Back to Journals » Clinical Ophthalmology » Volume 13
Three-dimensional ocular endoscope system for vitrectomy
Authors Kita M, Kusaka M, Yamada H, Hama S
Received 28 June 2019
Accepted for publication 12 August 2019
Published 27 August 2019 Volume 2019:13 Pages 1641—1643
DOI https://doi.org/10.2147/OPTH.S221197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Supplementary video 1: 3D endoscope system for vitreous shaving and cryopexy [ID 221197]
Views: 842
Mihori Kita, Mami Kusaka, Hiroshi Yamada, Sachiyo Hama
Department of Ophthalmology, National Organization Kyoto Medical Center, Kyoto, Japan
Correspondence: Mihori Kita
Department of Ophthalmology, National Organization Kyoto Medical Center, 1-1 Mukouhata-cho, Fukakusa, Fushimi-ku, Kyoto 612-8555, Japan
Tel +81 75 641 9161
Fax +81 75 643 4325
Email [email protected]
Purpose: To introduce a new three-dimensional (3D) endoscope system for vitrectomy.
Subjects and methods: We report a single-center, retrospective, consecutive surgical case series of 391 eyes that underwent 25G hybrid vitrectomy. To create 3D endoscopic images, a 3D converter HD-3D-A was connected to a monocular endoscopic system.
Results: The 3D endoscope system was successfully used to perform hybrid vitrectomy. No intra- or postoperative complications related to this system were encountered in any of the cases.
Conclusion: This 3D endoscope system appears to be a valuable and promising tool for use in vitrectomy.
Keywords: endoscope, 3D, vitrectomy, hybrid, wide-angle view
Introduction
We have previously reported on the efficacy of an ocular endoscope1–4 and on the combined use of a wide-angle viewing system and an endoscope, which we call “hybrid vitrectomy”.5,6 However, since an ocular endoscope can only provide a two-dimensional (2D) view, it is necessary to create an empirical stereoscopic vision during the endoscopy.
 |
Figure 2 A 3D converter NOVEL HD-3D-A creates 3D endoscopic images in real-time.Abbreviation: 3D, three-dimensional. |
Recently, Yoshida et al, reported using a novel three-dimensional (3D) image system for transurethral surgery.7 In our current report, we introduce a 3D ocular endoscope system for use in vitrectomy that utilizes their newly developed 3D converter.
Subjects and methods
This study was approved by the Ethics Committee of the National Hospital Organization Kyoto Medical Center, and all procedures involved adhered to the tenets of the Declaration of Helsinki. Written informed consent for the procedure and participation in our study was provided by all the patients after explaining the surgical procedures.
We report a single-center, retrospective, consecutive surgical case series of 391 eyes that underwent vitrectomy between May 2017 and June 2019. Indications for vitrectomy included rhegmatogenous retinal detachment or proliferative vitreoretinopathy (149 eyes), epiretinal membrane (85 eyes), proliferative diabetic retinopathy (71 eyes), macular hole (49 eyes), vitreous opacity or vitreous hemorrhage (22 eyes), vitreomacular traction syndrome (10 eyes), and luxation of intraocular lens (5 eyes).
Instruments
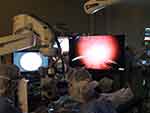
The Constellation Vision System (Alcon Laboratories, Fort Worth, TX, USA) was used to perform all of the 25G vitrectomy procedures. The NGENUITY 3D visualization system (Alcon Laboratories) was attached to a VISU 210 microscope (Carl Zeiss Meditec, Jena, Germany) and used to observe the surgical procedures (Figure 1). Images of the fundus on the NGENUITY 3D visualization system monitor were obtained through either a RESIGHT wide-angle viewing system (Carl Zeiss Meditec) or an ocular endoscope system, or by using both systems.
The 2D endoscopic images of the fundus were obtained through an ocular endoscope system consisting of an HD camera FC-304 (Fiber Tec, Tokyo, Japan), LED light unit FL-301 (Fiber Tec), 25G fiber Previt (Fiber Tec), and image processor FI-302, which can change the size and contrast of the images, and thereby distinguish the honeycomb pattern that forms the images (Fiber Tec).
To create 3D endoscopic images, a 3D converter NOVEL HD-3D-A (Shinko Optical, Tokyo, Japan) was connected to a monocular endoscopic system (Figure 2). The processed images were directly sent to a 3D display, which was 26 inches in size and positioned to the front and right of the surgeon at a distance of 1 m (Figure 1). Surgical staff wore polarized glasses during the surgical procedures.
Results
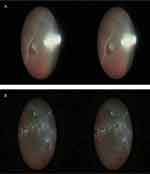
In all surgeries, the 3D endoscope system provided natural stereoscopic images of the fundus and was successfully used to perform hybrid vitrectomy. Especially in the manipulation at the peripheral area, including vitreous shaving (Figure 3A, Video S1), cryopexy (Video S1), laser photocoagulation, or silicone oil removal (Figure 3B, Video S2), 3D endoscope image was useful and resulted in the enhanced skills in vitrectomy. None of the surgical staff perceived either a time-lag resulting from the 3D processing or double vision in any of the cases.
There were no intra- or postoperative complications related to the 3D endoscopic system encountered in any of the cases.
Discussion
Although a wide-angle viewing system makes it possible to observe a panoramic fundus, the image is still quite small. In contrast, when using an endoscope, it is possible to enlarge the image by simply closing in on the retina. However, when closing in on the retina, the observation field will be narrow. Moreover, use of an endoscope makes it possible to observe the peripheral area of the fundus without any scleral indentation.1–4 Combining the use of a wide-angle viewing system and an endoscope as a visualization tool for each step of the surgery is referred to as “hybrid vitrectomy”, with the end result of a much more efficient procedure.5,6
The conventional ocular endoscope only provides a 2D view. The 25G ocular endoscope fiber is 0.5 mm in diameter, which is too small for equipping it with two optical axis cameras. The 3D processor used in our current study converts the raw 2D endoscopic image to a pair of images using a computer-based algorithm. When viewing these images, they appear to be from two different points that match the surgeon’s convergence angle,7 which is changeable. Thus, when utilizing this method, it makes it possible to provide real-time 3D endoscopic images. The depth sensation of the image helps the surgeon to more easily, rapidly, stably, and safely perform minute manipulations as compared to when using empirical stereoscopic vision. It could prevent to hit the retina or to make iatrogenic holes, in particular, the situations in which it is necessary to operate on the peripheral retina. We have previously reported that the digital 3D visualization system NGENUITY allows for both wide-angle viewing and endoscopic views on one large monitor at the same time, which can help make the surgeon more comfortable with regard to the ergonomics aspect, in addition to ensuring the hybrid vitrectomy is more efficient and safe.6 However, at the present time, the endoscopic view on the monitor of NGENUITY is only 2D. In the near future, 3D endoscopic images created by this processor are expected to be integrated into the monitors of NGENUITY, which will allow for safer manipulation and more comfortable conditions for the surgeons, as they will not need to look back and forth between the NGENUITY monitor and the 3D endoscope monitor during procedures.
In conclusion, this novel 3D endoscopic system for use in vitrectomy appears to be a valuable and promising tool.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kita M, Yoshimura N. Endoscope-assisted vitrectomy in the management of pseudophakic and aphakic retinal detachments with undetected retinal breaks. Retina. 2011;31:1347–1351. doi:10.1097/IAE.0b013e3182003c93
2. Yoshitake S, Oh H, Kita M. Endoscope-assisted vitrectomy for retinal detachment in an eye with microcornea. Jpn J Ophthalmol. 2012;56:613–616. doi:10.1007/s10384-012-0176-x
3. Kita M. Endoscope-assisted vitrectomy. World J of Ophthalmol. 2014;12:52–55. doi:10.5318/wjo.v4.i3.52
4. Kita M, Fujii Y, Hama S. Twenty five-gauge endoscopic vitrectomy for proliferative vitreoretinopathy with severe corneal opacity. Jpn J Ophthalmol. 2018;62:302–306. doi:10.1007/s10384-018-0578-5
5. Morishita S, Kita M, Yoshitake S, Hirose M, Oh H. 23-gauge vitrectomy assisted by combined endoscopy and a wide-angle viewing system for retinal detachment with severe penetrating corneal injury: a case report. Clin Ophthalmol. 2011;5:1767–1770. doi:10.2147/OPTH.S25373
6. Kita M, Mori Y, Hama S. Hybrid wide-angle viewing-endoscopic vitrectomy using a 3D visualization system. Clin Ophthalmol. 2018;12:313–317. doi:10.2147/OPTH.S156497
7. Yoshida S, Kihara K, Fukuyo T, Ishioka J, Saito K, Fujii Y. Novel three-dimensional image system for transurethral surgery. Int J Urol. 2015;22:14–15. doi:10.1111/iju.12612
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.