Back to Journals » Journal of Inflammation Research » Volume 15
Time to Occurrence of Phlebitis After Continuous Infusion of Total Nutrient Admixture Through Peripheral Veins: An Experimental Animal Study
Authors Guo JL, Yan XY, Zhao QL, Gao CN, Wei CH, Wei Z, Yue YT, Guo XJ
Received 25 October 2021
Accepted for publication 10 December 2021
Published 11 January 2022 Volume 2022:15 Pages 205—215
DOI https://doi.org/10.2147/JIR.S346186
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jin-Li Guo,1,* Xian-Yan Yan,1,* Qing-Li Zhao,1 Chao-Na Gao,1 Chen-Hui Wei,1 Zhuan Wei,1 Yi-Ting Yue,2 Xiu-Juan Guo1
1Department of Nursing, Second Hospital of Shanxi Medical University, Taiyuan, People’s Republic of China; 2Department of Nursing, Fenyang College of Shanxi Medical University, Fenyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jin-Li Guo
Department of Nursing, Second Hospital of Shanxi Medical University, No. 382 of Wuyi Road, Xinghualing District, Taiyuan, 030001, People’s Republic of China
Tel +86 13546395590
Email [email protected]
Objective: To study the limit time of phlebitis caused by continuous infusion of KabivenTM Pl and TNA (KabivenTM Pl+ alanyl glutamine + potassium aspartate) through a peripheral vein, and to provide a reference for clinical formulation of preventive measures for phlebitis.
Methods: White rabbits (n = 72) were randomly divided into three groups: group A (Normal saline), group B (Kabiven™ Pl), and group C (TNA). Blood was collected from the ear margin vein before administration and after three hours, four hours, five hours, and six hours of administration. CRP and TNF-ɑ were measured by enzyme-linked immunosorbent assay. Hematoxylin and eosin staining and immunohistochemical staining were performed on tissue samples taken from the insertion point of the indwelling needle, the tip of the indwelling needle, and 1 cm from the tip of the indwelling needle, closer to the heart, to analyze early pathological changes in blood vessels.
Results: (1) There were no visible inflammatory symptoms in groups A, B, or C within 6 hours. (2) Four hours after starting intravenous administration, the levels of inflammatory markers in groups B and C were higher than in group A, and (3) the degree of inflammatory cell infiltration in groups B and C was more severe than in group A. (4) In all groups, the inflammatory reaction at the tip of the indwelling needle was more severe than at the other two sites.
Conclusion: When the emulsions TNA and Kabiven™ Pl are infused through a peripheral vein, (1) four hours may be considered as the maximum time for continuous intravenous infusion in the same vein before inflammatory changes become evident, and (2) systematic assessment of the tip of the indwelling needle should be considered for inclusion in the nursing plan for phlebitis monitorings.
Keywords: peripheral vein, Kabiven™ Pl, TNA, phlebitis, limit time, CRP, TNF-ɑ
Introduction
Currently, short peripheral venous catheters (PVCs) are commonly used for intravenous infusion in hospitals, with a utilization rate as high as 70%.1 However, for such an important device PVCs remain highly susceptible to complications resulting in catheter failure, which has been reported in individual studies to be as high as 69%, but worldwide literature has never been systematically synthesized which may lead to an underappreciation of these rates.2 Phlebitis has the highest incidence, accounting for 24% of all complications,1 and the physical and chemical properties of the drugs being used is one of the main risk factors for phlebitis. Yanhong Wang studied the limit time of chemical phlebitis caused by strong acid drug amiodarone.3 In this study, the widely used intravenous nutrient solution, fat emulsion amino acid (17) and glucose (11%) injection (Kabiven™ Pl), which is strongly acid and has high osmotic pressure, was selected as the object.
The 2016 INS (Infusion Nursing Standard)4,5 stipulates that under the conditions of compatibility and stability of liquid, other emulsions may be used to prepare a parenteral nutrition solution and a central venous access device (CVAD) should be used as the puncture tool. However, if the medical environmental requirements for a CVAD puncture cannot be met and the nutritional treatment of the patient will be delayed, a short PVC infusion can be used. However, continuous use of PVC parenteral nutrition, parenteral nutrition with osmotic pressure <900 mOsm/L, and other irritants should be avoided to prevent chemical phlebitis.4 However, the guidelines do not specify a precise upper time limit for “continuous infusion” or “continuous use”. The nutrient solution is often prepared with alanyl glutamine, potassium aspartate, and other emulsions to create a total nutrient admixture (TNA) with high osmotic pressure and strong acid properties. However, through the previous investigation of the continuous infusion of such emulsions in clinical patients, it has been found that even if the continuous infusion of TNA through PVC meets the INS standard, the duration of continuous infusion of the treatment drug is generally more than 24 hours, which increases the risk of chemical phlebitis. Therefore, it is particularly important to investigate the safe time period for the intravenous infusion of TNA through a PVC and the time until the occurrence of phlebitis. In this study, considering the current clinical situation and the various limitations of the human body as the object of clinical research, animals were used as the objects of the experiment to provide a reference for the clinical development of phlebitis prevention measures.
Recent studies have suggested that6 CRP may participate in the modulation of lipoprotein uptake, eNOS function, and nitric oxide (NO) generation. NO is the most important vasodilator factor produced by endothelial cells. It is considered to be one of the most important indicators reflecting endothelial function. It is a vasoactive substance that regulates vascular tone, vascular wall inflammation, anti-thrombosis, and inhibits smooth muscle cell proliferation. Therefore,7 c-reactive protein (CRP) is considered a reliable predictor of endothelial dysfunction. More and more evidences show that8 the inflammatory cytokine tumor necrosis factor (TNF-a) plays a key role in destroying the circulation of large blood vessels and capillaries by stimulating the production of reactive oxygen species.
Then this study chose PVC to continuously infuse strong acid and high osmotic pressure drugs to detect the change trend of CRP and TNF-a to determine the limit time for phlebitis.
Materials and Methods
Drugs
For patients who are fasting before and after gastrointestinal surgery and require nutritional support, and adult patients with gastrointestinal dysfunction and contraindicated oral/intestinal nutrition, KabivenTM Pl with pH 4.84 and osmotic pressure of 815mOsm/L is usually used. Intravenous nutrient solution, total volume 1440mL, ingredients include refined soybean oil 35g, anhydrous glucose 68g, alanine 3.3g, arginine 2.4g, aspartic acid 0.71g, phenylalanine 1.6g, glutamine Acid 1.2 g, glycine 1.6 g, histidine 1.9 g, isoleucine 1.2 g, leucine 1.6 g, lysine 1.9 g, methionine 1.2 g, proline 1.4 g, serine 0.94 g, threonine 1.2 g, tryptophan 0.40 g, tyrosine 0.05 g, valine 1.5 g, sodium glycerophosphate (anhydrous) 1.0 g, calcium chloride 0.15 g, potassium chloride 1.2 g, magnesium sulfate 0.33 g, sodium acetate 1.0 g, 220 mmol of sodium, 17 mmol of potassium, 2.8 mmol of magnesium, 1.4 mmol of calcium, 7.5 mmol of phosphorus, 2.8 mmol of sulfate, 32 mmol of chlorine, 27 mmol of acetate, etc.
However, in the actual clinical treatment process, the formulation of the treatment plan often prepares drugs such as alanyl glutamine injection and potassium aspartate on the basis of KabivenTM Pl to form a TNA with a pH of 5.17 and an osmotic pressure of 800 mOsm/L.
Animal Mode
All experimental procedures are in accordance with the 8th edition of the US National Research Council’s Laboratory Animal Care and Use Guidelines Update Committee, and have passed the ethics committee of the Second Hospital of Shanxi Medical University. A total of 72 healthy New Zealand white rabbits of either sex were selected. Inclusion criteria: similar in age; weighing 2.5–3.0 kg; and the veins of the ear margin were thick, straight, elastic, and full. Exclusion criteria: venous malformation of the ear margin, subcutaneous blood stasis, induration, or scar mass. The rabbits were randomly divided into three groups (n = 24 in each): group A, normal saline; group B, Kabiven™ Pl; and group C, TNA. The emulsions were continuously infused through the ear vein of the rabbits. The three groups were further randomly divided into four groups: the three-hour, four-hour, five-hour, and six-hour groups (n = 6 in each). Based on the physicochemical properties, infusion speed, and body weight requirements of the emulsions, a single-channel injection pump was used to infuse the emulsions at a rate of 4 mL/kg/h.
A special rabbit box was used to limit the activity of the rabbits. This was operated by a assigned operator for each rabbit. Thick, straight, elastic, and easily fixed external ear veins were selected as the puncture vessels. Depilatory cream was applied evenly to the veins to be punctured and the surrounding skin. After 5–6 minutes, allowing time for the cream to work, sterile gauze was dipped into a 0.9% sodium chloride solution and used to clean the puncture site and surrounding skin. Both operators carried out the experiment in accordance with aseptic technical operation principles and technical disinfection specifications. They wore sterile operating clothes, sterile gloves, disposable surgical masks, and sterile operating caps and laid out sterile treatment towels. During the operation, one operator assisted by securing the ear, ensuring the scope of puncture operation area, and selecting qualified vessels for the venipuncture operation. The second operator punctured the rabbits and placed the PVC catheters. After the operation, the medical waste will be processed by the government designated agency.
Enzyme Linked Immunosorbent Assay (ELISA)
First, the three groups A, B, and C were drawn with 2~3mL venous blood before administration, and after the end of the 3h, 4h, 5h, and 6h time periods. The blood was left standing at room temperature for 30 minutes and then placed into a centrifuge for centrifugation. The supernatant was collected, stored in a refrigerator at −80°C, and tested using the ELISA method. The blood samples collected before administration were used to detect the baseline values of C-reactive protein (CRP) and tumor necrosis factor α (TNF-α). The antibody kit is produced by Boster Company in the United States. A microplate with a wavelength of 450 nm was used for repeated detection.
Histopathology Analysis
In groups A, B, and C, ear vein tissue specimen were collected at 2h after the four periods of administration were 3h, 4h, 5h, and 6h. Because the length of the cannula of the PVC catheter is about 2.5 cm, using the selected puncture vessel as the standard horizontal line, an area of 0.75 cm to the left and 0.75 cm to the right of the vessel was drawn, ie, a width of 1.5 cm. A rectangular tissue specimen with an area of about 1.5 × 3.5 cm was obtained, using the insertion point of the indwelling needle as the starting point (point I) and 1 cm from the tip of the indwelling needle, on the side closer to the heart, as the cut-off point (point O). The specimen was immersed in a formalin neutral fixative for 24 hours, was routinely dehydrated, and underwent routine paraffin embedding and sectioning. Three sections were made from each vessel. The sectioning positions were as follows: at point I, point T (at the tip of the indwelling needle) and point O, on the side closer to the heart. The specific location of venous blood vessel specimens is shown in Figure 1. H&E staining and optical microscopic qualitative detection were performed. The staining results were submitted to professional pathologists for diagnosis. The site at 0.5 cm from the insertion point and the site at the tip of indwelling needle catheter were used to compare the severity of mechanical and chemical phlebitis. The tip of the indwelling needle catheter and the site 0.5 cm nearer to the heart from the tip of the indwelling needle catheter were used to compare the most serious phlebitis skin parts.
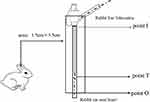 |
Figure 1 Schematic diagram of the three parts of the rabbit venous blood vessel specimens. |
In this study, a semi-quantitative scoring system was used to assess the severity of phlebitis, and the specific grading was 0–3.9 (0): no inflammatory reaction (only the perivascular connective tissue presents with hyperemia and edema), (1): mild inflammation (infiltration of lymphocytes and plasma cells is found in the perivascular connective tissue, and no inflammatory cells are found in the vascular wall and lumen), (2): moderate inflammation (lymphocytes, plasma cells, and a few neutrophils are infiltrated into the connective tissue and vascular wall), (3): severe inflammation (diffuse infiltration of lymphocytes and neutrophils can be seen in the perivascular connective tissue and various layers of the vascular wall and vascular lumen, and there are many exudates and necrotic cell fragments in the vascular lumen)
Immunohistochemical Technique
Adopting the same steps as in Statistical Analysis, a puncture site was made. At point I, point T (at the tip of the indwelling needle) and point O. Based on the principle of specific binding of antigens and antibodies, the proteins in the tissue cells were determined using a chemical reaction to color the TNF-α and the CRP antibody. CRP and TNF-α positive cells were counted in three high power field areas, and the average number of positive cells in each area was obtained.
Visual Monitoring Indexes
Because the subjects were animals, no pain scale could be used. Phlebitis was classified into five grades5: grade 0, no signs; grade 1, redness at the puncture site; grade 2, redness at or edema of the puncture site; grade 3, Dredness at the puncture site with a visible cord-like vein that could be touched; grade 4, redness at the puncture site with a visible cord-like vein that could be touched and was longer than 2.54 cm, and pus drained from the site.
Statistical Analysis
Data were presented as mean ± standard error of the mean (SE), following analyzed was performed by the statistical software IBM SPSS 22.0 (SPSS, Inc. Chicago, IL, USA). The severity of phlebitis was compared using the Mann–Whitney U-test. Normality of the data was test by the Kruskal–Wallis method. Multiple groups results were analyzed by one-way analysis of variance (ANOVA), followed by Least Significant Difference multiple comparison test. P-values less than 0.05 were considered as statistically significant.
Results
Visual Observation Results
Owing to the short time of continuous intravenous infusion, there were no visible inflammatory signs in any of the groups.
Determination of Inflammatory Markers
Change Trend of CRP
Repeated measurement analysis of variance revealed that there was an interaction between the groups and the time periods (F = 24.309, P < 0.05). The change trend of CRP over time was different between groups A, B, and C after four hours of administration. After 4 hours, the CRP of group B and C began to increase, higher than the baseline level before administration, while group A basically maintained the trend of level. The CRP levels in all three groups were compared in each time points. As shown in Table 1 and Figure 2. In group A, the difference in CRP between the time points was not statistically significant (P > 0.05). In groups B and C, there was no significant difference in CRP before administration and three hours after starting administration (Fbaseline = 0.210, P = 0.814; F3h = 0.102, P = 0.762), but the difference between before administration and after administration four, five, and six hours was statistically significant (F4h = 18.793, P < 0.05; F5h = 16.000, P = 0.01; F6h = 15.783, P = 0.011).
 |
Table 1 Change Trend of CRP Concentration in the KabivenTM Pl Group, TNA Group and Normal Saline Group (mg/l) |
 |
Figure 2 Change trend of CRP concentration. |
Change Trend of TNF-α
Repeated measurement analysis of variance revealed that there was an interaction between the groups and the time periods (F = 23.57, P < 0.05). The change trend of TNF-α over time was different between groups A, B, and C after four hours of administration. After 4 hours, the TNF-α of group B and C began to increase, higher than the baseline level before administration, while group A basically maintained the trend of level. The TNF-α levels in all three groups were compared in each time points. As shown in Table 2 and Figure 3, in group A, the difference in TNF-α between the time points was not statistically significant (P > 0.05). In groups B and C, there was no significant difference before administration and three hours after starting administration (Fbaseline = 3.321, P = 0.078; F3H = 1.55, P = 0.268), but the difference between before administration and after administration four, five, and six hours was statistically significant (F4h = 3.424, P < 0.05; F5h = 17.1, P = 0.009; F6h = 9.308, P = 0.028).
 |
Table 2 Change Trend of TNF-α Concentration in the KabivenTM Pl Group, TNA Group and Normal Saline Group (mg/l) |
 |
Figure 3 Change trend of TNF-α concentration. |
Pathological Results
Comparison of the Pathological Morphology and Inflammatory Reaction of the Marginal Vein in the Ear of a Rabbit Between the Different Time Points
The results revealed that at three hours after starting administration, there was no significant difference in the degree of vascular inflammatory reaction between the groups (P > 0.05). At four hours, the vascular inflammation in groups B and C was more severe than in group A (P < 0.05). In groups B and C, the integrity of the vascular intima was damaged, the vascular endothelial cells were partly exfoliated, and a small number of diffuse inflammatory cells were seen in the vascular lumen. At five and six hours, there was significant difference between group A and groups B and C (P < 0.05). As shown in Figures 4–7, the rate at which vascular endothelial cells fell off was increased, the cells began to present apoptosis, and the inflammatory reaction was aggravated.
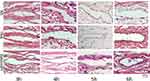 |
Figure 4 The place where the indwelling needle is inserted (point I). |
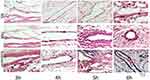 |
Figure 5 The tip of the indwelling needle (point T). |
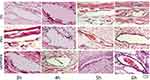 |
Figure 6 The site 1 cm from the tip of the indwelling needle near the heart (point O). |
 |
Figure 7 Three groups of statistical graphs of the degree of inflammation in different time periods. |
Groups B and C: After three hours of continuous infusion, the structure of the vascular endothelium was intact, the surrounding connective tissue presented with slight hyperemia and edema, and no inflammatory cell infiltration was found. After four hours of continuous infusion, the integrity of the vascular intima was damaged, the vascular endothelial cells were partly exfoliated, and a small number of diffuse inflammatory cells was seen in the vascular lumen. After five and six hours of continuous infusion, the rate at which endothelial cells fell off was increased, the cells began to present apoptosis, and the inflammatory reaction was aggravated.
Group A: The structure of the vascular endothelium was intact, the surrounding connective tissue presented with hyperemia and edema, and, with an increase in the continuous infusion time, a small amount of inflammatory cell infiltration could be seen.
Comparison of the Degree of Inflammatory Cell Infiltration in the Different Sites at the Same Time Point
Compare and analyze the pathology of point I, point T and point O of the three groups respectively. The results revealed that in groups A, B, and C, point T was more severe than at the other two sites. After HE analysis of the obtained pathological slices, it is obvious that compared with Point I and Point O, point T has more inflammatory cells, deeper vascular infiltration, and more severe damage to the integrity of the vascular intima. As shown in Figures 4–6, 8. The difference was statistically significant (P < 0.05).
 |
Figure 8 Three groups of different parts of the inflammatory response statistics chart. |
Comparison of the Degree of Apoptosis of the Endothelial Cells at Different Time Points
The results revealed that after four hours of continuous intravenous infusion, the vascular walls in groups B and C began to show a brown reaction. Over time, the range of this brown area of inflammatory markers in the vascular lumen increased gradually, the inflammatory reaction was exacerbated, and the apoptosis rate of the vascular endothelium was accelerated. There was no large brown area in the vascular lumen of group A (Figures 9 and 10).
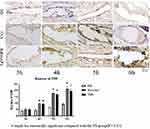 |
Figure 9 CRP at the tip of the indwelling needle. |
 |
Figure 10 TNF-α at the tip of the indwelling needle. |
Discussion
Phlebitis is the most common complication of PVC, including mechanical phlebitis, bacterial phlebitis, thrombophlebitis and chemical phlebitis. Among them, chemical phlebitis accounts for a relatively high incidence, which directly increases the patient’s additional pain and medical expenses, and seriously interferes with drug compliance and the patient’s quality of life.10 Arol Ann Oragano et al proposed that lack of scientific nursing guidance is an important external factor to increase the incidence of phlebitis. Then, the limit time of continuous infusion of stimulant drugs to cause phlebitis is one of the important research aspects.11
Kimberly Morrison et al proposed that there was no significant difference in the degree of vascular inflammatory reaction between 72 and 96 hours of continuous infusion of emulsions through the peripheral vein.12 However, a limit time was not proposed to prevent phlebitis. In this study, the rabbit ear-marginal vein was continuously infused with the drugs KabivenTM Pl and TNA, which have both strong acidity and high osmotic pressure. As the continuous intravenous infusion time increases, the stimulation of the drug solution to the inner wall of the blood vessel increases, which can directly damage HUVECs and increase Vascular permeability, causing the release of inflammatory markers CRP and TNF-α, leading to inflammation under the action of chemokines.13
The latest research shows that CRP may be involved in the production of nitric oxide (NO), regulating the function of eNOS and the activity of the pro-inflammatory transcription factor nuclear factor-kb. NO is a vasoactive substance produced by eNOs acting on L-arginine. It plays a role in regulating vascular tone, vascular wall inflammation, anti-thrombosis, and inhibiting the proliferation of smooth muscle cells. It is the most important vasodilator factor produced by endothelial cells. At four hours of administration, the inflammatory markers in groups B and C started to gradually increase: vascular endothelial cells were falling, and the integrity of the vascular intima deteriorated. With the prolongation of the administration time, the administration time of the two groups B and C reached five hours and six hours, the inflammatory factor CRP was highly released, and the production of BH4 was inhibited. However, eNOs in the body cannot produce NO when BH4 is deficient, but generate too much ROS to damage endothelial function. At the same time, CRP can promote the production of EMPs, thereby reducing the ability of endothelial cells to produce NO metabolites, resulting in impaired endothelial function. EMPs are small vesicles that are shed when endothelial cells are activated or apoptosis, and are biological factors for inflammation and thrombosis.6
Therefore, based on these results, if the clinical treatment emulsions exhibit these two physical and chemical properties, the nursing staff can judge the safe period for continuous intravenous infusion of the emulsions. The safe length of time for continuous intravenous infusion is three hours. To ensure effective clinical nursing treatment based on these scientific results, once the length of time exceeds four hours, to reduce the risk of phlebitis, the blood vessels should be evaluated, and a new site should be selected for venous access to ensure the continuity, timeliness, and safety of the treatment.
In this study, a comparative analysis of the morphology of the pathological tissue was conducted on the pathological sections taken from the point I and the point T and the point O. The results revealed that the inflammatory reaction of the insertion site was relatively mild (point I). The reasons may be that: (1) PVC puncture is an invasive technique. After successful puncture, the catheter remains in the vascular lumen, as a foreign body, for a long time. The catheter at the puncture site is in contact with the vascular wall, which can form friction stimulation.14 (2) In the case of PVC indwelling in the vascular lumen, the original direction of the blood flow in the vascular cavity is blocked by the catheter. Therefore, the blood and the catheter form a vortex, causing the blood to continuously impact the catheter and the vascular intima, leading to a relatively mild mechanical phlebitis at the puncture site.14 The most serious inflammatory reaction was the Point T. It is because the drug solution continues to stimulate the vascular intima, pro-inflammatory cytokines and TNF-α and interferon released by infiltrating leukocytes may contribute to the permanent and enhanced local vascular inflammation. TNF-α is synthesized by activated macrophages, monocytes, some T cells and NK cells, and it participates in pathological processes such as inflammation, immune response and vasculitis.15 The increase in the expression of TNF-α accelerates the upregulation of the vascular cell adhesion molecule and the human intercellular adhesion molecule-1 (ICAM-1) through the nuclear factor-κB signaling pathway; activates the inflammatory response;16 and exacerbates the vascular injury, where ICAM-1, expressed on lymphocytes; macrophages; and vascular endothelial cells, plays an important role in leukocyte adhesion and endothelial leukocyte migration.17 Especially in the acute stage, CRP increases sharply under the induction of TNF-α. The conformation of CRP at the junction of the endoplasmic reticulum changes and the affinity with the endoplasmic reticulum decreases.18 These activate the body’s inflammatory response mechanism, aggravate the inflammatory response, exacerbate vascular injury and spasm,11 and accelerate the rate of endothelial cell apoptosis.
Therefore, when the clinical nursing staff develop nursing measures relating to phlebitis, they should focus on the tip of the indwelling needle. Precise and scientific nursing should be provided to avoid further aggravation of the inflammatory reaction of the venous vessels to improve efficiency in the treatment of phlebitis, to standardize the prevention processes for phlebitis, to use the research results with good effect, and to reflect the technical and scientific nature of nursing work.
Conclusion
When PVC was used to continuously administer two types of physicochemical drugs, KabivenTM Pl and TNA, within three hours was the safe time for continuous intravenous infusion, and four hours was the limit time. If it exceeds four hours, there will be the risk of phlebitis, which improves the clinical Nursing staff can be early warning of the occurrence of phlebitis, so as to reduce the inflammatory reaction of blood vessels and reduce the incidence of phlebitis. At the same time, three different parts of the PVC indwelling blood vessel, the point I and the point T and the point O were compared and analyzed. The most serious part of phlebitis was the point T. In the development of clinical nursing treatment plan, the place where phlebitis had been treated was the point T.
Limitations and Perspectives for Future Research
In this study, venous serum and vascular tissue morphology were regarded as the starting point of the research. Therefore, our findings are indirect and cannot be immediately generalized to clinical practice. It is proposed that the next step in experimental project design will consider cells as the breakthrough point to explore the cellular pathway mechanism of phlebitis caused by the continuous infusion of emulsions through a peripheral vein to provide a more accurate and scientific reference for the clinical development of phlebitis prevention measures.
Ethics Approval
This study was conducted with approval from the Ethics Committee of Second Hospital of Shanxi Medical University (No: 2017046).
Funding
2017 Shanxi Province Applied Basic Research (No.: 201701D121172).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marsh N, Webster J, Ullman AJ, et al. Peripheral intravenous catheter non-infectious complications in adults: a systematic review and meta-analysis. J Adv Nurs. 2020;76(12):3346–3362. doi:10.1111/jan.14565
2. Marsh N, Webster J, Flynn J, et al. Securement methods for peripheral venous catheters to prevent failure: a randomised controlled pilot trial. J Vasc Access. 2015;16(3):237–244. doi:10.5301/jva.5000348
3. Wang Y, Wang J, Yang J, et al. Study on protection of human umbilical vein endothelial cells from amiodarone-induced damage by intermedin through activation of Wnt/β-catenin signaling pathway. Oxid Med Cell Longev. 2021;2021:8889408. doi:10.1155/2021/8889408
4. Infusion Nurses Society. Infusion therapy Standards of Practice. J Infus Nurs. 2016;29(1):s1–s100.
5. Zhang J, Shen J, Yin W, et al. The intervention research on treatment by Xianchen to rabbits model of chemotherapeutic phlebitis. Acta Cir Bras. 2016;31(8):549–556. doi:10.1590/S0102-865020160080000008
6. Xiaoting L, Liu Y, Zhao L, et al. Chronic psychological stress induces vascular inflammation in rabbits. Stress. 2013;16(1):87–98. doi:10.3109/10253890.2012.676696
7. Machalińska A, Pius-Sadowska E, Babiak K, et al. Correlation between flicker-induced retinal vessel vasodilatation and plasma biomarkers of endothelial dysfunction in hypertensive patients. Curr Eye Res. 2018;43(1):128–134. doi:10.1080/02713683.2017.1358372
8. Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007;49(2):304–310. doi:10.1161/01.HYP.0000252664.24294.ff
9. Miyabe K, Notohara K, Nakazawa T, et al. Histological evaluation of obliterative phlebitis for the diagnosis of autoimmune pancreatitis. J Gastroenterol. 2014;49(4):715–726. doi:10.1007/s00535-013-0818-x
10. Zhang ZX, Wang P, Zhang QS, Pan X, Zhao QX, Wang XK. Effects of anisodamine on the expressions of vascular endothelial growth factor and intercellular adhesion molecule 1 in experimental infusion phlebitis. Chin Med J. 2012;125(2):300–305.
11. Oragano CA, Patton D, Moore Z. Phlebitis in intravenous amiodarone administration: incidence and contributing factors. Crit Care Nurse. 2019;39(1):1–12. doi:10.4037/ccn2019381
12. Morrison K, Holt KE. The effectiveness of clinically indicated replacement of peripheral intravenous catheters: an evidence review with implications for clinical practice. Worldviews Evid Based Nurs. 2015;12(4):187–198. doi:10.1111/wvn.12102
13. Yanyan L, Chunyan H, Wubin H, Can T, Zhenya S. Experimental research on preventing mechanical phlebitis arising from indwelling needles in intravenous therapy by external application of mirabilite. Exp Ther Med. 2018;15:276–282.
14. Yang J, Guo JL. Experimental study on phlebitis induced by intravenous indwelling needle continuous infusion of amiodarone hydrochloride injection. Shanxi Taiyuan: Shanxi Medical University. 2001;20–21.
15. Li WJ, Liu Y, Wang JJ, et al. “Angiotensin II memory” contributes to the development of hypertension and vascular injury via activation of NADPH oxidase. Life Sci. 2016;149:18–24. doi:10.1016/j.lfs.2016.02.037
16. Lamoke F, Labazi M, Montemari A, Parisi G, Varano M, Bartoli M. Trans-Chalcone prevents VEGF expression and retinal neovascularization in the ischemic retina. Exp Eye Res. 2011;93(4):350–354. doi:10.1016/j.exer.2011.02.007
17. Ge GF, Shi WW, Yu CH, et al. Baicalein attenuates vinorelbine-induced vascular endothelial cell injury and chemotherapeutic phlebitis in rabbits. Toxicol Appl Pharmacol. 2017;318:23–32. doi:10.1016/j.taap.2017.01.013
18. Nabata A, Kuroki M, Ueba H, et al. C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: implications for the destabilization of atherosclerotic plaque. Atherosclerosis. 2008;196(1):129–135. doi:10.1016/j.atherosclerosis.2007.03.003
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
