Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Thoracic Paravertebral Block Decreased Body Temperature in Thoracoscopic Lobectomy Patients: A Randomized Controlled Trial
Authors Yan Y , Geng J , Cui X, Lei G, Wu L, Wang G
Received 11 October 2022
Accepted for publication 12 January 2023
Published 20 January 2023 Volume 2023:19 Pages 67—76
DOI https://doi.org/10.2147/TCRM.S392961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yanhong Yan,1 Jiao Geng,2 Xu Cui,1 Guiyu Lei,1 Lili Wu,1 Guyan Wang1
1Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Anesthesiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Guyan Wang, Department of Anesthesiology, Beijing Tongren Hospital, Capital Medical University, No. 1 Dongjiaomingxiang, Beijing, 100730, People’s Republic of China, Tel +86-13910985139, Fax +86-10-58268017, Email [email protected]
Purpose: Thoracic paravertebral block (TPVB) may be highly beneficial for thoracoscopic lobectomy patients, but it may increase the risk of hypothermia. Apart from its anesthetic-reducing effects, this randomized controlled trial aimed to investigate the hypothermic effect of TPVB, and thus optimize its clinical use.
Patients and methods: Adult patients were randomly allocated to two groups: TPVB + general anesthesia (GA) group or GA group. In the TPVB+GA group, the block was performed after GA induction by an experienced but unrelated anesthesiologist. Both the lower esophageal and axillary temperature were recorded at the beginning of surgery (T0) and every 15 min thereafter (T1-T8), and the end of surgery (Tp). The primary outcome was the lower esophageal temperature at Tp. The secondary outcomes included lower esophageal temperature from T0-T8 and axillary temperature from T0-Tp. The total propofol, analgesics, and norepinephrine consumption and the incidence of adverse events were also recorded.
Results: Forty-eight patients were randomly allocated to the TPVB+GA (n=24) and GA (n=24) groups. The core temperature at the end of the surgery was lower in the TPVB+GA group than the GA group (35.90± 0.30°C vs 36.35± 0.33°C, P< 0.001), with a significant difference from 45 min after the surgery began until the end of the surgery (P< 0.05). In contrast, the peripheral temperature showed a significant difference at 60 min after the surgery began till the end (P< 0.05). TPVB+GA exhibited excellent analgesic and sedative-sparing effects compared to GA alone (P< 0.001), though it increased norepinephrine consumption due to hypotension (P< 0.001).
Conclusion: Although thorough warming strategies were used, TPVB combined with GA remarkably reduced the body temperature, which is an easily neglected side effect. Further studies on the most effective precautions are needed to optimize the clinical use of TPVB.
Keywords: core temperature, peripheral temperature, general anesthesia, warming strategy
Plain Language Summary
- This randomized controlled trial verified that TPVB may trigger perioperative hypothermia in thoracoscopic surgery patients as the incidence of hypothermia was higher in the TPVB+GA group than the GA group.
- No patients in either group had myocardial infarction, stroke, or incision site infection and the blood loss was comparable. That is not to say the safety of hypothermia, due to the limited sample size.
- Further precautions to prevent hypothermia are needed to optimize the clinical use of TPVB.
Introduction
Inadvertent perioperative hypothermia (core temperature <36.0°C1) among thoracic surgery patients is a prevailing phenomenon that has been reported to occur in 35%2 or >70%3 of cases. Hypothermia may detrimentally affect prognosis, as it is associated with delayed drug metabolism and increased surgical incision site infection, cardiovascular morbidity, and bleeding (due to dysfunctional hemostasis).4–6 Therefore, any risk factors for perioperative hypothermia should be carefully taken into account.
According to 2021 French guidelines on enhanced recovery after pulmonary lobectomy,7 thoracic paravertebral block (TPVB) is recommended as a first-line analgesic option over thoracic epidural anesthesia due to its safety profile and equivalent analgesic efficacy (grade 2+ evidence). However, according to a retrospective study of 1467 patients undergoing thoracoscopic surgery,3 TPVB may be a risk factor for hypothermia, though this requires further confirmation.
This prospective randomized controlled trial (RCT) aimed to explore the effect of TPVB on body temperature in patients undergoing thoracoscopic lobectomy. The results may serve as a reference for optimizing the clinical use of TPVB.
Methods
Study Design and Ethics Approval
This single-center double-blinded (participants and outcome assessors were blinded) prospective RCT was conducted at Beijing Tongren Hospital. The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital (TRECKY2021-055). Before recruitment, the trial was registered in the Chinese Clinical Trial Registry (https://www.chictr.org.cn, Yanhong Yan) on April 20, 2021 (registration no. ChiCTR2100045662). Additionally, before enrollment, written informed consent was obtained from each patient. The research protocol complied with the Consolidated Standards of Reporting Trials (CONSORT) statement and the Helsinki Declaration.
Participants
Adult patients (>18 years old) undergoing elective thoracoscopic lobectomy under general anesthesia (GA) from June to October 2021 were enrolled in the study. Exclusion criteria comprised: puncture site infection, psychiatric disorder or unable to cooperate, severe thoracic spinal deformity, pleural effusion or pneumothorax, allergy to study drugs, and refusal to participate.
Randomization and Blinding
After providing signed informed consent, all eligible participants were randomly allocated to either the TPVB+GA group or the GA group, using block randomization with a ratio of 1:1 and a block size of 4. Before enrollment, a random number sequence was generated by a nurse who was not involved in the rest of the study using SAS v9.4 (SAS Institute, Cary, NC, USA). Group allocation was concealed in consecutively numbered (from 1 to 48) sealed opaque envelopes prepared by the same nurse.
Before the surgery, an anesthesia nurse opened the relevant envelope and prepared the instruments and medications according to the instructions in the envelope on whether or not to prepare medications for TPVB. After GA induction, an experienced attending anesthesiologist (operation times >100), who was not involved in the rest of the study, performed the interventions as allocated, in order to ensure that the participants were blinded to group allocation. All data were obtained and recorded by blinded assessors.
Temperature Monitoring
Axillary temperature (peripheral temperature) was continuously monitored using a wireless thermometer (iThermonitor WT701; Raiing Medical, Boston, MA, USA).8 Upon entering the operating room, a temperature monitoring pad was placed in the shaved axilla opposite the surgical site and fixed with a hypoallergenic patch. The patient was instructed to adduct their ipsilateral arm for at least 5 min until the temperature displayed became stable The pad collected temperature data every 4 s and wirelessly transmitted the data to a paired smartphone.
After GA induction, an esophageal temperature probe (ES-DG-09A; Exsense Medical Technology Co., Ltd., Zhuhai, Guangdong, China) was inserted into the lower esophagus, at a distance (cm) of 0.228×(standing height)−0.2.9 The intraoperative esophageal temperature (core temperature) was displayed on the monitor (CARESCAPE B850; GE Healthcare, Boston, MA, USA), though the value was masked to ensure the anesthesiologist could not observe it.
Both the axillary and esophageal temperatures were recorded at the beginning of surgery (T0) and every 15 min thereafter (T1-T8), and the end of surgery (Tp). When each patient was fully awake, the axillary pad and esophageal probe were removed.
Anesthesia Protocol
On entering the operating room, standard monitoring was conducted, ie, non-invasive blood pressure, heart rate, pulse oximetry, invasive arterial blood pressure, 5-lead electrocardiography (lead II), and bispectral index (BIS).
GA Induction
For all patients, midazolam (0.02–0.04 mg·kg−1), propofol (1.5–2.5 mg·kg−1), sufentanil (0.2–0.3 μg·kg−1), and rocuronium (0.6 mg·kg−1) were administered. After 3 min, a double-lumen endotracheal tube was inserted (assisted by a video laryngoscope), after which a fiberoptic bronchoscope was used for positioning. Volume-controlled ventilation was applied, with a tidal volume of 6–8 mL·kg−1, respiratory rate of 12–15 min−1, and positive end-expiratory pressure (PEEP) of 5 cm H2O to maintain normal SpO2 (≥90%) and PetCO2 (35–45 mmHg).
GA Maintenance
Anesthesia at an appropriate depth was maintained with continuous infusion of propofol (4–12 mg·kg−1·h−1) and remifentanil (0.1–0.3 μg·kg−1·min−1). Vasoactive drugs were titrated to ensure hemodynamic stability (within 20% of the basal levels). All patients were extubated after the surgery and transferred to the post anesthesia care unit (PACU).
Perioperative Warming Strategies
The ambient temperature of the operating room was maintained at 22–25°C using a laminar flow system. When in the waiting area, the patients were covered with a single-layer cotton blanket taken from a 42°C warmer. GA induction was performed after the patient underwent 20 min of prewarming with a full-length water mattress (Variotherm 550; Hirtz, Koln, Germany) set at 38°C on the operating table The mattress was used throughout the surgery. All fluids, both for infusion and irrigation, were kept in an incubator (MIR-162; Sanyo, Osaka, Japan) set at 38°C.
TPVB
In the TPVB+GA group, after GA induction, the block was performed in the lateral decubitus position by the attending anesthesiologist. A single-injection (T4–T5) technique was used under real-time ultrasound guidance (Navi S; Wisonic, Shenzhen, China) with a low-frequency 1–5 MHz curved transducer. A parasagittal out-of-plane technique was used. The transducer was covered with a sterile transparent film dressing (3L Medical Products Group Co., Ltd., Jiangxi, China); care was taken to avoid trapping air under the cover. Ultrasound gel served as the coupling medium. The transducer was placed 3–4 cm lateral to the midline and moved or tilted medially to clearly observe the pleura and the transverse process. The transducer was moved caudad or cephalad to the middle of the thoracic paravertebral space, guided by the median line shown on the screen. This was followed by measuring the depth from the skin to the pleura to avoid inserting the needle too deep. The needle (UniPlex NanoLine; PAJUNK, Geisingen, Germany) was then inserted at the midline of the transducer until the tip (shown as a bright spot) reached the thoracic paravertebral space. For confirmation, a hydrolocation technique was used, involving ventral displacement of the pleura by 1–2 mL of normal saline. After negative aspiration, 40mL of 0.375% ropivacaine (Nalox; AstraZeneca, Sodertalje, Sweden) was injected at the given site.
If the anesthesiologist failed twice (with no pleura displacement observed), the TPVB would be abandoned and the patient would be excluded from the study. If a serious complication occurred (eg, total spinal anesthesia or local anesthetic systematic toxicity), emergency treatment would immediately be provided, and the patient would also be excluded from the study.
Outcomes
The primary outcome was the core temperature (lower esophageal temperature) at the end of the surgery. The secondary outcomes included core temperature from the beginning to 120 min after the surgery began and intraoperative peripheral temperature (axillary temperature).
The incidence of hypothermia (lower esophageal temperature <36°C) at the end of the surgery was also recorded. The ambient temperature, total intraoperative drug (propofol, remifentanil, sufentanil, and norepinephrine) consumption, infusion volume, blood loss, urine volume, and surgery duration were also recorded.
After the surgery, the patients were transferred to the PACU, where the numeric rating scale (NRS) pain score (0–10), incidence of severe shivering (shivering score >2: moderate muscular activity in more than one muscle group, or the entire body),10 and incidence of nausea/vomiting were evaluated. The incidences of myocardial infarction, stroke, and incision site infection in the first 72 h were taken from the electronic medical records.
Sample Size
According to our pilot study, the lower esophageal temperature at the end of the surgery (the primary outcome) in TPVB+GA group or GA group was 35.91±0.31°C vs 36.25±0.29°C. Based on this, PASS 15.0 (NCSS LLC, Kaysville, UT, USA) was used for sample size calculation with a power (1-β) of 0.9 and significance level (α) of 0.05. Given a dropout rate of approximately 10% (ie the duration of surgery less than 2 hours or severe complications occurred intraoperatively) and a block size of 4, 48 cases with 24 cases in each group were needed.
Statistical Analysis
Statistical analysis was performed in SPSS for Windows v17.0 (IBM Corp., Armonk, NY, USA). Normally distributed continuous variables are expressed as mean±SD, and the t-test was used for between-group comparisons. Non-normally distributed continuous variables are expressed as median (interquartile range), and the Mann–Whitney U-test was used for between-group comparisons. Categorical variables are expressed as the number of cases (%), and the χ2 test was used for between-group comparisons. P<0.05 was considered statistically significant.
Patient and Public Involvement
The idea for the study was derived from clinical practice and the previous report.3 Patients or the public took no part in the design, conduct, report, or the dissemination of the study.
Results
A total of 56 patients undergoing elective thoracoscopic lobectomy were assessed for inclusion in this study. However, 3 patients had scoliosis, 3 had puncture site infection, and 2 refused to participate in the study. Therefore, 48 patients completed the study (24 in each group) (Figure 1). The two groups were comparable regarding demographics, American Society of Anesthesiologists (ASA) physical status, operation duration, and operating room temperature (P>0.05) (Table 1). They were also similar regarding fluid volumes, ie, infusion volume, blood loss, and urine volume (P>0.05) (Table 1).
 |
Table 1 Comparison of Baseline and Anesthetic Parameters Between the TPVB+GA and GA Groups |
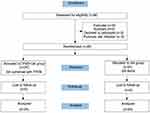 |
Figure 1 Flow chart. |
The lower esophageal temperatures are shown in Table 2 and Figure 2A. The lower esophageal temperature at the end of the surgery was lower in the TPVB+GA group than the GA group (35.90±0.30°C vs 36.35±0.33°C, P<0.001). In the TPVB+GA group, the lower esophageal temperature dropped sharply in the first hour of surgery (mean decrease: 0.46°C), and then essentially plateaued until the end of surgery (mean decrease: 0.08°C). In contrast, the lower esophageal temperature in the GA group remained almost stable throughout the surgery (mean decrease: 0.07°C) (Table 2, Figure 2A). There was a significant between-group difference at 45 min after the surgery began, which continued to the end (P<0.05).
 |
Table 2 Comparison of Lower Esophageal Temperature Between the TPVB+GA and GA Groups |
The same trends were seen for the axillary temperature, though the between-group decrease became significant at 60 min after the surgery began (a 15-min lag compared with the lower esophageal temperature) and continued to be significant until the end (mean decrease: 0.61°C) (Figure 2B).
There were dramatic decreases in propofol, sufentanil, and remifentanil consumption in the TPVB+GA group compared to the GA group (P<0.001), whereas the norepinephrine consumption sharply increased (P<0.001) (Figure 3).
Hypothermia was significantly more common in the TPVB+GA group than the GA group (58.3% vs 8.3%, P<0.001, Table 3). Nonetheless, there was no significant difference in the incidence of severe shivering (16.7% vs 8.3%, P=0.666, Table 3). GA patients were non-significantly more prone to nausea and vomiting in the PACU (12.5% vs 4.2%, P=0.530). The numeric rating scale (NRS) pain score was similar between the two groups (P>0.05) (Table 3). No patients in either group had myocardial infarction, stroke, or incision site infection within 72 h of surgery (Table 3).
 |
Table 3 Comparison of Adverse Events Between the TPVB+GA and GA Groups |
Discussion
Compared to GA alone, TPVB combined with GA significantly reduced both the core and peripheral temperatures in patients undergoing thoracoscopic lobectomy. Moreover, the incidence of hypothermia in the TPVB+GA group (58.3%) was about seven times that in the GA group (8.3%). To the best of our knowledge, there are few RCTs on the hypothermic effect of TPVB in patients undergoing GA. Previous studies mainly focused on the puncture technique,11 the analgesic effect12,13 and the prognostic impact14 of TPVB. Its effect on temperature is an easily overlooked but non-negligible issue. In 2019, Li et al3 conducted a single-center retrospective study on the incidence and risk factors of hypothermia in video-assisted thoracoscopic surgery, revealing that 72.7% of the patients experienced hypothermia, and TPVB (especially after GA induction) might be a risk factor due to its hypothermic effect involving core-to-periphery heat dissipation. Zhang et al15 confirmed the hypothermic effect of TPVB by assessing skin temperature differences among various areas using infrared thermography. In light of the intrinsic limitations of previous research, our RCT was conducted to show that TPVB may decrease the patients’ temperature during thoracic surgery despite thorough warming strategies.
A possible mechanism underlying the hypothermic effect of TPVB involves the fact that thoracic paravertebral space communicates with the epidural space, and so local anesthetics can spread to the epidural space after injection. Seidel et al16 simulated TPVB using cadavers by injecting 10–20 mL of Alcian blue staining solution via various routes to observe its diffusion. They showed that with a sagittal transducer position, medial needle guidance, and a puncture site close to the midline, the dye spreads to the epidural space in 77.8% of cases, and a higher volume induces more staining. In our RCT, the parasagittal out-of-plane technique was applied, with the puncture site at about 3.0 cm from the midline, and 40 mL of 0.375% ropivacaine was injected.
Consequently, it is likely that epidural diffusion might be existed in TPVB+GA group.
Epidural anesthesia impairs thermoregulation via three mechanisms. First, epidural anesthesia leads to abnormal perception in the blocking area, jeopardizing the transmission of cold signals to the central nervous system, and thus failing to initiate the thermoregulation program.17 Second, all autonomic thermoregulation mechanisms are nerve dependent, and epidural anesthesia causes temporary denervation in the blocking area, so the thermoregulation mechanisms do not work well.18 Lastly, epidural anesthesia reduces the shivering and vasoconstriction thresholds, thereby augmenting the temperature fluctuation range.19
In addition to the epidural anesthesia mechanism, the thoracic paravertebral space communicates with the intercostal space, so this may be involved in the hypothermic effect of TPVB. There is a lack of research on the effect of intercostal nerve block on the core temperature. Some researchers20 have suggested that peripheral nerve blocks (such as brachial plexus block) may induce hypothermia by affecting heat distribution and impairing vasoconstriction in the skin in the blocking area. However, Sessler et al21 suggested that peripheral nerve blocks alone have a limited effect on thermal regulation.
Other factors that may influence the development of hypothermia include: age, body mass index, operating room temperature, operation duration, fluid infusion and loss volumes, use of thermal insulation, and anesthetic agents.3,22 Anesthetic agents (such as propofol23 and opioids24) dose-dependently reduce the vasoconstriction and shivering threshold to around 34.5°C, which increases the core-to-periphery heat dissipation. Additionally, the metabolic heat production decreases by about 30%.25 Ultimately, the decreased heat production and increased heat loss contribute to hypothermia.
However, in our RCT, propofol, remifentanil, and sufentanil consumption levels were lower in the TPVB+GA group than the GA group, despite the TPVB+GA group having a higher incidence of hypothermia, so these agents may not be relevant to the higher incidence of hypothermia in our RCT. Additionally, there were no significant between-group differences in age, body mass index, operating room temperature, operation duration, and fluid infusion and loss volume (P>0.05). Moreover, identical warming strategies were used in the two groups. To sum up, TPVB is likely to have triggered the temperature difference between the two groups.
The overall incidence of hypothermia in the GA group (8.3%) was lower than in previous studies (50–75%).3,26 This may have been due to the thorough warming strategies that are standard practice at our center: each patient was prewarmed for 20 min before GA induction, a disposable warmed blanket was used to cover each patient, each patient laid on a perioperative water mattress (set at 38°C), and all liquids were kept in incubators set at 38°C. The effectiveness of these precautions, owing to the reduced core-to-periphery gradients, has been confirmed in previous studies.27–29
This study showed that the change in the peripheral temperature (axillary temperature) coincided with the change in the core temperature (lower esophagus temperature), but with a 15-min lag. The explanation may involve the following: (1) as anesthesia-related hypothermia mainly stems from core-to-periphery heat dissipation,18 there existed an earlier decrease in the core temperature and (2) the water mattress was near the peripheral temperature monitoring site, so the site was more susceptible to heating.
TPVB provides very good analgesia and reduces the required dosage of opioids without increasing the occurrence of adverse events.30–32 TPVB has a good adverse event profile; hypotension is among the most common side effects.33 In agreement with these previous findings, the TPVB+GA group consumed less propofol, sufentanil, and remifentanil, while the postoperative NRS pain score and the incidence of adverse events (shivering, nausea, and vomiting) were comparable to those in the GA group. Additionally, in agreement with the findings of Fang et al,34 the vasopressor (norepinephrine) consumption was significantly higher in the TPVB+GA group than the GA group, which may be attributable to the sympathetic nerve block.
There are several limitations in this study. First, to minimize discomfort and help to ensure participant blinding, TPVB was performed after GA induction, making it hard to evaluate its effectiveness; nonetheless, changes in body temperature, hemodynamic parameters, and anesthetic consumption may serve as indirect indicators. Second, postoperative body temperature was not recorded, as the core temperature monitoring procedure (involving an esophageal temperature probe) made this difficult; further studies are needed to address this issue. Lastly, the most appropriate TPVB regimen has not been confirmed, so further studies are needed to optimize the local anesthetic volume and dosage.
Conclusions
TPVB increases the incidence of hypothermia during thoracoscopic lobectomy, so additional effective warming strategies are required during this procedure. Monitoring of the core temperature provides timely data on body temperature changes, which can facilitate prompt precautionary interventions and thereby optimize patient outcomes.
Data Sharing Statement
The data are not available to the general public due to the regulations of our institution, but they are available to researchers on reasonable request by emailing Yanhong Yan ([email protected]).
Ethics Statement
The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital (TRECKY2021-055). Before recruitment, the trial was registered in the Chinese Clinical Trial Registry (https://www.chictr.org.cn, Yanhong Yan) on April 20, 2021 (registration no. ChiCTR2100045662). Before enrollment, written informed consent was obtained from each patient. The research protocol complied with the Consolidated Standards of Reporting Trials (CONSORT) statement and the Helsinki Declaration.
Acknowledgments
We thank Haixia Wang and Ying Liu for their help in preparing the envelopes (for allocation concealment) and the necessities for conducting TPVB.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This RCT was funded by the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (ZYLX202103) and the Beijing Hospitals Authority’s Ascent Plan (DFL20220203). Neither funder participated in study design, data collection, or publication of the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Borges FK, Spence J. Challenging dogma about perioperative warming during non-cardiac surgery. Lancet. 2022;399:1757–1759. doi:10.1016/S0140-6736(22)00607-9
2. Karalapillai D, Story DA, Calzavacca P, et al. Inadvertent hypothermia and mortality in postoperative intensive care patients: retrospective audit of 5050 patients. Anaesthesia. 2009;64:968–972. doi:10.1111/j.1365-2044.2009.05989.x
3. Li Y, Liang H, Feng Y. Prevalence and multivariable factors associated with inadvertent intraoperative hypothermia in video-assisted thoracoscopic surgery: a single-center retrospective study. BMC Anesthesiol. 2020;20:25. doi:10.1186/s12871-020-0953-x
4. Madrid E, Urrútia G, Roqué I Figuls M, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev. 2016;4:CD009016. doi:10.1002/14651858.CD009016.pub2
5. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. New Engl J Med. 1996;334:1209–1215. doi:10.1056/NEJM199605093341901
6. Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71–77. doi:10.1097/01.anes.0000296719.73450.52
7. Berna P, Quesnel C, Assouad J, et al. Guidelines on enhanced recovery after pulmonary lobectomy. Anaesth Crit Care Pain Med. 2021;40:100791. doi:10.1016/j.accpm.2020.100791
8. Pei L, Huang Y, Mao G, et al. Axillary temperature, as recorded by the iThermonitor WT701, well represents core temperature in adults having noncardiac surgery. Anesth Analg. 2018;126:833–838. doi:10.1213/ANE.0000000000002706
9. Mekjavic IB, Rempel ME. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol. 1990;69:376–379. doi:10.1152/jappl.1990.69.1.376
10. Kinjo T, Tadokoro T, Tokushige A, et al. Effects of perioperative administration of Acetaminophen on postoperative shivering. Anesth Analg. 2020;130:983–990. doi:10.1213/ANE.0000000000004306
11. Uppal V, Sondekoppam RV, Sodhi P, et al. Single-injection versus multiple-injection technique of ultrasound-guided paravertebral blocks. Reg Anesth Pain Med. 2017;42:575–581. doi:10.1097/AAP.0000000000000631
12. Chen N, Qiao Q, Chen R, et al. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: a randomized, double-blinded, clinical trial. J Clin Anesth. 2020;59:106–111. doi:10.1016/j.jclinane.2019.07.002
13. Huan S, Deng Y, Wang J, et al. Efficacy and safety of paravertebral block versus intercostal nerve block in thoracic surgery and breast surgery: a systematic review and meta-analysis. PLoS One. 2020;15:e0237363. doi:10.1371/journal.pone.0237363
14. Lee EK, Ahn HJ, Zo JI, et al. Paravertebral block does not reduce cancer recurrence, but is related to higher overall survival in lung cancer surgery. Anesth Analg. 2017;125:1322–1328. doi:10.1213/ANE.0000000000002342
15. Zhang S, Liu Y, Liu X, et al. Infrared thermography for assessment of thoracic paravertebral block: a prospective observational study. BMC Anesthesiol. 2021;21:168. doi:10.1186/s12871-021-01389-4
16. Seidel R, Wree A, Schulze M. Thoracic-paravertebral blocks: comparative anatomical study with different injection techniques and volumes. Reg Anesth Pain Med. 2020;45:102–106. doi:10.1136/rapm-2019-100896
17. Glosten B, Sessler DI, Faure EAM, et al. Central temperature changes are poorly perceived during epidural anesthesia. Anesthesiology. 1992;77:10–16. doi:10.1097/00000542-199207000-00003
18. Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387:2655–2664. doi:10.1016/S0140-6736(15)00981-2
19. Joris J, Ozaki M, Sessler DI, et al. Epidural anesthesia impairs both central and peripheral thermoregulatory control during general anesthesia. Anesthesiology. 1994;80:268–277. doi:10.1097/00000542-199402000-00006
20. Cho CK, Chang M, Sung TY, et al. Incidence of postoperative hypothermia and its risk factors in adults undergoing orthopedic surgery under brachial plexus block: a retrospective cohort study. Int J Med Sci. 2021;18:2197–2203. doi:10.7150/ijms.55023
21. Sessler DI, Warner DS, Warner MA. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–338. doi:10.1097/ALN.0b013e31817f6d76
22. Simegn GD, Bayable SD, Fetene MB. Prevention and management of perioperative hypothermia in adult elective surgical patients: a systematic review. Ann Med Surg. 2021;72:103059. doi:10.1016/j.amsu.2021.103059
23. Matsukawa T, Kurz A, Sessler DI, et al. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169–1180. doi:10.1097/00000542-199505000-00012
24. Kurz A, Go JC, Sessler DI, et al. Alfentanil slightly increases the sweating threshold and markedly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:293–299. doi:10.1097/00000542-199508000-00009
25. Sessler DI, Todd MM. Perioperative heat balance. Anesthesiology. 2000;92:578–596. doi:10.1097/00000542-200002000-00042
26. Emmert A, Gries G, Wand S, et al. Association between perioperative hypothermia and patient outcomes after thoracic surgery. Medicine. 2018;97:e0528. doi:10.1097/MD.0000000000010528
27. Horn EP, Bein B, Broch O, et al. Warming before and after epidural block before general anaesthesia for major abdominal surgery prevents perioperative hypothermia. Eur J Anaesthesiol. 2016;33:334–340. doi:10.1097/EJA.0000000000000369
28. Thiel B, Mooijer BC, Kolff-Gart AS, et al. Is preoperative forced-air warming effective in the prevention of hypothermia in orthopedic surgical patients? A randomized controlled trial. J Clin Anesth. 2020;61:109633. doi:10.1016/j.jclinane.2019.109633
29. Becerra Á, Valencia L, Saavedra P, et al. Effect of prewarming on body temperature in short-term bladder or prostatic transurethral resection under general anesthesia: a randomized, double-blind, controlled trial. Sci Rep. 2021;11:20762. doi:10.1038/s41598-021-00350-2
30. Zhao H, Xin L, Feng Y. The effect of preoperative erector spinae plane vs. paravertebral blocks on patient-controlled oxycodone consumption after video-assisted thoracic surgery: a prospective randomized, blinded, non-inferiority study. J Clin Anesth. 2020;62:109737. doi:10.1016/j.jclinane.2020.109737
31. Wong J, Cooper J, Thomas R, et al. Persistent postsurgical pain following thoracotomy: a comparison of thoracic epidural and paravertebral blockade as preventive analgesia. Pain Med. 2019;20:1796–1802. doi:10.1093/pm/pny293
32. Ozen V, Derdiyok O, Karacalar S. Ultrasound-guided thoracal paravertebral block for awake thoracoscopic lobectomy in a high-risk patient: the first reported case. J Minim Access Surg. 2021;17:562–565. doi:10.4103/jmas.JMAS_106_21
33. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96:418–426. doi:10.1093/bja/ael020
34. Fang B, Wang Z, Huang X. Ultrasound-guided preoperative single-dose erector spinae plane block provides comparable analgesia to thoracic paravertebral block following thoracotomy: a single center randomized controlled double-blind study. Ann Transl Med. 2019;7:174. doi:10.21037/atm.2019.03.53
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


