Back to Journals » Infection and Drug Resistance » Volume 14
Thyroid Profile and Factors Associated with Hypothyroidism Among Multidrug-Resistant Tuberculosis Patients Attending Saint Peter’s Specialized Hospital Addis Ababa, Ethiopia
Authors Biranu E , Wolde M, Negesso AE , Hailu Tola H , Molla Sisay M
Received 26 March 2021
Accepted for publication 18 June 2021
Published 12 July 2021 Volume 2021:14 Pages 2675—2684
DOI https://doi.org/10.2147/IDR.S310404
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Endalkchew Biranu,1,2 Mistire Wolde,1 Abebe Edao Negesso,1 Habteyes Hailu Tola,3 Million Molla Sisay2
1Addis Ababa University College of Health Sciences Department of Medical Laboratory Sciences, Addis Ababa, Ethiopia; 2St. Peter’s Specialized Hospital, Research and Evidence Generation Directorate, Addis Ababa, Ethiopia; 3Ethiopian Public Health Institute, Tuberculosis/HIV Research Directorate, Addis Ababa, Ethiopia
Correspondence: Mistire Wolde Snr
Department of Medical Laboratory Sciences, College of Health Sciences, Addis Ababa University, P.O. Box 30538, Addis Ababa, Ethiopia
Tel +251-911-69 97-10
Email [email protected]
Background: The emergence of MDR-TB is a global public health problem. Hypothyroidism is one of the severe adverse drug reactions (ADRs) in MDR-TB patients on treatment. Representative data on hypothyroidism and its associated factors among MDR-TB patients are lacking.
Objective: To determine thyroid profiles and associated risk factors among multidrug-resistant TB patients during therapy with anti-MDR-TB regimen in Saint Peter Specialized Hospital Addis Ababa, Ethiopia from January to November 2020.
Methods: A cross-sectional study was conducted in MDR-TB patients in Addis Ababa, Ethiopia. A total of 162 patients, who were older than 18 years, had bacteriologically confirmed MDR-TB and on treatment for more than one month were enrolled consecutively from the TB registration book. However, critically sick patients and those who were receiving additional drugs known to cause severe ADRs were excluded. Simple descriptive statistics were used to present the socio-demographic and clinical characteristics of the patients. A logistic regression model was used to assess the association between independent and dependent variables. A p-value < 0.05 was considered as statistically significant in all analyses.
Results: Mean age of the study participant was 35.9 ± 13.6 years. The prevalence of hypothyroidism was 32 (19.8%). The presence of co-morbidity, being underweight, and prothionamide use were significantly associated with hypothyroidism in MDR-TB patients on treatment.
Conclusion: Hypothyroidism occurs commonly among MDR-TB patients. Presence of co-morbidity, being underweight, and prothionamide drug use are the factors associated with hypothyroidism. Monitoring of thyroid function test during MDR-TB treatment and factors associated with hypothyroidism require attention to prevent complication.
Keywords: multidrug-resistant, tuberculosis, thyroid profile, hypothyroidism
Background
Tuberculosis (TB) has become the single infectious disease causing morbidity and mortality among millions across the world. A recent World Health Organization (WHO) global TB estimate indicates 10 million new cases and 1.4 million deaths occurred worldwide in 2019.1 The emergence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant TB (XDR-TB) is becoming a global public health due to the contagious nature of the diseases, difficulty related to diagnosis and treatment.2 MDR-TB is refers to the Mycobacterium tuberculosis strain resistant to the two most potent frontline TB drugs such as isoniazid and rifampicin.3 About 3.3% of the new and 18% of the previously treated TB cases developed MDR-TB in 2019.1
The most challenges in MDR-TB treatment are the adverse drug reactions (ADR) related to its drugs.4 Hypothyroidism is among the severe ADR that occurred during MDR-TB treatment.4,5 Endocrine abnormalities do not always reflect a direct infection of the gland, but could occur due to a physiological response of the system or drugs used for the treatment.6 Hypothyroidism is a deficiency in the secretion and action of thyroid hormones. This disorder is associated with increased level of thyroid stimulating hormone (TSH) and decreased free triiodothyronine (T3) and free thyroxine (T4) level. As a result, reduced T4 and T3 concentration in the serum leads to hyper-secretion of pituitary TSH and notable elevation in the serum TSH concentration.7 ADR related to MDR-TB drugs are also the main cause of treatment discontinuation.8 This is the most significant determinant of poor treatment outcomes such as prolonged morbidity, drug resistance development, treatment failure, and mortality.9 Monitoring TSH rates in MDR-TB patients is essential for managing the side effects of MDR-TB drugs.
In the patients on second-line MDR-TB drugs up to 58% developed hypothyroidism due to para-aminosalicylic acid, ethionamide, and prothionamide.10,11 Moreover, 54% and 69% of MDR-TB patients developed hypothyroidism.12–14 A recently published review study indicated 17% of MDR-TB patients developed hypothyroidism.15 In addition, a previously reported study from Ethiopia showed the prevalence of hypothyroidism is 17.2% in patients treated for MDR-TB.16
Although some studies have reported the burden of hypothyroidism among MDR-TB patients on treatment, there is limited evidence on the burden and the factors associated with the high proportion of hypothyroidism. Moreover, the burden and the risk factors of hypothyroidism could vary from setting to setting due to different factors such as nutrition status of the population in a given geographic area.17,18 Thus, an area-specific study could well explain the risk factors associated with high hypothyroidism in MDR-TB patients on treatment. Therefore, this study aimed to determine the prevalence of hypothyroidism and its risk factors in MDR-TB patients on treatment.
Materials and Methods
Study Area
This study was conducted in Addis Ababa, the capital city of Ethiopia, from January to November 2020 at Saint Peter Specialized Hospital. Saint Peter hospital was established in 1953. It is located in the Gullele sub-city of Addis Ababa. It is administered under the Ethiopia Federal Ministry of Health (FMoH). It is the first national hospital that stated MDR-TB treatment in Ethiopia in April 2009. It was a center of excellence and training during the scaling up of MDR-TB treatment initiation centers in the country.16 This hospital is a referral hospital that receives patients from all parts of the country. During the study period there were 197 MDR-TB patients on treatment in the hospital, of whom 30 were in patients.
Study Design and Population
A cross-sectional study was conducted in Saint Peter Specialized Hospital. MDR-TB patients who were bacteriologically diagnosed and treated with second-line anti-TB treatments at Saint Peter Specialized Hospital were the study population. MDR-TB patients older than 18, bacteriologically confirmed, had full data on the key variables, and on treatment for more than one month were included into this study. However, critically sick patients, those who were not initiated anti-MDR tuberculosis drugs, and receiving additional drugs known to have ADR were excluded from this study.
Sample Size Calculation and Sampling Method
Sample size was determined by single population proportion using the Epi-info sample size calculator by considering a population prevalence of hypothyroidism of 17.2% in Ethiopia,16 95% confidence level, and 0.06 desired precision. As a result, a total of 151 sample size was determined. The sample size was also further increased to 166 after considering 10% contingency sample due to registration errors and refusal. However, 162 patients were included into this study after 4 patients refused to participate in the study.
Data and Specimen Collection
Socio-demographic and clinical data were collected in structured questions. Five milliliters of peripheral venous blood was drawn from the cubital vein of each participants using an aseptic technique in a plain gel vacutainer tube. The blood samples were then centrifuged at 3000 rpm to separate the serum within one hour after collection and stored at 20 °C in a refrigerator until the thyroid profile was tested. Samples were collected, handled, and transported to the laboratory according to guidelines of the Clinical and Laboratory Standards Institute/NCCLS (National Clinical Chemistry Laboratory Standards).19,20
The professionals who collected the data and specimens were trained on the purpose of the study, participant selection, blood sample collection and transportation, and ethical issues. All data and blood samples were collected under the supervision of the appointed research directorate and principal investigator.
Thyroid Profiles Testing
Thyroid function tests such as TSH, free T3 and T4 were measured within 24 hours by Cobas e601 analyzer. This is a fully automated discrete immunoassay that deals with the electrochemiluminescence concept. The electrochemiluminescence competitive low molecular weight analyte principle was used for FT3 and FT4. The high molecular weight analyte sandwich principle was used for TSH test.21
Data Quality Assurance
The specimen was collected and transported based on the recommended procedure and the instrument was established based on manufacturer recommendation. Commercially quality control materials were used in the same manner as patient specimens to ensure the precision and accuracy of the instrument. Finally, the results were recorded and handled appropriately and stored in a secured place until entered into statistical software. Moreover, to assure the quality of the data socio-demographic and clinical data, blood sample collection and laboratory test process were supervised by the principal investigator. The collected data were checked regularly for any error, and the necessary correction was taken on the same date as errors occurred.
Data Analysis
Data were double entered into IBM SPSS statistics version 23 separately into two different databases by different persons, and analyzed by the same statistical package. Patients’ characteristics and thyroid profile tests of the patients were analyzed by mean ± standard deviation (SD) and percentage based on measurement level of the variables. Bivariate and multivariate logistic regression model were used to assess the association between hypothyroidism and independent variables. The p-value <0.05 was considered statistically significant in all analyses.
Ethical Consideration
This study was approved by the Department of Research and Ethics Committee (DREC) of Medical Laboratory Sciences, College of Health Sciences Addis Ababa University (Approval number-DRERC/479/19/MLS) and Saint Peter Specialized Hospital (approval number-V143/28/01/2020). Informed consent was obtained from each participant, and sensitive information that could identify the patients was not disclosed to protect confidentiality, and our study complied with the Declaration of Helsinki.
Definitions
Thyroid profiles: measurable or quantifiable characteristics that include T3, T4, and TSH.
Euthyroid: normal TSH, free T3, and free T4.
Primary hypothyroidism: high TSH with low free T4 and free T3.
Subclinical hypothyroidism: high TSH and normal free T4 and free T3.
Sick euthyroid: low free T3 with normal TSH and free T4 or low free T4 and free T3 with normal TSH).22
Results
Patients’ Characteristics
Table 1 depicts the socio-demographic and clinical characteristics of the participants. A total of 162 MDR-TB patients were included in this study. Of 162 patients, 99 (61.1%) were males and the mean age was 35.9 ± 13.6 years with age range 18 to 79 years. The majority of the patients (64.8%) were single and 76 (46.9%) unemployed. One hundred and thirty-nine (85.8%) of the patients were urban dwellers. Monthly income of 54 (33.3%) patients was less than 1000 Ethiopian Birr. Forty (24.7%) patients had co-morbidities. Of the 40 patients who had co-morbidities 26 (16%) were HIV positive, 10 (6.2%) had diabetes mellitus, and 4 (2.5%) had other diseases. Of the total, 16 (9.9%) patients were smokers and 21 (13%) had a history of alcohol consumption. One hundred and fifty-one (92.6%) patients had pulmonary TB, 8 (4.9%) had extrapulmonary, and 3 (1.8%) had both. Twenty-three (14.2%) patients had previous history of MDR-TB treatment, and 122 (75.3%) patients were on treatment of MDR-TB for more than three months (Table 1). The mean height of the patients was 1.7 (±8.2) meters, while the mean of body weight was 53.4 (±10.3) kilograms and the mean of body mass index (BMI) was 19.26 (±3.2 m2/kg).
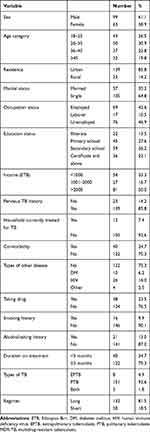 |
Table 1 Socio-Demographic Characteristics of MDR-TB Patients at Saint Peter’s Specialized Hospital in Addis Ababa, Ethiopia, 2020 (n = 162) |
Table 2 shows the MDR-TB drugs currently taken by patients. Thirty-six (22.2%) patients were taking prothionamide (Pto) and only one (0.6%) patients was ppara-aminosalicylate sodium (PAS). Six (3.7%) patients were on capreomycin (Cm), while 151 (93.2%) levofloxacin (Lfx), and 24 (14.8%) Moxifloxacin (Mfx). The majority (95.1%) patients were taking cycloserine (Cs), while 156 (96.3%) clofazimine (Cfz), and 153 (94.4%) Linezolid (Lzd). All drugs were given based on patients’ weight per kilogram (Table 2).
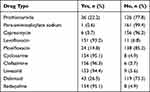 |
Table 2 TB Drugs in Use MDR-TB Patients at Saint Peter’s Specialized Hospital in Addis Ababa, Ethiopia, 2020 (n = 162) |
Thyroid Profile
Table 3 depicts the mean distribution of thyroid profiles. The mean level of thyroid stimulating hormone (TSH) was 3.0 (±2.9 µU/mL), while mean of thyroxine (T4) was 7.8 (±2.1 µg/dL), and triiodothyronine (T3) 1.3 (±0.36 ng/mL). Of the total patients, 19.8% had high TSH (Figure 1A), while 8 (4.9%) had low T4 (Figure 1B) and 6 (3.7%) had low T3 (Figure 1C). Of the total 36 patients on prothionamide, 15 (41.7%) developed hypothyroidism.
 |
Table 3 Mean Distribution of Thyroid Profile of MDR-TB Patients at Saint Peter’s Specialized Hospital in Addis Ababa, Ethiopia, 2020 (n = 162) |
 |
Figure 1 (A) TSH, (B) T4 and (C) T3 levels based on reference values and (D) clinical classification of hypothyroidism distribution among MDR-TB patients at Saint Peter’s Specialized Hospital. |
Clinical Classification Based on Thyroid Profile
Normal range for TSH was 0.27–4.2 μU/mL, whereas for T3 was 0.8–2.0 ng/mL and for FT4 was 5.1–14.1 µg/dL. A patient with high serum TSH level (4.2–10 μU/mL) and normal FT3 and FT4 levels was considered as had subclinical hypothyroidism, while patients with high TSH (>10 μU/mL) and low FT3 and FT4 levels were classified as patients with overt hypothyroidism. Further, patients with normal TSH, FT3, and FT4 were considered euthyroid. Thus, the prevalence of hypothyroidism in patients with MD-TB was 19.8% and 28 (17.3%) patients had subclinical hypothyroidism (Figure 1D).
Factors Associated with Hypothyroidism
Presence of any co-morbidity (COR 2.6, 95% CI 1.1–5.9, p = 0.022), HIV sero-reactive (COR 3.5, 95% CI 1.4–8.9, p = 0.008), drug used other than MDR-TB drugs (COR 3.4, 95% CI 1.5–7.9, p = 0.003), duration on MDR-TB treatment ≥ 3 months (COR 3.8, 95% CI 1.1–5.7, p = 0.034), being underweight (COR 2.5, 95% CI 1.1–5.7, p = 0.034), and prothionamide drug use (COR 4.6, 95% CI 2.0–10.6, p = 0.001) were significantly associated with hypothyroidism in bivariate analysis (Table 4).
 |
Table 4 Risk Factors of Hypothyroidism Based on Bivariate Analysis in MDR-TB Patients at Saint Peter’s Specialized Hospital, Addis Ababa, Ethiopia, 2020 (n = 162) |
Presence of co-morbidity (AOR = 3.8; 95% CI 1.5–9.9; p = 0.006), being underweight (AOR = 2.6; 95% CI 1.0–6.6; p = 0.050), and prothionamide use (AO = 5.4; 95% CI 2.0–14.4; p < 0.001) were significantly associated with hypothyroidism in MDR-TB patients on treatment after controlling for the other variables in the model (Table 5).
 |
Table 5 Risk Factors of Hypothyroidism Based on Multivariable Analysis of MDR-TB Patients at Saint Peter’s Specialized Hospital, Addis Ababa, Ethiopia, 2020 (n = 162) |
Discussion
The need for thyroid disease diagnosis in MDR-TB patients should not be underestimated because studies have shown that subclinical hypothyroidism raises the risk of depression and decreases adherence to treatment with MDR-TB and HIV.23,24 The current study revealed that the prevalence of hypothyroidism among MDR-TB patients on treatment was 19.8%. Presence of co-morbidity, being underweight, and on prothionamide treatment were significantly associated with hypothyroidism in MDR-TB patients on treatment.
The prevalence of hypothyroidism in MDR-TB in the present study was 19.8% which is slightly higher than the previous study reported from Ethiopia by Meressa et al in 2009 (17.2%).16 However, the prevalence of hypothyroidism in the current study was comparable with a study reported by Ben-nan et al in 2020 (19.78%) from China.25 Another study reported from Lesotho by Satti et al in 2012 (69%)12 also showed higher prevalence of hypothyroidism in MDR-TB patients on treatment.
In the current study, 17.3% of MDR-TB patients had subclinical hypothyroidism and 4 (2.5%) had overt hypothyroidism. The study reported by Ige et al in 2016 (7.8%) from Nigeria26 indicated a lower proportion of subclinical hypothyroidism than our study finding. Moreover, the study reported by Bares et al in 201627 showed higher subclinical (40%) and overt (38%) hypothyroidism than this study. A study from India indicated a comparable proportion (2.4%) of overt hypothyroidism with our study.28 However, the same study reported from India showed a lower euthyroid proportion (58.21%)28 than the current study.
Presence of co-morbidity was significantly associated with hypothyroidism in MDR-TB patients. Although there is no evidence, this association between co-morbidities and hypothyroidism in MDR-TB patients could be due to the drug–drug reaction and the effect of other disease drugs. For instant, stavudine is the risk factor to hypothyroidism in HIV patients.29 This finding is similar with our finding in which MDR-TB patients co-infected with HIV developed hypothyroidism more than those who have no any co-morbidities. Moreover, studies reported that HIV and TB co-infections could cause alteration of thyroid function.30,31 Being underweight was also significantly associated with hypothyroidism in MDR-TB patients. Although we could not find the evidence that shows the effect of MDR-TB and its treatment on the thyroid profile, being underweight could lead to low iodine concentration in the serum.32,33 Thus, it could lead to hypothyroidism in MDR-TB patients, and require follow up during the treatment.
In the present study, being on prothionamide treatment was significantly associated with hypothyroidism in MDR-TB patients. This finding was similar with the study reported by Ben-nan et al in 2020 (71.4%) from China in which prothionamide and para-aminosalicylic acid were significantly associated with hypothyroidism.25 Moreover, the study reported by Cheung et al (2019) from Australia34 indicated the significant association between prothionamide and hypothyroidism in MDR-TB patients on treatment.
The main strength of this study is assessing the factors associated with hypothyroidism in patients on MDR-TB treatment. In addition, the result of this study is less likely influenced by selection bias, because all patients on treatment during the study period were enrolled into the study. Several risk factors were included in the multivariable model to control the confounding effect of each variable, which relatively minimizes the effect of confounders. This study was conducted in a single hospital, which could limit the generalizability of this finding. Important risk factors of hypothyroidism such as dietary habits and physical exercises were not studied. This could limit the conclusiveness of risk factors of hypothyroidism.
Conclusion
This study revealed that the prevalence of hypothyroidism is considerable in MDR-TB patients on treatment. Prothionamide, co-morbidity, and being underweight were significantly associated with hypothyroidism. Monitoring of the thyroid profile for better patient management and treatment outcome among MDR-TB patients at baseline and follow up is required. Nutritional supplementation for patients with low BMI is necessary to reduce the risk of hypothyroidism and to increase treatment success. In order to determine the possible role of hypothyroidism among MDR-TB patients with thyroid disease and its associated risk factors, a large-scale observational study is required.
Acknowledgments
The authors would like to acknowledge that the Addis Ababa University College of Health Science, the Directorate of St. Peter’s Specialized Hospital Research & Evidence Generation and the staff of St. Peter’s Specialized Hospital Laboratory for their support during the implementation of this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The sources of budgets were Addis Ababa University, Saint Peter’s hospital and personal.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research.
References
1. World Health Organization. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization; 2020.
2. Chaudhry LA, Zamzami M, Aldin S, Pazdirek J. Clinical consequences of non-compliance with directly observed therapy short course (DOTS): story of a recurrent defaulter. Int J Mycobacteriol. 2012;1(2):99–103. doi:10.1016/j.ijmyco.2012.05.003
3. Mishra G, Ghorpade SV, Xdr-tb: MJ. An outcome of programmatic management of TB in India. Indian J Med Ethics. 2014;11(1):47–52. doi:10.20529/IJME.2014.013
4. Dela AI, Tank ND, Singh AP, et al. Adverse drug reactions and treatment outcome analysis of DOTS-plus therapy of MDR-TB patients at district tuberculosis center: a four year retrospective study. Lung India. 2017;34(6):522–526. doi:10.4103/0970-2113.217569
5. Drucker D, Eggo MC, Salit IE, et al. Ethionamide-lnduced Goitrous Hypothyroidism. Ann Internal Med. 1984;100(6):837–839. doi:10.7326/0003-4819-100-6-837
6. Sultan KM. Assessment of body mass index and nutritional status in pulmonary tuberculosis patients. J Faculty Med. 2012;54(3):204–208.
7. Demers LM, Spencer C. The Thyroid: Pathophysiology and Thyroid Functiontesting. Tietz Textbook of Clinical and Molecular Diagnostics.
8. Dela AI, Tank ND, Singh AP, et al. Adverse drug reactions and treatment outcome analysis of DOTS-plus therapy of MDR-TB patients at district tuberculosis centre: a four year retrospective study. Lung India. 2017;34(6):522–526. doi:10.4103/0970-2113.217569
9. Chaudhry LA, Zamzami M, Aldin S, et al. Clinical consequences of non-compliance with directly observed therapy short course (DOTS): story of a recurrent defaulter. International Journal of Mycobacteriology. 2012;1(2):99–103.
10. Drucker D, Eggo MC, Salit IE. Ethionamide-lnduced Goitrous Hypothyroidism. Ann Intern Med. 1984;100(6):837–839.
11. Macgregor A, Somner A. The AntiThyroid Action of Para-Aminosalicylic Acid. Lancet. 1954;264(6845):931–936. doi:10.1016/S0140-6736(54)92552-0
12. Satti H, Mafukidze A, Jooste PL, et al. High rate of hypothyroidism among patients treated for multidrug-resistant tuberculosis in Lesotho. Int J Tuberculosis Lung Dis. 2012;16(4):468–472. doi:10.5588/ijtld.11.0615
13. Andries A, Isaakidis P, Das M, et al. High Rate of Hypothyroidism in Multi-drug-Resistant Tuberculosis Patients Co-Infected with HIV in Mumbai, India. PLoS One. 2013;10:e78313. doi:10.1371/journal.pone.0078313
14. Modongo C, Zetola NM. Prevalence of hypothyroidism among MDR-TB patients in Botswana. Int j Tuberculosis Lung Dis. 2012;16(11):1561. doi:10.5588/ijtld.12.0403
15. Tola HH, Holakouie-Naieni K, Lejisa T, et al. Is hypothyroidism rare in multidrug resistance tuberculosis patients on treatment? A systematic review and meta-analysis. PLoS One. 2019;14(6):218–487. doi:10.1371/journal.pone.0218487
16. Meressa D, Hurtado RM, Andrews JR, et al. Achieving high treatment success for multidrug-resistant TB in Africa: initiation and scale-up of MDR TB care in Ethiopia—an observational cohort study. Thorax. 2015;70(12):1181–1188. doi:10.1136/thoraxjnl-2015-207374
17. Wang X, Mo Z, Mao G, et al. Geographical influences on thyroid abnormalities in adult population from iodine-replete regions: a cross-sectional study. Sci Rep. 2021;11(1):1–7. doi:10.1038/s41598-020-79139-8
18. Hwang S, Lee EY, Lee WK, et al. Correlation between iodine intake and thyroid function in subjects with normal thyroid function. Biol Trace Elem Res. 2011;143:1393–1397. doi:10.1007/s12011-011-8997-x
19. Surks MI, Ortiz E, Daniels GH. SCH: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi:10.1001/jama.291.2.228
20. National committee for Clinical Laboratory Standards. Procedures for the Collection of Diagnostic Blood Specimens by Vein Puncture: Approved Standard.
21. Chaudhry LA, Zamzami M, Aldin S, Pazdirek J. e- Lab Doc - Roche manual. J Mycobacteriol. 2012;1(2):99–103.
22. Lee S, Farwell AP. Euthyroid sick syndrome. ComprPhysiol. 2016;6(2):1071–1080.
23. Yaqoob A. Subclinical hypothyroidism and its consequences. Public Health Biol Sci. 2012;1(2):53–60.
24. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88(6):2438. doi:10.1210/jc.2003-030398
25. Kumar R. Clinical analysis of hypothyroidism after anti-tuberculosis treatment in patients with multi drug resistance tuberculosis. Chin J Antituberc. 2020;42(5):465–471.
26. Ige OM, Akinlade KS, Rahamon SK, et al. Thyroid function in multidrug-resistant tuberculosis patients with or without human immunodeficiency virus (HIV) infection before commencement of MDR-TB drug regimen. Afr Health Sci. 2016;16(2):596–602. doi:10.4314/ahs.v16i2.30
27. Bares R, Khalid N, Daniel H, et al. Hypothyroidism during second-line treatment of multidrug-resistant tuberculosis: a prospective study. Int J Tuberculosis Lung Dis. 2016;20(7):876–881. doi:10.5588/ijtld.15.0692
28. Dash M, Behera P, Kumar R. Thyroid profile in pulmonary tuberculosis patients: a prospective study in a tertiary medical college of southern Odisha. Int J Clin Trials. 2020;7(2):66–71. doi:10.18203/2349-3259.ijct20200538
29. Grappin M, Piroth L, Verges B, et al. Increased Prevalence of subclinical hypothyroidism in HIV patients treated with highly active antiretroviral therapy. PubMed. 2000;14(8):1070–1072. doi:10.1097/00002030-200005260-00026
30. Sajid KM, Parveen R, Sabih DE, et al. Thyroid function in pulmonary tuberculosis. J Coll PhysiciansSurg Pak. 2006;16(10):633–636.
31. Hoffman CJ, Brown TT. Thyroid function abnormalities in HIV-Infected patients. Clin Infect Dis. 2007;45(4):488–494. doi:10.1086/51997
32. Chaker L, Korevaar TI, Medici M, et al. Thyroid function characteristics and determinants: the Rotterdam Study. Thyroid. 2016;26(9):1195–1204. doi:10.1089/thy.2016.0133
33. Ríos-Prego M, Anibarro L, Sánchez-Sobrino P. Relationship between thyroid dysfunction and body weight: a not so evident paradigm. Int J Gen Med. 2019;12:299. doi:10.2147/IJGM.S206983
34. Cheung YM, Van K, Lan L, et al. Hypothyroidism associated with therapy for multi-drug resistant tuberculosis in Australia. Int Med j. 2019;49(3):364–372. doi:10.1111/imj.14085
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
