this variance could be linked to a reduced prehospital delay in the early group, considering that 10% more patients in this group arrived at the trauma center compared with the same patients in the late group who were within 10 kilometers. This suggests that early treatment and reduced prehospital delay may contribute to the higher recovery rate in the early group, as these are found to be crucial elements in improving recovery and reducing hospital stays for TBI patients.16 Additionally, it is important to consider other factors, such as the severity of injuries and the quality of medical care provided, to determine the exact reasons behind this difference in recovery rates.
Regarding time up to surgery versus functional in-hospital recovery, previous studies suggest lower functional recovery among the early groups (24% versus 51%).15 However, in this study, early versus late classification was not significant regarding hospital recovery in nearly equal proportion. The lower functional recovery observed in the former study could probably be because the study participants in the previous study were isolated subdural hematoma brain injuries unlike this one. In another study, the presence of isolated subdural hematomas showed patients were more likely to have worse outcomes.24 The less functional recovery observed in the previous study could be because timely intervention might have been given for patients who are assumed to have complicated clinical conditions.15 In another study, patients were compared as early versus late to surgery groups at 200 minutes as a cutoff point and a higher rate of mortality was observed among the late group unlike this study.25 Whereas in this study all (the four) deaths were among the early surgery group. This observed difference could be because the previous study was conducted among isolated severe head injury patients and a late surgical intervention to severe head injury could undoubtedly result in a higher mortality rate.
This study illustrates that TBI grade and diastolic blood pressure to be an independent predictor of time to recovery. Several studies suggest TBI grade as an important predictor of recovery, which is in line with this study.25,26 In a previous study, SBP instead of DBP were found to be statistically significant outcome predictor.27 This difference could be due to the different time points used to take vital signs between the two studies. In this study, vital signs were assessed immediately before surgery once the patient get resuscitation at the emergency department. In general, this study suggests there is no statistically significant difference in early recovery between early (within four hours) and lately (after four hours) treated TBI patients. In line with this, we recommend future studies and researchers to reassess the impact of timing of surgical interventions with a higher level of statistical power at a wider range. Moreover, this study identifies TBI grade, oxygen saturation, prehospital time, mechanism of injury, and type of injury as predictors of time to recovery.
One of the strengths of this study is that variables that were neglected in previous studies were addressed. Eg, the dependent variable itself and time variables. On the other side a more similar cohort of patients were studied. Ie, patients with neurosurgical interventions alone were recruited. Data were captured longitudinally, and most importantly a prospective cohort study design was used.
There were also some limitations; due to the emergence of the COVID-19 pandemic at the start of the study which had put a limit on transportation, perhaps road traffic collisions as a cause of injury could be underestimated in this study. Some of the study variables that demand active involvement by the data collector during clinical procedures, Eg, anesthesia duration, were collected from documented patient records due to feasibility issues. The follow up in this study was limited to an in-hospital stay and this study does not assess long-term functional recovery due to high financial and time limitations.
Conclusion
This study assessed factors affecting surgical outcomes in 117 traumatic brain injury (TBI) patients treated at Addis Ababa’s ALERT Trauma Center. While the timing of surgery did not significantly impact recovery time, predictors of prolonged recovery included TBI grade and lower diastolic blood pressure. Observations noted a 2% recovery rate difference between early and late surgery groups, potentially attributed to prompt treatment initiation and reduced prehospital delay in the former. The study, contrary to some previous findings, emphasized the independent predictive value of TBI grade and diastolic blood pressure for recovery. Notable strengths included addressing variables neglected in prior studies and employing a prospective cohort design, while limitations included pandemic-related challenges and a short-term follow-up period. Overall, this research contributes nuanced insights into TBI outcomes, laying a foundation for future investigations with increased statistical power and broader scope.
Data Sharing Statement
Deidentified participant data generated during this study are available from the corresponding author upon a reasonable request.
Acknowledgment
PI has undertaken this research as part of an academic requirement for a postgraduate degree program.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jerome E, Laing GL, Bruce JL, Sartorius B, Brysiewicz P, Clarke DL. An audit of traumatic brain injury (TBI) in a busy developing-world trauma service exposes a significant deficit in resources available to manage severe TBI. South Afr Med J. 2017;107(7):621. doi:10.7196/SAMJ.2017.v107i7.10562
2. Adegboyega G, Zolo Y, Sebopelo LA, et al. The burden of traumatic brain injury in Sub-Saharan Africa: a scoping review. World Neurosurg. 2021;156:e192–e205. doi:10.1016/j.wneu.2021.09.021
3. USA government official website. Traumatic brain injury (TBI); 2023.
4. Robert P, Granacher J. Traumatic Brain Injury: Methods for Clinical and Forensic Neuropsychiatric Assessment.
5. Saatman K, Duhaime A, Bullock R, Maas A, Valadka A, Manley G. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–738. doi:10.1089/neu.2008.0586
6. Jacobsson LJ, Westerberg M, Lexell J. Health-related quality-of-life and life satisfaction 615 years after traumatic brain injuries in northern Sweden. Brain Inj. 2010;24(9):1075–1086. doi:10.3109/02699052.2010.494590
7. Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehab. 2010;25(2):72–80. doi:10.1097/HTR.0b013e3181ccc8b4
8. Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723–1730. doi:10.1056/NEJMra1109343
9. Capone-Neto A, Rizoli S. Linking the chain of survival: trauma: trauma as a traditional role model for multisystem trauma and brain injury. Curr Opinion Crit Care. 2009;15(4):290e4.
10. McCrea M, Iverson GL, McAllister TW, et al. An integrated review of recovery after mild traumatic brain injury (MTBI): implications for clinical management. Clin Neuropsychol. 2009;23(8):1368–1390. doi:10.1080/13854040903074652
11. Cramer SC, Sur M, Dobkin BH. Harnessing neuroplasticity for clinical applications. Brain. 2011;134(6):1591–1609. doi:10.1093/brain/awr039
12. Parag P, Hardcastle TC. Interpretation of emergency CT scans of the head in trauma: neurosurgeon vs radiologist. World J Surg. 2022;46(6):1389–1395. doi:10.1007/s00268-022-06525-w
13. World Health Organization. Emergency and trauma care Emergency care systems for universal health coverage: ensuring timely care for the acutely ill and injured; 2019: 9–14.
14. Wilson L, Boase K, Nelson LD. A manual for the Glasgow outcome scale-extended interview. J Neurotrauma. 2021;38(17):2435–2446. doi:10.1089/neu.2020.7527
15. Dent DL, Croce MA, Menke PG, et al. Prognostic factors after acute subdural hematoma. J Trauma. 1995;39(1):36–43. doi:10.1097/00005373-199507000-00005
16. Mehmood A, Rowther AA, Kobusingye O, Ssenyonjo H, Zia N, Hyder AA. Delays in emergency department intervention for patients with traumatic brain injury in Uganda. Trauma Surg Acute Care Open. 2021;6(1):e000674. doi:10.1136/tsaco-2021-000674
17. Kleinbaum DG, Klein M. Survival Analysis. A Self-Learning Text. Springer; 2012:711.
18. Demlie TA, Alemu MT, Messelu MA, Wagnew F, Mekonen EG. Incidence and predictors of mortality among traumatic brain injury patients admitted to Amhara region Comprehensive Specialized Hospitals, northwest Ethiopia, 2022. BMC Emerg Med. 2023;23(1):55. doi:10.1186/s12873-023-00823-9
19. Mitiku GK, Miresa BD, Kisito QKJM. Factors affecting traumatic brain injury outcome among patients treated for head injury at surgical side, in Nekemte Referral Hospital, Oromia, Ethiopia. J Spine Neurosci. 2020;1(2):1–13. doi:10.14302/issn.2694-1201.jsn-20-3554
20. Landes M, Venugopal R, Berman S, Heffernan S, Maskalyk J, Azazh A. Epidemiology, clinical characteristics and outcomes of head injured patients in an Ethiopian emergency centre. Afr J Emergency Med. 2017;7(3):130–134. doi:10.1016/j.afjem.2017.04.001
21. National Library of Medicine. Traumatic brain injury; 2020.
22. Wagner AK, Sasser HC, Hammond FM, Wiercisiewski D, Alexander J. Intentional traumatic brain injury: epidemiology, risk factors, and associations with injury severity and mortality. J Trauma. 2000;49(3):404–410. doi:10.1097/00005373-200009000-00004
23. Matei VP, Rosca AE, Pavel AN. Risk factors and consequences of traumatic brain injury in a Swiss male population cohort. BMJ Open. 2022;12(7):e055986. doi:10.1136/bmjopen-2021-055986
24. Lee JJ, Segar DJ, Morrison JF, Mangham WM, Lee S, Asaad WF. Subdural hematoma as a major determinant of short-term outcomes in traumatic brain injury. J Crit Care. 2017;45:1–14. doi:10.1016/j.jcrc.2017.11.035
25. Matsushima K, Inaba K, Siboni S, et al. Emergent operation for isolated severe traumatic brain injury: does time matter? J Trauma Acute Care Surg. 2015;79(5):838–842. doi:10.1097/TA.0000000000000719
26. Qureshi JS, Ohm R, Rajala H, et al. Head injury triage in a sub Saharan African urban population. IJSU. 2020;11(3):265–269.
27. Dinh MM, Bein K, Roncal S, Byrne CM, Petchell J, Brennan J. Redefining the golden hour for severe head injury in an urban setting: the effect of prehospital arrival times on patient outcomes. Injury. 2013;44(5):606–610. doi:10.1016/j.injury.2012.01.011
28. Feigin VL, Barker-Collo S, Krishnamurthi R, Theadom A, Starkey N. Epidemiology of ischaemic stroke and traumatic brain injury. Best Pract Res Clin Anaesthesiol. 2010;24(4):485–494.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.