Back to Journals » Vascular Health and Risk Management » Volume 14
Thromboangiitis obliterans episode: autoimmune flare-up or reinfection?
Authors Mohareri M, Mirhosseini A, Mehraban S , Fazeli B
Received 23 April 2018
Accepted for publication 12 June 2018
Published 28 September 2018 Volume 2018:14 Pages 247—251
DOI https://doi.org/10.2147/VHRM.S172047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Video abstract presented by Mehran Mohareri & Ali Mirhosseini.
Views: 1199
Mehran Mohareri,1 Ali Mirhosseini,1 Saeedeh Mehraban,1 Bahare Fazeli1,2
1Immunology Research Center, Inflammation and Inflammatory Diseases Division, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran; 2Vascular Independent Research and Education, European Foundation, Milan, Italy
Background: The possible role of infectious pathogens in the development of thromboangiitis obliterans (TAO) was considered soon after the disease was first described. However, it is not yet known whether infectious pathogens induce thrombotic vasculitis or if they cause a type of autoimmune disease. To investigate whether TAO relapses are more likely due to reinfection or autoimmune flare, the serum levels of toll-like receptor (sTLR) 4, sTLR2, C-reactive protein (CRP), and neopterin were evaluated in TAO patients during both the acute and quiescent phases of the disease as well as in a gender-, age-, and smoking habit-matched control group.
Methods: Following a cross-sectional study design, 28 patients in the acute phase of TAO and 23 patients in the quiescent phase participated in this study. In addition, 31 matched controls were enrolled.
Results: Toll-like receptor (TLR) 4 was significantly higher in patients in the acute phase of the disease than in patients in the quiescent phase (P=0.012). Also, TLR4 was significantly higher in the patients with CRP >7 µm/mL than in the patients with lower CRP (P=0.031). Notably, TLR4 in the patients in the quiescent phase of TAO was significantly lower than in the controls (P=0.006). No significant difference in the level of TLR2 was found among the groups (P>0.05). Neopterin was significantly higher in the acute phase of TAO in comparison to the quiescent phase (P=0.003) and the controls (P=0.005).
Conclusion: These findings indicate that the trigger of TAO might be Gram-negative bacteria, which can be hidden or immunologically suppressed in the quiescent phase of TAO, leading to a lower level of TLR4 accompanying the normal level of neopterin. However, relapses might develop according to toxic or hypoxic cell injuries. Hence, TLR4 shedding will increase, and therefore, sTLR4 could become closer to the level demonstrated in the controls.
Keywords: thromboangiitis obliterans, Buerger’s disease, TLR2, TLR4, neopterin, CRP, peripheral arterial diseases, vasculitis, innate immunity, infection
Introduction
Thromboangiitis obliterans (TAO), or Buerger’s disease, is an episodic, non-atherosclerotic segmental inflammatory and occlusive vascular disease that affects small- and medium-sized vessels of the extremities.1 The intervals between flare-up episodes of TAO ranged from 6 months to 12 years.2 Such episodes typically occur following changes in smoking habit, lifestyle, or mental stress.3,4 Currently, TAO is most prevalent in the Middle East, Southeast Asia, the Far East, and Eastern Europe.1 The etiology of TAO remains unknown.1 Although there is a close relationship between TAO and smoking, smoking explains neither the low prevalence of TAO among smokers nor its geographical distribution.4 The possible role of infectious pathogens in the development of TAO has been considered since the disease was first described.4 However, it is not yet known whether infectious pathogens induce thrombotic vasculitis or if they cause a type of autoimmune or autoinflammatory disease.5 The two main challenging pathogens, according to recent studies, are Porphyromonas gingivalis, which is a commensal oral bacteria6,7 (in which case autoinflammatory diseases would be more likely), and Rickettsials,8–11 which might either induce the disease itself or induce autoimmune immunity because of its close structural similarity with human mitochondria.12
However, without considering which of these pathogens might be responsible for the development of TAO, the clues that point to the role of infectious pathogens in TAO suggest Gram-negative pathogens.5,6,8,9 Owing to the fact that innate immunity only recognizes the pathogen’s category and not its precise species,13 studying the innate immunity of TAO patients might clarify whether TAO is an autoinflammatory disease, an autoimmune disease, or an infectious disease.
Toll-like receptor (TLR) 4 is a transmembrane protein that mainly mediates the innate immune response against the lipopolysaccharide (LPS) of Gram-negative bacteria. TLR2 is usually responsible for Gram-positive bacterial recognition, although Gram-negative bacteria can also activate the TLR2 signaling pathway.14,15 While TLR4 and TLR2 are transmembrane proteins, they are also normally detectable in serum due to the shedding of the cell surface receptor and the normal regulatory function of the cells.16 It has been demonstrated that the LPS of Gram-negative bacteria will downregulate the gene expression of TLR4 and, consequently, deplete TLR4.17 However, hypoxic or toxic cell injury will increase TLR4 shedding.18
The aim of this study was to evaluate the serum levels of TLR4 (sTLR4) and TLR2 (sTLR2) in TAO patients in comparison with controls. Also, to investigate whether TAO relapses are more likely due to reinfection or autoimmune reaction, sTLR4, sTLR2, high-sensitivity C-reactive protein (hsCRP), and neopterin, which assists adaptive immunity in eliminating intracellular pathogens,19 were compared between patients in the acute and quiescent phases of TAO.
Materials and methods
From February 2016 through August 2017, patients who had been clinically diagnosed with TAO, according to Shionoya’s criteria (including disease onset under the age of 50, history of tobacco use, infrapopliteal arterial occlusion, either upper limb involvement or phlebitis migrans, and absence of atherosclerotic risk factors other than smoking)20 with imaging confirmation were enrolled in the study. All patients and controls signed a written informed consent form. The study was approved by the Ethics Committee for Clinical Research of the Mashhad University of Medical Sciences (no 941335).
Patients who experienced pain at rest, painful ischemic ulcers, gangrene, or progressive claudication were included in a study group designated as being in the acute phase of TAO. The quiescent phase group comprised patients with a prior history of hospital admissions who were called and invited to participate in the study after undergoing a physical exam. Both groups of patients were matched by age and smoking habit, according to the influence of age and smoking on the serum levels of neopterin, TLR2, and TLR4. Additionally, controls were included who were matched according to age, gender, and smoking habit.
sTLR2 and sTLR4 were evaluated by the ELISA method (ZellBio GmbH, Ulm, Germany). The serum level of neopterin was evaluated using the GenWay ELISA kit (GenWay Biotech Inc, San Diego, CA, USA) with the normal range being 0–10 nmol/L. Also, hsCRP was determined using the Monobind Kit (Monobind Inc, Lake Forest, CA, USA) according to its instructions, with the normal range being 0–3 µg/mL. SPSS version 11.5 (SPSS Inc, Chicago, IL, USA) was used for analysis of the patient data. The descriptive data are presented as mean ± standard error. According to the distribution of the data, ANOVA, t-tests, and Mann–Whitney and Kruskal–Wallis tests were used.
Results
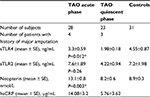
A total of 94 serum samples from 51 patients who had been clinically diagnosed with TAO and 31 healthy, matched controls were selected for the current study. Of the 51 TAO patients, 28 were in the acute phase of the disease, while 23 patients were in the quiescent phase. Seven of the TAO patients had a history of more than one hospital admission as a result of disease flare-ups. In addition, seven patients underwent below-knee amputation either before or during the study. Of the seven TAO patients who underwent below-knee amputation, three were in the quiescent phase with amputation background and four were in the acute phase of the disease. All patients and controls were Caucasian men and current smokers at the time of blood sampling. The chief symptoms among the TAO patients in the acute phase were burning pain at rest (32.1%), toe or limb gangrene with rest pain (35.7%), chronic ulcer with pain (28.6%), and progressive claudication (3.6%).
The mean ages of the patients in the acute phase group, the quiescent phase group, and the control group were 40.6±1.1, 43±1.8, and 40.3±0.5 years, respectively. No significant difference was observed relative to age among the studied groups (P=0.34). The mean number of cigarettes smoked per day in the acute phase group was 17.7±1.2 vs 18.9±3.3 in the quiescent phase group and 18.8±0.79 in the control group. No significant difference in terms of cigarette consumption was found among the studied groups (P=0.85).
The mean level of sTLR4 in the acute phase patients was 3.3±0.59 ng/mL, while in the quiescent phase group it was 1.98±0.18 ng/mL, and in the control group it was 4.55±0.87 ng/mL. Interestingly, sTLR4 in the acute phase group was significantly higher than in the patients in the quiescent phase of the disease (P=0.012). However, notably, the level of sTLR4 in the quiescent phase group was significantly lower than in the controls (P=0.006). Nonetheless, no correlation was demonstrated between the sTLR4 level in the acute phase group and the controls (P=0.96). The mean levels of sTLR2 in the acute phase group, the quiescent phase group, and the healthy controls were 7.6±1.89, 4.22±0.94, and 7.2±1.98 ng/mL, respectively. However, no significant difference between the levels of sTLR2 was found (P>0.05).
The mean serum levels of neopterin in patients in the acute phase, the quiescent phase, and the matched controls were 13.1±0.8, 8.2±0.6, and 8.9±0.3 nmol/L, respectively. The neopterin level was significantly higher in the patients in the acute phase of the disease in comparison with the patients in the quiescent phase (P=0.003) and the controls (P=0.005).
Moreover, 73.1% of patients were positive for C-reactive protein (CRP) and 26.9% were negative. The mean serum level of hsCRP was 16.6±2.5 µg/mL in positive subjects and 1.77±0.29 µg/mL in hsCRP-negative patients. The mean level of hsCRP in the quiescent and active phase of the disease was 5.76±3.62 and 14.08±3.2 µg/mL, respectively. However, no significant difference was found between the hsCRP levels in the active and quiescent phases of TAO (P=0.29).
Table 1 shows an overview of the summarized data on sTLR4, sTLR2, hsCRP, and neopterin for TAO patients in the acute and quiescent phase of the disease and the control group.
The mean sTLR4 in hsCRP-positive subjects was 2.05±0.3 ng/mL, while in the hsCRP-negative patients it was 2.97±1.1 ng/mL, without any statistically significant difference between them (P=0.95). Additionally, the mean level of TLR2 in hsCRP-positive subjects was 3.8±0.68 ng/mL, and in hsCRP-negative patients it was 8±4.7 ng/mL (P=0.57).
According to several studies,21–23 the hsCRP cutoff value for differentiating infections from autoimmune disease flare-up has been considered >5–7 µg/mL. Hence, the mean sTLR4 and sTLR2 were evaluated and compared in patients with positive and negative hsCRP according to the revised cutoff point of 7 µm/mL. The mean sTLR4 in the revised positive hsCRP patients was 2.79±0.6 ng/mL, and in the hsCRP-negative patients it was 1.68±0.18 ng/mL (P=0.031). The mean level of TLR2 in positive adjusted hsCRP subjects was 6.6±2.6 ng/mL, while in the hsCRP-negative patients it was 2.8±0.3 ng/mL (P=0.08) (Table 2).
Discussion
The role of infectious pathogens in the development of TAO was first hypothesized by Leo Buerger in 1908, who reported that the thrombosis of the patients he studied looked like syphilis thrombosis, although he did not detect treponema pallidum in their tissue specimens.24 While the relationship between smoking and TAO was noted and emphasized by other angiologists, in 1924, Buerger concluded that smoking might be a predisposing factor for – but not the main etiology of – TAO development.25 However, studies that investigated pathogens inside the vascular tissue of TAO patients were rare, possibly owing to a lack of facilities and scientific methods for conducting molecular tests.
During the 1980s, when immunochemistry techniques were being refined, Bartolo et al detected antibodies against Rickettsials in the serum of TAO patients in two different study sets.8,9 This work failed to draw significant attention. More recently, the footprint of Rickettsials has been detected using more precise tests, such as microimmunofluorescence and tissue PCR tests.10,11
In addition, Iwai et al demonstrated the presence of patients’ oral bacteria, P. gingivalis, in the thrombosis of their occluded arteries using tissue PCR.6 In another study in 2016 involving intravenous administration to beagles of the bacteria responsible for periodontitis, Chen et al demonstrated that this bacteria might have direct effects on platelet activation and, consequently, increase the risk of thrombosis.7
Given this information, if we consider infectious pathogens as a possible trigger for the development of TAO, we must identify the underlying mechanism to determine appropriate treatment.
Although TLRs are the main components of innate immunity, their potential role in the pathogenesis of autoimmune and autoinflammatory diseases has also been noted. For instance, it has been demonstrated that TLR4 plays a role in the induction of T helper 17, which is known as a key player in the development of autoimmune diseases.26,27 In a recent study, a significantly high expression of TLR2 and a significantly low expression of TLR4 on the monocytes’ surface – but not in the serum levels – of patients with systemic lupus erythematosus (SLE) were demonstrated. However, no association was found between TLR2 and TLR4 expression and SLE activity.28
As previously mentioned, neopterin is a biomarker of cellular immunity, being produced by human monocytes/macrophages and endothelial cells upon stimulation with interferon gamma, which is the key cytokine in cellular adaptive immunity.19 Evidence also points to an increase in the production of neopterin during acute Gram-negative bacterial infections, although the enhanced neopterin production is not specific to a particular infectious disease but only indicates that an immunological process is ongoing.29–32 An elevated concentration of neopterin in rheumatoid arthritis and SLE during disease activity has also been demonstrated,33,34 although its underlying mechanism is not well understood.
CRP is an inflammatory biomarker that is synthesized by the liver following IL-6 regulation. A high serum level of CRP has been reported in both infections and autoimmune diseases, such as SLE. However, as mentioned previously, several studies have been conducted on serum levels of CRP with the aim of differentiating bacterial infection from disease flare-ups in patients with autoimmune diseases, and the best cutoff value of hsCRP for this purpose has been reported as 5–7 µg/mL.21–23,35
The results of the current study demonstrated that neopterin and TLR4 were significantly increased in TAO patients in the acute phase of the disease in comparison with the TAO patients in the quiescent phase. Notably, in this study, sTLR4 was significantly higher in patients with hsCRP >7 µg/mL in comparison to the patients with hsCRP <7 µg/mL. However, sTLR4 was significantly lower in patients in the quiescent phase of TAO in comparison with the controls. These findings indicate that the trigger of TAO might be intracellular Gram-negative bacteria, which can be hidden or immunologically suppressed in the quiescent phase of the disease, leading to a lower level of TLR4 accompanying the normal level of neopterin. However, relapses might develop according to a cell injury trigger. This trigger might be a toxic trigger following the smoking of an unprocessed or low-quality cigarette, a hypoxic trigger due to cold-induced vasoconstriction, or the presence of an infectious pathogen. Following cell injury, TLR4 shedding will increase, and therefore, sTLR4 could become closer to the level demonstrated in controls.
Conclusion
The data of the current study do not support the claim that TAO relapses are due to reinfection, but they do support the important role of infectious pathogens in TAO development. Further studies on the possible infectious trigger behind TAO development are highly recommended to achieve improved disease management.
Acknowledgments
The authors would like to thank Prof Hasan Ravari and Dr Hossein Taheri for helpful cooperation in setting up the Biobank of Buerger’s disease. Also, they would like to thank Dr Rahim Rezaee and Hiva Sharebiani for their helpful advice and comments.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Fazeli B, Ravari H. Mechanisms of thrombosis, available treatments and management challenges presented by thromboangiitis obliterans. Curr Med Chem. 2015;22(16):1992–2001. | ||
Fazeli B, Ravari H, Assadi R. Natural history definition and a suggested clinical approach to Buerger’s disease: a case-control study with survival analysis. Vascular. 2012;20(4):198–202. | ||
Fazeli B, Modaghegh H, Ravrai H, Kazemzadeh G. Thrombophlebitis migrans as a footprint of Buerger’s disease: a prospective-descriptive study in north-east of Iran. Clin Rheumatol. 2008;27(1):55–57. | ||
Fazeli B. Buerger’s disease as an indicator of socioeconomic development in different societies, a cross-sectional descriptive study in the north-east of Iran. Arch Med Sci. 2010;6(3):343–347. | ||
Fazeli B, Rezaee SA. A review on thromboangiitis obliterans pathophysiology: thrombosis and angiitis, which is to blame? Vascular. 2011;19(3):141–153. | ||
Iwai T, Inoue Y, Umeda M, et al. Oral bacteria in the occluded arteries of patients with Buerger disease. J Vasc Surg. 2005;42(1):107–115. | ||
Chen YW, Iwai T, Umeda M, et al. Elevated IgG titers to periodontal pathogens related to Buerger disease. Int J Cardiol. 2007;122(1):79–81. | ||
Bartolo M, Antignani PL, Todini AR, Ricci G. [Buerger’s disease: etiologic role of the rickettsiae?] J Mal Vasc. 1987;12(1):82–84. French. | ||
Bartolo M, Guarnaccia G, Zardi O. [Possible rickettsial etiology of Buerger’s disease]. J Mal Vasc. 1982;7(4):309–311. French. | ||
Fazeli B, Ravari H, Ghazvini K. Rickettsia infection could be the missing piece of the Buerger’s disease puzzle. Int Angiol. 2017;36(5):410–416. | ||
Fazeli B, Ravari H, Farzadnia M. Does a species of Rickettsia play a role in the pathophysiology of Buerger’s disease? Vascular. 2012;20(6):334–336. | ||
Emelyanov VV. Evolutionary relationship of Rickettsiae and mitochondria. FEBS Lett. 2001;501(1):11–18. | ||
Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. | ||
Neyen C, Lemaitre B. Sensing Gram-negative bacteria: a phylogenetic perspective. Curr Opin Immunol. 2016;38:8–17. | ||
Kang SS, Sim JR, Yun CH, Han SH. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch Pharm Res. 2016;39(11):1519–1529. | ||
Tedder TF. Cell-surface receptor shedding: a means of regulating function. Am J Respir Cell Mol Biol. 1991;5(4):305–306. | ||
Zager RA, Johnson AC, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 2007;292(1):F304–F312. | ||
Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164(7):3476–3479. | ||
Michalak Ł, Bulska M, Strząbała K, Szcześniak P. Neopterin as a marker of cellular immunological response. Postepy Hig Med Dosw. 2017;71(1):727–736. | ||
Shionoya S. Diagnostic criteria of Buerger’s disease. Int J Cardiol. 1998;66(Suppl 1):S243–S245; discussion S247. | ||
Joo K, Park W, Lim MJ, Kwon SR, Yoon J. Serum procalcitonin for differentiating bacterial infection from disease flares in patients with autoimmune diseases. J Korean Med Sci. 2011;26(9):1147–1151. | ||
Suh CH, Jeong YS, Park HC, et al. Risk factors for infection and role of C-reactive protein in Korean patients with systemic lupus erythematosus. Clin Exp Rheumatol. 2001;19(2):191–194. | ||
Suh CH, Chun HY, Ye YM, Park HS. Unresponsiveness of C-reactive protein in the non-infectious inflammation of systemic lupus erythematosus is associated with interleukin 6. Clin Immunol. 2006;119(3):291–296. | ||
Buerger L. Thrombo-angiitis obliterans: a study of the vascular lesions leading to presenile spontaneous gangrene. Am J Med Sci. 1908;136:567–580. | ||
Maddock WG, Coller FA. Peripheral vasoconstriction by tobacco and its relation to thrombo-angiitis obliterans. Ann Surg. 1933;98(1):70–81. | ||
Powrie F, Coffman RL. Cytokine regulation of T-cell function: potential for therapeutic intervention. Trends Pharmacol Sci. 1993;14(5):164–168. | ||
Banus S, Stenger RM, Gremmer ER, et al. The role of Toll-like receptor-4 in pertussis vaccine-induced immunity. BMC Immunol. 2008;9(1):21. | ||
Fawzy SM, Khalifa RH. Expression of TLR2 and TLR4 on CD14 monocytes in female SLE patients E. Rheumatology. 2016;6(3):201. | ||
Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3(2):175–187. | ||
Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351(1–2):17–29. | ||
El-Lebedy D, Hussein J, Ashmawy I, Mohammed AM. Serum level of neopterin is not a marker of disease activity in treated rheumatoid arthritis patients. Clin Rheumatol. 2017;36(9):1975–1979. | ||
Nagy G, Brózik M, Tornóci L, Gergely P. Diagnostic value of combined evaluation of neopterin and anti-DNA antibody levels for assessment of disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18(6):699–705. | ||
Latz E, Visintin A, Lien E, et al. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the Toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277(49):47834–47843. | ||
Nomura F, Akashi S, Sakao Y, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J Immunol. 2000;164(7):3476–3479. | ||
Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.