Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Time to Euthyroidism and Its Determinants Among Thyrotoxicosis Patients on Anti-Thyroid Drug Who Attend to Medical and Ambulatory Clinics of South Tigrai General Hospitals
Authors Maldey H , Tadesse S, Alem AZ , Hagezom HM , Hagos Gufue Z
Received 29 March 2021
Accepted for publication 22 June 2021
Published 15 October 2021 Volume 2021:17 Pages 1091—1101
DOI https://doi.org/10.2147/TCRM.S312810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Hiluf Maldey,1 Sumeya Tadesse,2 Adugnaw Zeleke Alem,3 Haftamu Mamo Hagezom,4 Zenawi Hagos Gufue5
1Department of Clinical Pharmacy, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 2Department of Clinical Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Epidemiology and Biostatistics, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 4Department of Psychiatry, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 5Department of Public Health, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia
Correspondence: Hiluf Maldey Email [email protected]
Purpose: To determine the time to euthyroidism and its determinants among thyrotoxicosis patients on anti-thyroid drug attending the medical and ambulatory clinics of South Tigrai General Hospitals, Ethiopia, 2015– 2020.
Methods: A multi-center retrospective cohort study was conducted from March 30, 2020, to July 30, 2020, among adult thyrotoxicosis patients who received anti-thyroid medications in the medical and chronic ambulatory clinics of South Tigrai General Hospitals, Ethiopia. Epi-data manager version 3.1 software was used for data entry and then exported to STATA version 15 for windows for analysis. Cox proportional hazards regression model was used to determine the determinants of time to euthyroidism and the summary measures of the adjusted hazard ratio with a 95% CI were presented, and P-value < 0.05 used to declare the statistical significance.
Results: One hundred and seventy-five (51.9%) thyrotoxicosis patients become euthyroid with the median time to euthyroidism of 9 months, IQR (6– 12) months, with a total of 5542 person-months of observation. Age greater than 40 years (AHR = 0.7; 95% CI 0.54– 0.96), toxic multi-nodular goiter (AHR = 0.69; 95% CI 0.60– 0.87), World Health Organization goiter size three (AHR = 0.78; 95% CI 0.54– 0.82) and baseline free thyroxin (AHR = 0.96; 95% CI 0.80– 0.99) were the independent determinants of delayed time to euthyroidism.
Conclusion: The time to euthyroidism was longer than the expected time. High baseline free thyroxin, toxic multinodular goiter, elderly patients, and patients with World Health Organization goiter size three were determinants of delayed time to euthyroidism.
Keywords: thyrotoxicosis, propylthiouracil, euthyroid, South Tigrai, Ethiopia
Introduction
The term “thyrotoxicosis” refers to a clinical state that results from inappropriate high thyroid hormone action in tissues mainly due to excessively high tissue thyroid hormone levels.1 It is a common endocrine disorder, affecting about 2% of women and 0.2% of men from the general population.2 It can be classified as overt thyrotoxicosis and subclinical thyrotoxicosis.1 In the United States, the prevalence is approximately 1.2% of which is 0.5% overt and 0.7% subclinical respectively.1,3 It is also one of the routine thyroid disorders encountered in the African continent including Ethiopia.4
The precise causes of thyrotoxicosis are not always reliably defined,3 but the most common causes include Graves’ disease (GD), toxic multinodular goiter (TMNG), and toxic adenoma (TA).1,2 Other less common causes of thyrotoxicosis include the entities of painless and subacute thyroiditis, which occur due to inflammation of thyroid tissue with release of the pre-formed hormone into the circulation.5
Ethiopia is among the top iodine-deficient countries in the world despite the passing of new legislation in 2011 under the National Nutrition Programme which led to greater availability of iodized salt, however, the eradication of iodine deficiency disorder (IDD) in Ethiopia remains a significant challenge.6 A study conducted in 2017 reported that although over 89.2% of salt in Ethiopia contains iodine, coverage of adequately iodized salt remains very low (26%).7 Tigrai region is one of the top iodine-deficient areas in Ethiopia and goiter prevalence in that area was greater than 30%.8
A systematic review study reported that the diagnosis and management of thyroid disorders in the African continent remain suboptimal.4 If thyroid function tests are not normalized early thyrotoxicosis can result in serious complications such as thyroid storm, cardiac dilation, hypertension, arrhythmia, congestive heart failure (CHF), and sudden cardiac arrest, osteoporosis, atrial fibrillation, weight loss, embolic events, muscle weakness, tremor, neuropsychiatric symptoms, and rarely cardiovascular collapse and death.1,9
According to the 2014 Ethiopian standard treatment guideline, propylthiouracil (PTU) is used only when methimazole or carbimazole is not appropriate but practically it is the only anti-thyroid drug available locally at the present time.10 Previous studies indicate that normalization of thyroid function test (TFT) using PTU was suboptimal or takes a long period and it does not reverse thyrotoxicosis as rapidly as methimazole or carbimazole and it has more side effects.11
Various studies done in Canada, Sweden, Elias University, and Nigeria show that normalization of thyroid function test was suboptimal and was taking a longer period than expected.12–14 A study done in Gondar also indicates that all TFTs were normalized in less than one-third of the participants meaning that patients who were euthyroid were sub-optimal and recovery of TFT took a longer period than expected from taking PTU.9
Generally in Ethiopia, there is limited research on time to euthyroidism and determinants among thyrotoxicosis patients and most of the previous studies were conducted in western countries in a single center with a small sample size relative to the current study. Therefore this study aimed to determine the time to euthyroidism and determinants among thyrotoxicosis patients on the anti-thyroid drug who attend medical and chronic ambulatory clinics of South Tigrai General Hospitals. This might be serve as a baseline reference, and guide for other researchers.
Methods and Materials
An institutional-based multi-centered retrospective cohort study was conducted from March 30, 2020 to July 30, 2020, among adult thyrotoxicosis patients, who were attending the Southern Zone of Tigrai General Hospitals from January 1, 2015, to January 1, 2020. The study was conducted at three general hospitals of South Tigrai and based on the information obtained from Tigrai regional health bureau there are only three general hospitals in the Southern Zone of Tigrai. These general hospitals were similar in their services, human resources, and medical equipment to each other. The selected general hospitals were Lemlem Karl, Korem, and Alamata general hospitals.
Sample Size Determination
The sample size was determined by applying a single population proportion formula using Epi Info version 7.2.4.0 software,11 with the assumptions of a 95% level of confidence, a 5% margin of error. Taking the proportion of thyrotoxicosis patients who attend the medical ward, ambulatory clinic, and normalized their thyroid function tests from the study conducted at the University of Gondar hospital,9 the proportion of thyrotoxicosis and who became euthyroid (0.33), accordingly the final sample size was 336.
Sampling Technique
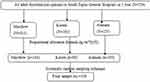
A total of 729 adult thyrotoxicosis patients were counted from all institutions, the data was taken from the Health Management Information System (HMIS) computer and logbook which was registered from January 1, 2015, to January 1, 2020. Proportional allocation was used to determine the final sample to be drawn from each institution. Finally, a systematic random sampling technique was used to draw the final sample from a prepared list of medical record number which was coded from 1 to N. The sampling interval was determined by dividing the number of units in the population by the desired sample size (K = N/n) and it was 2 or (K = 2) in all three institutions. The first card was selected randomly and the remaining sample was selected for every second medical record (see Figure 1).
Data Collection Tools and Procedure
The data collection instrument was pretested on 5% (20 medical records which were not included in the final analysis) one week before the data collection time in Lemlem Karl Hospital which gives a slightly higher service than the other two, then the data collection instrument was modified based on the results of the pretest. During data collection, the data on the tool and the medical records were rechecked for accuracy, then after data collection for each sample each variable was recoded before data entry to minimize manual data entry error.
Before data collection, training was given for data collectors (10 BSc nurses) on the contents of the data extraction checklist, how to abstract data from patient medical records and health facility logbooks for two days before the data collection period by the principal investigator and three experienced supervisors who have a master’s degree and working in the medical and ambulatory clinics of the three hospitals were involved. The checklist contains four parts: socio-demographic variables; clinical related features; medication-related questions, and time to thyroid function test normalization questions.
Operational Definitions
Thyrotoxicosis: Thyroid Stimulating Hormone (TSH) level < 0.4 mU/L.1,9 Euthyroid (event): is considered if free thyroxin (FT4) and free triiodothyronine (FT3) are within the normal range.1,5 Normal range: TSH (0.4–5.0 mU/L), FT4 (10.4–19.6pmol/l) and FT3 (4–5.3 pmol/L). Censored: Those subjects who were discharged against medical advice, discharged with significant problems, death other than thyrotoxicosis (accident or any cause not related to thyrotoxicosis), referred to other health facilities or event-free until the end of the study were considered as censored.
Time to event: The total time from initiation of treatment to the occurrence of an event. Complication: is a condition that arises during the hospital stay that prolongs the length of stay.16 Comorbidity: is a pre-existing condition that affects the treatment received and/or prolongs the length of stay.16 World Health Organization (WHO) goiter size: according to WHO goiter classification goiter size was categorized as grade 0, 1, 2, and 3.17 Grade 0: The goiter is not palpable or visible even when the neck is extended. Grade 1: The goiter is detected on palpation and/or visible when the neck is extended. Grade 2: Goiter is visible when the neck is in the normal position. Grade 3: Large goiter visible from distance. Classification of the different types of goiter size was mainly made based on clinical grounds (i.e. based on clinical examination) and was taken from the medical records.
Data Quality Assurance and Analysis
Data were entered using Epi data manager software version 3.1 and exported to STATA version 15 for analysis. Descriptive statistics of numeric variables were presented using mean with standard deviation or median with interquartile range, whereas categorical variables were expressed as a frequency and percentages. The outcomes of each patient are dichotomized into euthyroid or censored. Kaplan–Meier method was used to estimate the overall time to euthyroidism and a Log rank test was used to compare the observed difference in survival time between categorical variables of adult thyrotoxicosis patients.
Assumptions of the Cox Proportional hazards were checked by Schoenfeld residual test. To assess the association among baseline variables and patient survival two strategies were used. First, each baseline variable that did not violate the assumptions of the Cox proportional hazards regression model was entered into a separate Cox regression model. Second, variables that have a P-value < 0.2 in the bivariate analysis were taken to multivariable analysis. A Cox regression model was used to determine factors associated with time to euthyroidism.
The estimated crude (CHR) and adjusted hazard ratios (AHR) with a 95% confidence interval were presented, and P-value <0.05 was used to declare statistical significance. Furthermore, multi-collinearity between the independent variables was assessed with variance inflation factor (VIF) to identify and avoid redundant variables that may affect our estimate, the overall mean VIF was 1.15 which was within the acceptable range (1–5).18
Results
Socio-Demographic and Clinical Characteristics
A total of 336 patients were included in this study, the mean age of the patients was 46.7 ± 11.94 years and there were a high proportion of females (88.7%). Three hundred and forty-three (93.1%) patients were non-smokers, 69.4% have goiter and 36.6% have WHO goiter size two. The common cause of thyrotoxicosis was toxic multinodular goiter in two-thirds of patients (66.6%) (Table 1).
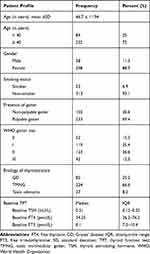 |
Table 1 Baseline Characteristics of Thyrotoxicosis Patients Attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n = 336) |
Baseline Sign and Symptoms
From the baseline sign and symptoms, heat intolerance and palpitation occurred in around two-thirds of the patients (66.3%) and (62.7%), respectively. Increased appetite, nervousness, and diffuse goiter occurred in less than one-third of patients (Table 2).
 |
Table 2 Frequency of Baseline Signs and Symptoms Among Thyrotoxicosis Patients Attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n = 336) |
Comorbidity and Complication
A total of 96 patients had different comorbidities, among these, diabetes mellitus was the most common comorbidity identified among 11% of patients followed by hypertension which was developed among 7.7% (Table 3). A total of 32 patients developed different complications, among these congestive heart failure was the most common complication which occurred in 4.7% of patients (Figure 2).
 |
Table 3 Common Comorbidities Among Thyrotoxicosis Patients Attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n=96) |
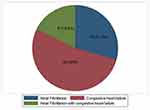 |
Figure 2 Common complications detected among thyrotoxicosis patients attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n=336). |
Medication-Related
Propylthiouracil and atenolol were prescribed for 100% and 58.71% patients, respectively. The minimum initial daily dose of PTU was 200 mg and the maximum was 600 mg (n = 2). Most of the patients (55.76%) were taking an initial daily dose of 200 mg (n = 187) and the remaining 43.7% (n = 147) were taking 350 mg. The minimum maintenance dose of PTU was 100 mg and the maximum was 300 mg, most of the patients (76.95%) were taking a maintenance daily dose of 200 mg. Metoprolol, furosemide, and spironolactone were the most commonly prescribed drugs respectively for the management of comorbidity and complications (Table 4).
 |
Table 4 Commonly Prescribed Medications for the Treatment of Comorbidities and Complications Among Thyrotoxicosis Patients Attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n = 336) |
Time to Euthyroidism
One hundred and seventy-five (51.9%) thyrotoxicosis patients become euthyroid with the median time to euthyroidism of 9 months with IQR (6–12) months and with 4427 person-months of observation. The median time to euthyroidism among thyrotoxicosis patients with WHO goiter size one was 8 months, IQR (6–14) months, for patients with goiter size two the median time to euthyroidism was 9 months, IQR (6–12) months. Finally, the median time to euthyroidism among thyrotoxicosis patients with goiter size three was 9 months, IQR (6–12) months.
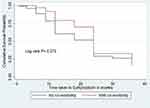
There was a statistically significant difference in the survival time between thyrotoxicosis patients with and without comorbidity at baseline (Log rank test = 4.88, df = 1, P = 0.027) (Figure 3). There was no statistically significant difference in the survival time among thyrotoxicosis patients on anti-thyroid drugs with and without palpable goiter (Log rank test = 0.19, df = 1, P = 0.66) (Figure 4). There was a statistically significant difference in the survival time between thyrotoxicosis with age ≥ 40 years and age <40 years (Log rank test = 7.08, df = 1, P = 0.014) (Figure 5).
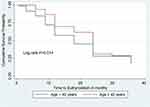 |
Figure 5 Kaplan–Meier survival curve of time to euthyroidism based on age category among thyrotoxicosis patients attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n = 336). |
Determinants of Time to Euthyroidism
In the final multivariable Cox proportional hazards regression model those variables which were clinically important and which had p-value < 0.2 in the bivariate analysis and non-collinear independent variables were included. The variables selected for the multivariable analysis were age, etiology, gender, smoking, comorbidity, initial PTU dose, WHO goiter size, baseline TSH, and baseline FT4.
Accordingly, the chance of achieving normalization of thyroid function tests early in the age group above the age of 40 years was decreased by 29.8% compared with the patients with age below 40 years (AHR = 0.702; 95% CI 0.544–0.962). The chance of achieving normalization early among TMNG patients’ was decreased by 30.8% compared with patients with Graves’ disease (AHR = 0.692; 95% CI 0.603–0.869). The chance of achieving normalization early among WHO goiter size three patients was decreased by 17.9% compared with WHO goiter size zero patients (AHR = 0.779; 95% CI 0.543–0.821). The chance of achieving normalization early, for one unit increase in the baseline FT4, was decreased by 3.6% (AHR = 0.964, (0.807–0.998), P = 0.035) (Table 5).
 |
Table 5 Determinants’ of Time to Euthyroidism Among Thyrotoxicosis Patients Attending South Tigrai General Hospitals, Ethiopia from 2015–2020 (n = 336) |
Discussions
This study provided important information about the time to euthyroidism among thyrotoxicosis patients attending South Tigrai general hospitals’ medical and ambulatory clinics. Most of the patients in the current study were elderly with a dominance of females, non-smokers, and with WHO goiter size two. At the baseline, common signs and symptoms encountered were heat intolerance and palpitation in the majority of the patients. Our study is similar to a retrospective study done in Saudi Arabia, which revealed a high proportion of female to male ratio of (161:42) 3.8:1, but Graves’ disease (GD) was the underlying cause in the majority of the patients, 140 (69%) patients, which differs from our study.19
In the current study, most of the patients (55.76%) were taking an equal initial and maintenance daily dose, which was 200 mg. The reason could be because frequent measurements of thyroid function tests were not done, measurements were taken after two or three rounds of repeated follow-up time. This could be due to cost or chemical reagent issues. As a result, patients were classified as non-euthyroid even if their thyroid function tests were improved. Different guidelines recommend that thyroid function tests should be checked 4–6 weeks after initiation of therapy and then every 2–3 months once the patient is euthyroid.20 That might be the reason for having equal maintenance and initial doses in most of the patients in the present study.
In the present study, 51.9% (75) patients became euthyroid, which is lower than a study conducted in Saudi Arabia which reported that 66 (72%) patients became euthyroid. This difference might be, in this study the most common etiology of thyrotoxicosis was TMNG, but the study done in Saudi Arabia reported that GD was the underlying cause in the majority of patients.19 In regions of iodine deficiency, the toxic multinodular goiter may be more common than GD.1 Our study area is one of the top iodine-deficient areas in Ethiopia8 and this may be the reason for the high proportion of TMNG in the present study and results in a lower outcome.
The chance of early normalization decreases by 17.9% in thyrotoxicosis patients with WHO goiter size three compared with WHO goiter size zero, which is consistent with a Swedish retrospective study, patients with goiter size two had a worse prognosis compared with goiter size zero (51.2% vs 68.9% in remission after 5 years; P = 0.014).21
In the current study, all the patients were taking PTU for the treatment of thyrotoxicosis. However, the 2014 Ethiopian standard treatment guideline for general hospitals recommends carbimazole or methimazole as the first-line drugs for the management of thyrotoxicosis and PTU is an alternative drug. Despite this, PTU is the only anti-thyroid drug (ATDs) available in Ethiopia at the moment.9
Our study shows that the treatment modality in all the patients was PTU and 51.9% of patients became euthyroid, which was higher than a retrospective study conducted at two Canadian centers which showed that only 22 (33%) became euthyroid.12 Our study also outperforms a descriptive study conducted in Nigeria, which found that 10 (9%) of patients with Graves’ disease were hypothyroid.14
The high proportion of euthyroid patients in the present study is due to the large sample size and difference in outcome measurement used by the facility set up because of the service differences which are described. Euthyroidism was measured in this report by normalization of FT4 and FT3 but in the above two studies, it was measured as if the three thyroid function tests (TSH, FT4, and FT3) were within the normal range. Therefore, the difference is mainly due to TSH which takes a long period to normalize, because TSH levels often remain suppressed for several months and therefore do not provide a sensitive index of early treatment response.5
In the present study, the median (IQR) time taken to become euthyroid was 9 months, IQR (6–12) months, which is lower than the study conducted in Gondar comprehensive specialized hospital which was 11.53 (13.39) months.9 This small difference might be due to the difference in the mean age of the participants which was (46.7 ± 11.94) years vs (47.25±14.26) years respectively. That may affect the outcome and it could be due to time measurement variation. The response is expected in 4 to 6 weeks of starting ATDs in most patients and after 6 weeks of starting ATDs, 90% of patients with GD become normalized but more than 24 months of ATD treatment is usually necessary to normalize TFTs in patients with TMNG.20,22
In the present study, the most common etiology identified was TMNG, this may be the reason for the longer time to euthyroidism than expected. In patients with TMNG, the levels of FT3 or FT4 increase two to three times from the normal.23 Baseline FT4 significantly affects the time to euthyroidism, which decreases the normalization by 3.6%. This finding was in line with the retrospective study conducted at the Elias University Hospital and Gondar university comprehensive specialized hospital, which reported that the level of FT4 at diagnosis was the reason for delayed TSH normalization.9,13
Our study was also similar to a retrospective study conducted in Edinburgh which reported that patients with a higher baseline of FT4 and FT3 were found to be hyperthyroid after they were treated with carbimazole for 4–6 weeks.24 In contradiction to our finding, a retrospective study conducted in Sweden reported higher FT4 and FT3 levels were not identified as significant prognostic factors.21 The cause of thyrotoxicosis in the current study was toxic multinodular goiter, whereas the cause of thyrotoxicosis in the Saudi Arabia study was Graves’ disease, which may be the reason for FT4 to be a determinant factor in the current study.
Age greater than 40 years reduces the time to euthyroidism by 29.8%, which is similar to the findings of a retrospective study conducted at the University of Gondar Hospital. Older age was found to slightly reduce the chances of achieving TSH normalization.9 On the other hand, our study is contrary to a study conducted at a teaching hospital in Bucharest, which showed that patients’ age did not seem to influence the thyroid test recovery.13
This finding was also in contradiction with another retrospective study conducted at Queen Elizabeth Hospital, Birmingham among Graves’ disease hyperthyroid patients, which found that patients under the age of 40 were more likely to fail to respond to medical treatment.15 Similarly, our findings differed from a retrospective study conducted in Edinburgh, which discovered that after 4–6 weeks of carbimazole treatment, 50% of patients younger than 30 years remained biochemically hyperthyroid, compared with 14% of patients older than 30 years.24
Although the exact cause of how older age affects the time to euthyroidism is unknown, the response of the hypothalamic-pituitary-thyroid axis to T4 and T3 levels may have changed due to changes in the setpoints by age.25 In the present study, there is a high proportion of people of older ages and TMNG is high in this age group. This might be the reason for the current study results being different from the other studies.
In this study, the minimum follow-up time was 4 weeks and the maximum follow-up time was 36 months because after ATD initiation, patients may show a response after 4 weeks but some patients did not show any response at this time, as TMNG patients may stay for greater than 24 months. The time to euthyroidism was determined when two thyroid function tests (FT4 and FT3) became normal. In our study, the three thyroid function tests were not done at the same time in most of the patients, this might be because of cost and shortage of chemical reagent. FT4 and FT3 levels were commonly measured in our participants, and the guidelines recommend that the dose of ATD be titrated based on free T4 and free T3 levels.17 That is why euthyroidism was measured by FT4 and FT3 only in the present study.
There were no adverse drug reactions recorded, but that does not mean that all the patients did not develop adverse drug reactions. This might be due to lack of documentation and in most of the patients complete blood count and organ function tests were not done frequently for follow-up after the initiation of the drug. This finding suggests that establishing timely follow-up of patients with full monitoring parameters and capability of providing comprehensive treatment services for thyrotoxicosis patients can reduce the risk of underreporting adverse drug reactions if the set-up is used.
Strength and Limitations of the Study
Strength
This is the second local study that tried to estimate time to euthyroidism and its determinants among thyrotoxicosis patients with a large sample size relative to the previous similar studies and, mainly, it is a multicenter study. The outcome of this study was measured using a standard tool and the most recent guidelines.
Limitation
The results of this study are not without limitations. Due to the retrospective nature of the study and relying on medical records, we did not address the adherence of the patients to the anti-thyroid drugs, which could be one of the determinants of the delayed time to euthyroidism. Variables such as goiter size and location may have been assessed and recorded differently because all data came from medical files from multiple doctors.
Conclusions
In general, more than half of the patients in this report were euthyroid, but it took longer than expected. High baseline FT4, toxic multinodular goiter, elderly patients, and patients with WHO goiter size three were associated with delayed time to euthyroidism. Therefore, close follow-up is important for such types of patients.
Data Sharing Statement
The datasets used and analyzed during this study are available from the corresponding author if needed.
Ethics Approval
The study was conducted after obtaining ethical clearance from the ethics review committee of the Department of Clinical Pharmacy, the University of Gondar with reference number 040/2020 and the Tigrai regional health bureau, medical director, chief executive officer, Matron, and head of the medical ward and ambulatory clinics of each institution. The data were collected from the medical records, in which patient consent was not required by the University and the Hospitals. Data were handled with strong confidentiality, neither the case records nor the data extracted were used for any other purpose and all the collected patient information was stored anonymously. This study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments.
Acknowledgments
Our gratitude goes to the chief executive officer, medical directors, medical record room workers, HMIS workers, data collectors of each institution for their unreserved support in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All the authors declared that they have no competing interests.
References
1. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. doi:10.1089/thy.2016.0229
2. Amballi A. Thyrotoxicosis-a review. Middle East J Sci Res. 2007;2(3–4):98–103.
3. Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301. doi:10.1038/nrendo.2018.18
4. Ogbera AO, Kuku SF. Epidemiology of thyroid diseases in Africa. Indian J Endocrinol Metab. 2011;15(Suppl2):S82. doi:10.4103/2230-8210.83331
5. Kahaly GJ, Bartalena L, Hegedüs L, Leenhardt L, Poppe K, Pearce SH. 2018 European thyroid association guideline for the management of graves’ hyperthyroidism. Eur Thyroid J. 2018;7(4):167–186. doi:10.1159/000490384
6. Andersson M, Karumbunathan V, Zimmermann MB. Global iodine status in 2011 and trends over the past decade. J Nutr. 2012;142(4):744–750. doi:10.3945/jn.111.149393
7. Dessie G, Amare D, Dagnew AB, et al. Prevalence of goiter among children in Ethiopia and associated factors: a systematic review and meta-analysis. BMC Public Health. 2019;19(1):1191. doi:10.1186/s12889-019-7505-7
8. Workie SB, Abebe YG, Gelaye AA, Mekonen TC. Assessing the status of iodine deficiency disorder (IDD) and associated factors in Wolaita and Dawro Zones School Adolescents, southern Ethiopia. BMC Res Notes. 2017;10(1):156. doi:10.1186/s13104-017-2480-5
9. Gebreyohannes EA, Ayele EM, Tesfaye SA, Seid MA. Normalization of thyroid function tests among thyrotoxicosis patients attending a University Hospital in North-West Ethiopia. Thyroid Res. 2019;12(1):3. doi:10.1186/s13044-019-0064-2
10. Food M, Administration HC, Ethiopia CAo. Standard Treatment Guidelines for General Hospitals. 2014.
11. Ross DS. Patient education: antithyroid drugs (Beyond the Basics).
12. Al Jassim A, Wallace T, Bouhabel S, et al. A retrospective cohort study: do patients with graves’ disease need to be euthyroid prior to surgery? J Otolaryngol Head Neck Surg. 2018;47(1):37.
13. Martin S, Sirbu A, Albu A, et al. The time to thyroid-stimulating hormone recovery during medical treatment in graves’disease and autonomous hyperthyroidism. Acta Endocrinol. 2013;9(3).
14. Ogbera AO, Fasanmade O, Adediran O. Pattern of thyroid disorders in the southwestern region of Nigeria.
15. Allahabadia A, Daykin J, Holder RL, Sheppard MC, Gough SC, Franklyn JA. Age and gender predict the outcome of treatment for graves’ hyperthyroidism. J Clin Endocrinol Metab. 2000;85(3):1038–1042.
16. Available from: https://hmsa.com/portal/provider/zav_pel.fh.COM.600.htm#:~:text=For%20the%20purposes%20of%20codingptlos.
17. Patel KN, Yip L, Lubitz CC, et al. The American Association of endocrine surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271(3):e21–e93.
18. Akinwande MO, Dikko HG, Samson A. Variance inflation factor: as a condition for the inclusion of suppressor variable (s) in regression analysis. Open J Stat. 2015;5(7):754. doi:10.4236/ojs.2015.57075
19. Akbar DH, Mushtaq MA, Al-Sheikh AA. Etiology and outcome of thyrotoxicosis at a university hospital. Saudi Med J. 2000;21(4):352–354.
20. Chisholm-Burns MA. Pharmacotherapy principles & practice/editors, Marie A. Chisholm-Burns, Terry L. Schwinghammer, Barbara G. Wells, Patrick M. Malone, Jill M. Kolesar, Joseph T. DiPiro. 2016.
21. Mohlin E, Filipsson Nyström H, Eliasson M. Long-term prognosis after medical treatment of graves‘ disease in a northern Swedish population 2000–2010. Eur J Endocrinol. 2014;170(3):419–427. doi:10.1530/EJE-13-0811
22. Bgwjt D, DiPiro TLSCV. Pharmacotherapy Handbook Ninth Edition, Barbara G. Wells, Pharmd, FASHP, FCCP, 2015 by Mcgraw-Hill Education. McGraw-Hill Education; 2015.
23. Begum F, Sultana S, Nahar N, et al. Protocol for management of hyperthyroidism by radioactive iodine (RAIT)-SNMB guidelines. Bangladesh J Nucl Med. 2015;18(1):85–88. doi:10.3329/bjnm.v18i1.34944
24. Bringmann I, Van Leeuwen B, Hennemann G, Beckett G, Toft A. Outcome of treatment of hyperthyroidism. J Endocrinol Invest. 1999;22(4):250–256. doi:10.1007/BF03343552
25. Wiersinga WM. T4 + T3 combination therapy: any progress? Endocrine. 2019;66(1):70–78. doi:10.1007/s12020-019-02052-2
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.