Back to Journals » Clinical Ophthalmology » Volume 17
Through the Eyes of Patients: Understanding Treatment Burden of Intravitreal Anti-VEGF Injections for nAMD Patients in Norway
Authors Reitan G, Kjellevold Haugen IB, Andersen K, Bragadottir R, Bindesbøll C
Received 16 March 2023
Accepted for publication 12 May 2023
Published 25 May 2023 Volume 2023:17 Pages 1465—1474
DOI https://doi.org/10.2147/OPTH.S409103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Gudrun Reitan,1 Inga Britt Kjellevold Haugen,2 Kristoffer Andersen,3 Ragnheidur Bragadottir,4,5 Christian Bindesbøll3
1Department of Molecular Medicine, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway; 2Department of Research, The Norwegian Association of the Blind and Partially Sighted, Oslo, Norway; 3Roche Norway, F Hoffmann-La Roche AG, Oslo, Norway; 4Department of Ophthalmology, Oslo University Hospital, Oslo, Norway; 5Department of Ophthalmology, Institute for Clinical Medicine, University of Oslo, Oslo, Norway
Correspondence: Christian Bindesbøll, Email [email protected]
Purpose: Patients with neovascular age-related macular degeneration (nAMD) are treated with frequent intravitreal injections to maintain vision. The treatment frequency impacts the life of patients and caregivers and poses a major capacity challenge for Norwegian ophthalmic clinics. The purpose of this survey was to document patient-reported outcomes on how the disease and the treatment impact nAMD patients in Norway.
Methods: Norwegian nAMD patients voluntarily completed the survey. The patients reported the time spent on each treatment appointment, the need for caregiver support, treatment intervals, and the emotional impact of the treatment. There was no active selection of patients to the survey. Respondents had to confirm the nAMD diagnosis prior to submitting the response. All data was included in the analysis as submitted by the respondents. This survey was market research involving anonymous patient data, and no participants were identifiable.
Results: In total, 130 patients responded to the survey. The majority of patients reported to receive nine or more injections per year (48.8%), and many patients needed caregiver support for every treatment appointment (37.7%). Patients reported to be anxious one day (25.4%), two days (8.5%), one week (10.8%) or more than one week (3.1%) prior to treatment. The week before the treatment, 33.1% of patients reported to be stressed and 15.4% struggled to sleep. The majority of patients reported the treatment as uncomfortable (54.6%) or as somewhat painful (26.2%). The results on yearly number of injections, time used each treatment day and need for caregiver support suggested a variation between Norwegian hospital regions.
Conclusions: This survey uncovers how treatment with intravitreal injections represents a substantial burden for Norwegian patients with nAMD. Future research on how the treatment burden impacts nAMD patients may lead to more patient-centered care and help guide treatment decisions. New treatments with longer intervals between injections are likely to both reduce the treatment burden and improve capacity in ophthalmology clinics.
Keywords: retina, nAMD, burden, intravitreal, quality of life, questionnaire
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of central vision loss in people above 50 years.1 Without treatment, late-stage AMD causes central vision loss and legal blindness. This can have a substantial impact on patient’s quality of life and functional independence in daily activities, contributing to a significant emotional impact.2,3
AMD is classified as either geographic AMD or neovascular AMD (nAMD), also called wet AMD. nAMD is an advanced form of AMD, affecting 10% to 15% of AMD patients.4 It is characterized by choroidal neovascularization (CNV) which is subject to macular leakage and hemorrhage, resulting in accelerated vision loss in nAMD without treatment. Growth factors, such as Vascular Endothelial Growth Factor (VEGF) and Angiopoietin 2 (Ang-2), are key mediators in the development and progression of CNV and visual impairment in patients with nAMD.5 nAMD can be treated with intravitreal injections to block these growth factors. This type of treatment has greatly improved the prognosis and visual function of patients with nAMD.6 In Norway, intravitreal injections are performed in an ambulatory setting as previously described.7
nAMD is a chronic disease, and many patients are treated with frequent anti-VEGF injections, as often as every fourth week, for many years. Approximately one in every four patients with nAMD treated at the Oslo University Hospital received follow-up treatment eight years after treatment initiation.8 The need for repetitive anti-VEGF injections poses a burden for patients, caregivers and the healthcare system.9–12
The most common barriers for patients with nAMD receiving anti-VEGF injections include anxiety related to treatment and adverse events and long travel distance to the nearest ophthalmic clinic.13 The information on how treatment affects patients with nAMD in Norway is scarce. It is crucial to understand the impact that treatment has on patients and the healthcare system. This knowledge will enable optimal treatment selection and help to optimize resource use in challenged healthcare systems. This survey aimed to document the patient perspective of treatment burden of intravitreal anti-VEGF injections in nAMD in Norway. More knowledge on how treatment affects patients both practically and emotionally is important because it may enable treatment strategies to become more patient-centered and improve patient health outcomes.
Methods
The survey was designed in collaboration with the Norwegian Association of the Blind and Partially Sighted (NABPS), which is the patient organization for people living with visual impairment in Norway. NABPS supported the awareness of the survey by presenting information about the survey and its purpose in their digital channels. There was no active selection of patients to the survey. Respondents had to confirm the nAMD diagnosis prior to submitting the response. The participation was voluntary and unpaid and did not involve any direct contact between the parties involved. The questionnaire was anticipated to be completed in approximately 15 minutes via an online platform for survey data collection. It included questions related to diagnosis, demographics, average time spent each treatment day, need for caregiver support, treatment interval and the emotional impact of the treatment. Questions about patient costs were not included in this survey questionnaire. Health care in Norway is publicly funded, with the government covering the majority of healthcare expenses. The funding is primarily derived from taxes and is distributed to the different regions of the country through a government-operated health enterprise. This means that, in Norway, people have access to high-quality medical care regardless of their income or ability to pay. In addition, patients and caregivers can apply to have treatment-related costs reimbursed.
There were no open-ended questions. The questions had pre-specified alternatives, and some of them allowed alternatives to be selected. At the time the survey was conducted, anti-VEGF therapies were the only intravitreal injection therapy available for patients with nAMD, hereafter referred to as anti-VEGF injection therapy.
There was no monitoring of the data. All data was included in the analysis as submitted by the respondents. Data was recorded if respondents completed the full questionnaire and submitted their response. The data collected were anonymous. Therefore, no participants were identifiable, taking into account all the means that may reasonably be envisaged used to identify the person concerned.
This survey was market research involving anonymous patient data and is not in scope for submission and approval from the regional ethics committee (REC) in Norway; use of anonymous information and assessments about health conditions is exempted from submission to REC and preapproval.
According to the Health Research Act §20, written consent from the participant was not required for use of anonymous data.14 However, the participants were informed about the purpose of the survey before voluntarily deciding to participate. Anonymous data are not in the scope of the General Data Protection Regulation (GDPR), according to article 4.
When assessing regional differences, counties were merged into health regions. Norway is divided into four health regions based on the regional health enterprise’s responsibility to provide health services to the population in a limited geographical area. Each regional health enterprise has the responsibility for its own health region. The health regions contain the following counties: Southern and Eastern Norway Regional Health Authority (RHA) (n = 69; Innlandet, Oslo, Vestfold og Telemark, Viken, Agder), Northern Norway RHA (n = 9; Nordland, Troms, Finnmark), Western Norway RHA (n = 22; Rogaland, Vestlandet), and Central Norway RHA (n = 27; Møre og Romsdal, Trøndelag).
Results
Patient Demographics
The questionnaire used in this survey is available in Supplementary Table 1. In total, 130 patients participated in the survey, of which 129 confirmed the diagnosis of nAMD (Table 1). The majority of respondents were women (90%). All of the patients that participated in the survey were older than 50 years, and the majority of patients were between 70 and 79 years (56.9%). The counties were evenly represented, with the exception of Agder and Nordland counties, which were underrepresented when adjusting for the number of inhabitants. The majority of patients reported to be diagnosed 1–3 years (47.7%) or 4–6 years (26.9%) prior to answering the survey. Most of patients were treated with intravitreal injection therapy (97.7%).
 |
Table 1 Baseline Characteristics of Survey Participants |
Quality of Life Related to Anti-VEGF Injection Therapy
The number of injections reported by participants was 1–4 times per year (18.1%), 5–8 times per year (33.1%), 9–12 times per year (33.1%), and more than 12 times per year (15.7%) (Figure 1). To get an estimate of the time used per treatment, patients provided the average time spent for preparations, transport, waiting time, treatment and recovery following treatment. The majority of patients reported to spend 1–3 hours (43.1%), followed by 4–6 hours (30.8%), 7–9 hours (13.1%), 12–15 hours (9.2%) and more than 15 hours (3.8%). Only 12.3% needed to take time off work on treatment days, while the remaining responders did not need to take time off or were retired. Approximately half of the patients required caregiver support every visit (37.7%) or occasionally (14.6%). The caregiver was reported as their partner (54.6%), other family member (11.5%), a friend (7.7%) or other (26.2%). Only 6.9% of caregivers had to take time off work on the treatment day. Some patients felt uncomfortable asking caregivers for assistance every time (15.4%), only initially (12.3%), whereas the majority did not (72.3%).
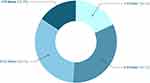 |
Figure 1 Yearly number of intravitreal injections in patients with nAMD. |
The majority of patients reported the treatment as uncomfortable (54.6%) or as somewhat painful (26.2%). Few patients reported the treatment as very painful (3.1%). Prior to treatment visits, patients felt anxious every time (38.5%), occasionally (17.5%), seldom (31.5%) or never (12.3%) (Figure 2A). The everyday life of patients was not affected (30.8%), somewhat affected (43.1%), moderately affected (23.8%) or very affected (2.3%) by the treatment. Patients were not nervous prior to treatment (42.3%), or nervous one day (35.4%), two days (8.5%), one week (10.8%) or more than one week (3.1%) prior to treatment. Patients could select one or more options about how they felt prior to their latest anti-VEGF injection and reported that they were unable to relax (20.8%), could not think about anything but the injection (7.7%), felt stressed (33.1%), struggled to sleep (15.4%), experienced reduced concentration (6.9%), headache (1.5%) or other/none of these alternatives (52.3%) (Figure 2B). Among patients that responded to one or several alternatives, 30.8% chose one, 6.2% two, 9.2% three, 0.8% four and 0.8% five alternatives.
Regional Differences in the Treatment with Anti-VEGF Injections
Regional differences in treatment interval, time used each treatment day, and the need for caregiver support were analyzed (Figure 3A–D). Some counties had few respondents. Accordingly, when assessing regional differences counties were merged into health regions as explained in methods. The four health regions are depicted in Figure 3A. The Northern Norway RHA had the highest number of patients reporting injections one to four times per year, whereas the Southern and Eastern Norway RHA had the highest number of patients reporting frequent injections (18.8%) (Figure 3B).
In the Southern and Eastern Norway RHA, comprising the majority of patients, 36.6% spent one to three hours, 36.6% four to six hours, 15.5% seven to nine hours and 11.3% spent more than nine hours each treatment day (Figure 3C). No patients reported to spend more than nine hours on a treatment day in the Western Norway RHA. The two regions with the highest number of patients that spent more than nine hours on a treatment day were the Northern Norway RHA (33.3%) and the Central Norway RHA (22.2%).
The need for caregiver support was highest in the Western Norway RHA where ~74% reported that they needed caregiver support every time or occasionally and lowest in the Northern Norway RHA, where 67% reported that they did not need caregiver support on the treatment day (Figure 3D).
Discussion
Based on the patient-reported data, results from this survey reveal how the treatment with anti-VEGF injections represents a substantial burden for patients with nAMD in Norway. The results support findings from other countries, demonstrating the high emotional and practical burden that retinal diseases place on patients.15–20
Patients with retinal diseases often need frequent anti-VEGF injections to stop progression of visual impairment. Healthcare providers in Norway have previously reported injection frequency as often as every four to eight weeks with present anti-VEGF therapies.21 In this survey, approximately half of the patients reported receiving anti-VEGF injections nine or more times per year, which indicates treatment every six weeks or more frequently. These frequent injections and visits pose a high burden on patients, caregivers and healthcare providers.21,22 The high treatment burden and undertreatment in clinical practice contribute to a decline in treatment frequency over time.9–12,23,24 However, the main reason is likely that nAMD patients treated with anti-VEGF experience deterioration of vision over time with progression of geographic atrophy.25
Patients have reported common barriers to anti-VEGF injection therapy, including fear of intravitreal injection, anxiety for negative examination results, difficulties in travelling to and from the hospital, fear of getting worse if the treatment did not work and possible injection-related side effects.13,26,27 Little is known about the emotional and practical impacts that anti-VEGF injection therapy place on nAMD patients in Norway. Accordingly, this survey included questions about emotions related to treatment, time spent each treatment day, and the need for caregiver support. Patients reported that they were nervous or struggled to sleep prior to treatment. This is in line with results from other countries.20 The visual impairment caused by nAMD is associated with poor quality of life and a higher risk of depression.3,28 This may be related to the treatment burden, but also the effects of vision loss on daily activities such as reading, driving, and recognizing faces.2,3
Another barrier for treatment is the time used per treatment day. A study that included patients from Germany, UK and Italy revealed that 53% needed to take at least one day off work and that they spent on average four and a half hours on the treatment day.20 The majority of the patients in this survey report that they spend more than four hours on the treatment day. Our data show that the highest proportion of patients who report to use more than nine hours per treatment day resides in health regions with the longest median distance to the hospital as measured by Statistics Norway. The Northern Norway RHA had most patients with both long travel distance and long treatment intervals. This may be caused by regional differences in treatment, but also that patients with long travel distance and with a need for frequent injections have lower compliance and therefore miss their treatment.
Some studies have reported that about 70% of patients with nAMD need caregiver support related to treatment visits.11,20 In Norway, patients and caregivers get reimbursed for treatment-related expenses through a national patient service, including travel costs and loss of income due to treatment-related absence from work. In this survey, approximately half of the patients reported a need for caregiver support every time or occasionally. This indicates that the value of a caregiver includes other aspects than practical support for patients with nAMD, for example the need for emotional support. Moreover, the need for caregiver support may be higher than reported, as the patients in this survey were younger than in clinical practice.29,30 This might suggest that the results present a more optimistic view in terms of caregiver support and the emotional impact of the treatment.
Treatment with anti-VEGF injections represents a great burden for many patients with nAMD but is also one of the main drivers behind growing capacity challenges for ophthalmic clinics in Norway.21,22,31 Capacity challenges in the treatment of retinal diseases are not only a problem in Norway but are a growing challenge globally, pushed forward by an increasingly elderly population, resource-intensive treatment alternatives, in combination with inadequate amount of new ophthalmologists.32 Real-world data from Norway and other representative Nordic countries suggest that patients in Norway are treated with frequent anti-VEGF injections.21,29 According to results from the largest retinal clinic in Norway, the mean number of injections in 2018 for patients receiving aflibercept and bevacizumab was 8.8 and 6.7, respectively.21 These numbers correspond well with the numbers reported in this survey and align more closely with randomized controlled trials compared to real-world data from some other countries.33,34 The frequent injections are indicative of Norwegian patients receiving an appropriate treatment regimen, which may result in better visual outcomes than patients in countries where treatment frequency is lower. However, ophthalmic clinics are experiencing a high demand for treatments, challenging both resources and costs, while patients are exposed to a substantial treatment burden. With the current capacity challenges and a growing number of patients, future patients may receive suboptimal treatment and subsequent loss of visual acuity.22
The capacity challenges combined with the treatment burden revealed in this survey are underlining the demand for new treatments that are less invasive or have longer durability. Such treatment options will reduce both the treatment burden for patients and improve capacity in ophthalmic clinics. There are explorative and more durable newly available treatments that will hopefully assist in alleviating these challenges.35 By documenting the impact of anti-VEGF injections on nAMD patients in Norway, like this survey seeks to do, the value of new treatments can be considered in a greater perspective. An example is a recent economic evaluation from the US considering three anti-VEGF medicines. In this report, valuation and inclusion of some of the central elements from this survey, for example the time used by patients and caregivers and travel costs, clearly demonstrated how the differences in costs between the treatments increased substantially as compared to only considering the medicine costs.36 In this survey, approximately half of responders needed caregiver assistance on treatments visits, many reported substantial time and a considerable emotional burden. If these factors were included in a Norwegian economic evaluation, it is plausible that differences in treatment costs in favor of treatments that require fewer injections would be identified.
There are limitations to this survey. The patient number is small compared to the prevalence of nAMD in Norway.37 However, the number of respondents was similar to a European survey on patients with retinal diseases.20 The survey is based on patient-reported data, considered truthful. Respondents had to confirm the nAMD diagnosis before submitting the questionnaire; however, it cannot be excluded that patients without nAMD participated in the survey. Access to the internet and understanding of the digital survey may vary between age groups. The patients included in this survey were also younger than the mean age of patients in clinical practice.29,30 The majority of responders were women. The gender representation is considerably skewed, even when considering a higher nAMD diagnosis rate among women versus men.29 The yearly report (2021) from the Swedish Macula Registry documents that 64% of nAMD patients were women and 36% men,29 suggesting that more women than men are diagnosed with the disease, and these numbers can be representative for the Norwegian patient population. In general, women are more likely to respond to surveys,38–40 very old people are associated to participate less often in digital surveys than younger age-groups,41,42 and higher education is positively correlated with survey participation.43–46 As such, the population included in this survey is likely to represent a subgroup of the nAMD patient population, and it is plausible that the responses are more positive than what will be the case in the patient population as a whole.
The impact of life quality of nAMD patients treated with intravitreal injections may vary between different countries due to factors such as treatment offered, accessibility of treatment, support systems, treatment capacity, ophthalmologist density, travel distances to retinal services, and geographical differences. Specific support strategies might vary accordingly. To our knowledge, this is the first publication reporting the impact that intravitreal injection therapy has on nAMD patients in Norway. Regional differences in the need for caregiver support and the time used per treatment day suggest that region-specific support strategies are needed to alleviate the burden for nAMD patients and caregivers in different parts of the country. Knowledge about the treatment burden and contributing factors may enable optimal treatment selection, support strategies, and help to optimize resource use in challenged healthcare systems.
Conclusion
This survey documents how treatment with anti-VEGF injections poses a substantial treatment burden for nAMD patients in Norway. Reducing the treatment burden and improving the support for patients would benefit those receiving intravitreal injection therapies for nAMD. The results from this survey provide additional considerations for ophthalmologists in Norway when deciding on the optimal treatment regime for their patients. Treatments associated with fewer injections represent a possibility to reduce the treatment burden for patients and their caregivers. In addition, treatments with longer durability may also reduce the economic burden and improve the capacity in ophthalmic clinics.
Acknowledgments
The authors acknowledge the Norwegian Association of the Blind and Partially Sighted for presenting information about the survey and its purpose in their digital channels, thereby allowing for increased awareness of how patients with nAMD perceive living with the disease.
Disclosure
Ragnheidur Bragadottir has been a member of a Roche advisory board in Norway in 2021 and 2022. Christian Bindesbøll and Kristoffer Andersen are employees of F Hoffmann-La Roche AG. The authors report no other conflicts of interest in this work.
References
1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e34. doi:10.1016/S2214-109X(17)30393-5
2. Gopinath B, Liew G, Burlutsky G, Mitchell P. Age-related macular degeneration and 5-year incidence of impaired activities of daily living. Maturitas. 2014;77(3):263–266. doi:10.1016/j.maturitas.2013.12.001
3. Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4:97. doi:10.1186/1477-7525-4-97
4. Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606–2617. doi:10.1056/NEJMra0801537
5. Joussen AM, Ricci F, Paris LP, Korn C, Quezada-Ruiz C, Zarbin M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: a review of preclinical data. Eye. 2021;35(5):1305–1316. doi:10.1038/s41433-020-01377-x
6. Hoeg TB, Ellervik C, Buch H, et al. Danish rural eye study: epidemiology of adult visual impairment. Ophthalmic Epidemiol. 2016;23(1):53–62. doi:10.3109/09286586.2015.1066396
7. Blom K, Bragadottir R, Sivertsen MS, Moe MC, Jorstad OK. Does pharmaceutical compounding of vascular endothelial growth factor inhibitors for intravitreal use alter the risk of post-injection endophthalmitis? Ocul Immunol Inflamm. 2022;30(3):713–716. doi:10.1080/09273948.2020.1820530
8. Berg K, Roald AB, Navaratnam J, Bragadottir R. An 8-year follow-up of anti-vascular endothelial growth factor treatment with a treat-and-extend modality for neovascular age-related macular degeneration. Acta Ophthalmol. 2017;95(8):796–802. doi:10.1111/aos.13522
9. Gohil R, Crosby-Nwaobi R, Forbes A, Burton B, Hykin P, Sivaprasad S. Caregiver burden in patients receiving ranibizumab therapy for neovascular age related macular degeneration. PLoS One. 2015;10(6):e0129361. doi:10.1371/journal.pone.0129361
10. Daien V, Finger RP, Talks JS, et al. Evolution of treatment paradigms in neovascular age-related macular degeneration: a review of real-world evidence. Br J Ophthalmol. 2021;105(11):1475–1479. doi:10.1136/bjophthalmol-2020-317434
11. Prenner JL, Halperin LS, Rycroft C, Hogue S, Williams Liu Z, Seibert R. Disease burden in the treatment of age-related macular degeneration: findings from a time-and-motion study. Am J Ophthalmol. 2015;160(4):725–31 e1. doi:10.1016/j.ajo.2015.06.023
12. Okada M, Mitchell P, Finger RP, et al. Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration: a mixed-methods systematic review. Ophthalmology. 2021;128(2):234–247. doi:10.1016/j.ophtha.2020.07.060
13. Polat O, Inan S, Ozcan S, et al. Factors affecting compliance to intravitreal anti-vascular endothelial growth factor therapy in patients with age-related macular degeneration. Turk J Ophthalmol. 2017;47(4):205–210. doi:10.4274/tjo.28003
14. The Health Research Act. Anonymous human biological material and health data; 2008:20.
15. Awdeh RM, Elsing SH, Deramo VA, Stinnett S, Lee PP, Fekrat S. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item national eye institute visual function questionnaire. Br J Ophthalmol. 2010;94(3):319–323. doi:10.1136/bjo.2007.135913
16. Deramo VA, Cox TA, Syed AB, Lee PP, Fekrat S. Vision-related quality of life in people with central retinal vein occlusion using the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2003;121(9):1297–1302. doi:10.1001/archopht.121.9.1297
17. Fenwick EK, Xie J, Pesudovs K, et al. Assessing disutility associated with diabetic retinopathy, diabetic macular oedema and associated visual impairment using the vision and quality of life index. Clin Exp Optom. 2012;95(3):362–370. doi:10.1111/j.1444-0938.2012.00742.x
18. Hariprasad SM, Mieler WF, Grassi M, Green JL, Jager RD, Miller L. Vision-related quality of life in patients with diabetic macular oedema. Br J Ophthalmol. 2008;92(1):89–92. doi:10.1136/bjo.2007.122416
19. Klein R, Moss SE, Klein BE, Gutierrez P, Mangione CM. The NEI-VFQ-25 in people with long-term type 1 diabetes mellitus: the Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2001;119(5):733–740. doi:10.1001/archopht.119.5.733
20. Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–946. doi:10.2147/OPTH.S100168
21. Jorstad OK, Steffensen LA, Eriksen K, Bragadottir R, Moe MC. Thirteen years of intravitreal anti-vascular endothelial growth factor therapy: the promises and burdens of a paradigm shift told from the perspective of the largest retina service in Norway. Acta Ophthalmol. 2020;98(8):774–779. doi:10.1111/aos.14177
22. Kristiansen IS, Haugli Braten R, Jorstad OK, Moe MC, Saether EM. Intravitreal therapy for retinal diseases in Norway 2011–2015. Acta Ophthalmol. 2020;98(3):279–285. doi:10.1111/aos.14262
23. Ciulla TA, Hussain RM, Pollack JS, Williams DF. Visual acuity outcomes and anti-vascular endothelial growth factor therapy intensity in neovascular age-related macular degeneration patients: a real-world analysis of 49 485 eyes. Ophthalmol Retina. 2020;4(1):19–30. doi:10.1016/j.oret.2019.05.017
24. Khanani AM, Skelly A, Bezlyak V, Griner R, Torres LR, Sagkriotis A. SIERRA-AMD: a retrospective, real-world evidence study of patients with neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2020;4(2):122–133. doi:10.1016/j.oret.2019.09.009
25. Tan CS, Ngo WK, Chay IW, Ting DS, Sadda SR. Neovascular age-related macular degeneration (nAMD): a review of emerging treatment options. Clin Ophthalmol. 2022;16:917–933. doi:10.2147/OPTH.S231913
26. Boyle J, Vukicevic M, Koklanis K, Itsiopoulos C, Rees G. Experiences of patients undergoing repeated intravitreal anti-vascular endothelial growth factor injections for neovascular age-related macular degeneration. Psychol Health Med. 2018;23(2):127–140. doi:10.1080/13548506.2016.1274040
27. Droege KM, Muether PS, Hermann MM, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1281–1284. doi:10.1007/s00417-012-2177-3
28. Casten RJ, Rovner BW. Update on depression and age-related macular degeneration. Curr Opin Ophthalmol. 2013;24(3):239–243. doi:10.1097/ICU.0b013e32835f8e55
29. Inger Westborg SA, Jonsson T, Karlsson N, Libert C, Lövestam-Adrian M, Rung L. Årsrapport 2021 - Svenska Makularegistret [Annual report 2021. The Swedish Macula Register]; 2021 Swedish.
30. Ziemssen F, Feltgen N, Holz FG, et al. Demographics of patients receiving Intravitreal anti-VEGF treatment in real-world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmol. 2017;17(1):7. doi:10.1186/s12886-017-0401-y
31. Bolme S, Austeng D, Morken TS, Follestad T, Halsteinli V. Cost consequences of task-shifting intravitreal injections from physicians to nurses in a tertiary hospital in Norway. BMC Health Serv Res. 2023;23(1):229. doi:10.1186/s12913-023-09186-0
32. Group TE. Vision for change: meeting the growing demand for eye care. Economist Impact; 2022.
33. Mehta H, Tufail A, Daien V, et al. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog Retin Eye Res. 2018;65:127–146. doi:10.1016/j.preteyeres.2017.12.002
34. Ciulla TA, Huang F, Westby K, Williams DF, Zaveri S, Patel SC. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2018;2(7):645–653. doi:10.1016/j.oret.2018.01.006
35. Patel P, Sheth V. New and innovative treatments for neovascular age-related macular degeneration (nAMD). J Clin Med. 2021;10:11. doi:10.3390/jcm10112436
36. Meer EA, Oh DH, Brodie FL. Time and distance cost of longer acting anti-VEGF therapies for macular degeneration: contributions to drug cost comparisons. Clin Ophthalmol. 2022;16:4273–4279. doi:10.2147/OPTH.S384995
37. Erke MG, Bertelsen G, Peto T, Sjolie AK, Lindekleiv H, Njolstad I. Prevalence of age-related macular degeneration in elderly Caucasians: the Tromso Eye Study. Ophthalmology. 2012;119(9):1737–1743. doi:10.1016/j.ophtha.2012.03.016
38. Brussaard JH, Brants HA, Bouman M, Lowik MR. The study population: general characteristics and potential confounding factors. Eur J Clin Nutr. 1997;51(Suppl 3):S19–24.
39. Korkeila K, Suominen S, Ahvenainen J, et al. Non-response and related factors in a nation-wide health survey. Eur J Epidemiol. 2001;17(11):991–999. doi:10.1023/A:1020016922473
40. Sogaard AJ, Selmer R, Bjertness E, Thelle D. The Oslo health study: the impact of self-selection in a large, population-based survey. Int J Equity Health. 2004;3(1):3. doi:10.1186/1475-9276-3-3
41. Kelfve S, Kivi M, Johansson B, Lindwall M. Going web or staying paper? The use of web-surveys among older people. BMC Med Res Methodol. 2020;20(1):252. doi:10.1186/s12874-020-01138-0
42. Poli A, Kelfve S, Klompstra L, Stromberg A, Jaarsma T, Motel-Klingebiel A. Prediction of (Non)participation of older people in digital health research: exergame intervention study. J Med Internet Res. 2020;22(6):e17884. doi:10.2196/17884
43. Barton J, Bain C, Hennekens CH, et al. Characteristics of respondents and non-respondents to a mailed questionnaire. Am J Public Health. 1980;70(8):823–825. doi:10.2105/AJPH.70.8.823
44. Bostrom G, Hallqvist J, Haglund BJ, Romelsjo A, Svanstrom L, Diderichsen F. Socioeconomic differences in smoking in an urban Swedish population. The bias introduced by non-participation in a mailed questionnaire. Scand J Soc Med. 1993;21(2):77–82. doi:10.1177/140349489302100204
45. Sonne-Holm S, Sorensen TI, Jensen G, Schnohr P. Influence of fatness, intelligence, education and sociodemographic factors on response rate in a health survey. J Epidemiol Community Health. 1989;43(4):369–374. doi:10.1136/jech.43.4.369
46. van den Akker M, Buntinx F, Metsemakers JF, Knottnerus JA. Morbidity in responders and non-responders in a register-based population survey. Fam Pract. 1998;15(3):261–263. doi:10.1093/fampra/15.3.261
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.