Back to Journals » Local and Regional Anesthesia » Volume 16
Thermal Imaging to Predict Failed Supraclavicular Brachial Plexus Block: A Prospective Observational Study
Authors Gamal M, Hasanin A , Adly N, Mostafa M, Yonis AM, Rady A, Abdallah NM , Ibrahim M, Elsayad M
Received 26 January 2023
Accepted for publication 1 June 2023
Published 9 June 2023 Volume 2023:16 Pages 71—80
DOI https://doi.org/10.2147/LRA.S406057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Stefan Wirz
Medhat Gamal, Ahmed Hasanin, Nada Adly, Maha Mostafa, Ahmed M Yonis, Ashraf Rady, Nasr M Abdallah, Mohammed Ibrahim, Mohamed Elsayad
Department of Anesthesia and Critical Care Medicine, Cairo University, Cairo, Egypt
Correspondence: Ahmed Hasanin, Department of Anesthesia and Critical Care Medicine, Faculty of Medicine, Cairo University, 01 Elsarayah Street, Elmanyal, Cairo, 11559, Egypt, Fax +20224168736, Email [email protected]
Background: Successful brachial plexus blockade produces sympathetic blockade, resulting in increased skin temperature in the blocked segments. This study aimed to evaluate the accuracy of infrared thermography in predicting failed segmental supraclavicular brachial plexus block.
Methods: This prospective observational study included adult patients undergoing upper-limb surgery under supraclavicular brachial plexus block. Sensation was evaluated at the dermatomal distribution of the ulnar, median, and radial nerves. Block failure was defined as absence of complete sensory loss 30 min after block completion. Skin temperature was evaluated by infrared thermography at the dermatomal supply of the ulnar, median, and radial nerves at baseline, 5, 10, 15, and 20 min after block completion. The temperature change from the baseline measurement was calculated for each time point. Outcomes were the ability of temperature change at each site to predict failed block of the corresponding nerve using area under receiver-operating characteristic curve (AUC) analysis.
Results: Eighty patients were available for the final analysis. The AUC (95% confidence interval [CI]) for the ability of temperature change at 5 min to predict failed ulnar, median, and radial nerve block was 0.79 (0.68– 0.87), 0.77 (0.67– 0.86), and 0.79 (0.69– 0.88). The AUC (95% CI) increased progressively and reached its maximum values at 15 min (ulnar nerve 0.98 [0.92– 1.00], median nerve 0.97 [0.90– 0.99], radial nerve 0.96 [0.89– 0.99]) with negative predictive value of 100%.
Conclusion: Infrared thermography of different skin segments provides an accurate tool for predicting failed supraclavicular brachial plexus block. Increased skin temperature at each segment can exclude block failure in the corresponding nerve with 100% accuracy.
Keywords: supraclavicular brachial plexus block, infrared thermography, skin temperature, block failure, analgesia, upper-limb surgery
Introduction
Peripheral nerve blocks are commonly used as sole anesthetic technique because they provide dense anesthesia, potent postoperative analgesia, and avoid the hazards of systemic analgesic drugs. Insufficient nerve block is an important factor in decreased patient satisfaction when regional anesthesia is used for upper-extremity surgery.1 Therefore, early detection of block failure would allow application of rescue techniques in timely fashion, which would improve patient satisfaction and save time in the operating theater.
The common approach for evaluation of block success is through assessment of motor and sensory components of the desired segments. However, this approach is usually delayed, subjective, and not possible if the patient is sedated or uncooperative.2 Therefore, there is increased interest in the use of objective methods for discriminating successful and failed blocks.3–5 Successful block is associated with sympathetic blockade in the blocked segments, resulting in increased skin temperature at the blocked segments.5,6 Therefore, evaluation of skin temperature after peripheral nerve block had been hypothesized to discriminate failed and successful blocks. This hypothesis was confirmed in epidural anesthesia7 and paravertebral block.8 However, similar studies in the brachial plexus showed conflicting results.9,10 Skin temperature was able to predict failure of infraclavicular brachial plexus block,6,9 but failed to achieve the same purpose after interscalene brachial plexus block.10 This conflicting evidence suggests that the thermal response might differ according to the block site. Lange et al found that successful block of ulnar and median nerves was associated with increased skin temperature in their specific segments, while radial and musculocutaneous nerve block was not,11 denoting heterogeneity in the effect of different nerve blockades on the temperature of the corresponding skin.
Therefore, the accuracy of skin temperature changes for detecting block success should be evaluated separately for each block site as well as for each dermatome affected by this block before its implementation in clinical practice. Supraclavicular brachial plexus block is a common block for upper-extremity operations and preferred by many physicians because it is easy to perform through a single-puncture approach and has a high success rate.12 Evaluating this block using thermal imaging has not previously been done. Hence, this study aimed to evaluate the accuracy of thermal imaging in predicting failed supraclavicular brachial plexus block. We hypothesized that in supraclavicular brachial plexus block, temperature changes at different skin dermatomes can predict failed block of the corresponding nerve.
Methods
This prospective observational study was conducted at Cairo University Hospital after institutional ethics committee approval (MD-376-2020) from February 2021 to March 2022. Written informed consent was obtained from all patients before enrolment. Participants were consecutive adult (aged 18–65 years) patients scheduled for elective upper-limb procedures under ultrasound-guided supraclavicular brachial plexus block. Patients with wound or inflammation at the examination site, peripheral vascular disease, or peripheral neuropathy were excluded.
In the anesthesia preparation room, routine monitoring was applied (electrocardiography, noninvasive blood pressure, and pulse oximetry) and vascular access was secured. Patients received 0.05 mg/kg midazolam as appropriate before temperature measurement and block performance. The preparation room temperature was usually 21–23°C. All skin coverage and bandages were removed from the hand and forearm. The patient was allowed to adapt to the room temperature for about 20 min before temperature measurement and block performance.
Supraclavicular brachial plexus block was performed by an experienced operator using a high-frequency linear transducer (8–12 MHz) connected to a Siemens ultrasound machine (Acusonx 300). The patient was placed in a semisitting position, with their head turned to the contralateral side of the block. After sterilization of the block site and local infiltration with local anesthetic, the transducer was placed in the coronal plane superior to the clavicle. The subclavian artery, pleura, and first rib were visualized, and the brachial plexus was identified as a hypoechoic structure posterior and superficial to the subclavian artery. An insulated block needle (22 G) was advanced in-plane to the transducer from lateral to medial toward the brachial plexus. A 25mL local anesthetic solution (bupivacaine 0.5% and lidocaine 2% at a ratio of 1:1) was injected under vision after negative aspiration at the pocket between the subclavian artery and the first rib, as well as at the nerve cluster superficial to the artery (double-point injection). The distribution of local anesthetic volume between the two injections was left to operator preference.
Motor and sensory functions were evaluated at 5-min intervals after block performance for 30 min. Motor function was evaluated by: the ability of the patient to extend the wrist joint against resistance (radial nerve), flex the distal interphalangeal joint of the little digit against resistance (ulnar nerve), oppose the index finger against resistance (median nerve), and flex the elbow joint against resistance (musculocutaneous). The blocked limb was compared to the other limb during evaluation of all nerves.
Sensory function was evaluated at the dermatomal distribution of each of the ulnar, median, radial, and musculocutaneous nerves using pieces of ice and pinprick. The area for sensory assessment was away from that for temperature assessment. Block failure was defined as failure of the block to provide complete sensory loss after 30 min of local anesthetic injection. In the case of tourniquet pain or segmental nerve sparing that did not involve the operative area, supplemental analgesia (50 µg fentanyl) was provided as appropriate. General anesthesia was provided if any of the following occurred: failure of supplementary analgesia, segmental nerve sparing involving the operative area, or complete block failure.
Evaluation of Skin Temperature
Skin temperature was evaluated by an assistant blinded to the block results using an FLIR C2 compact thermal camera (FLIR Systems, Oregon, USA). Prior to temperature measurement, the focal length of the thermal camera was adjusted at 0.5 meters from the patient’s upper extremity. The camera was calibrated to eliminate the effect of the room temperature before use in each patient, scanning for each area was conducted for 10 seconds, and the temperature was recorded after reaching a stable reading. The temperature was recorded at three points: tip of the little finger (ulnar nerve), tip of the index finger (median nerve), and base of the dorsal aspect of the index finger (radial nerve). Temperature was recorded at baseline before local anesthetic injection and at 5, 10, 15, and 20 min after local anesthetic injection. Evaluation of skin temperature was done for both the ipsilateral and contralateral sides to the block.
Temperature change was calculated as: the temperature measurement at each point − the baseline measurement. We did not perform specific evaluation for the temperature at the musculoskeletal nerve segments. Musculocutaneous nerve block does not produce temperature change at its skin territory according to Lange et al.11 Therefore, we did not include it to reduce the examination time. The temperature recording was completed 20 min after block performance. If complete motor and sensory loss had been achieved by then, the surgery was allowed without completing the 30 min period of assessment.
The primary outcome of our study was the ability of temperature change at the tip of the little finger 5 min after the block to predict failed ulnar nerve blockade. Secondary outcomes included the ability of temperature change at each site 5, 10, 15, and 20 min after the block to predict failed block of the corresponding nerve. Temperature change at the little finger, index finger, and dorsal base of the index finger were evaluated in patients with failed and successful musculocutaneous nerve block after removal of overlapping failure with other segments.
Sample Size
Sample size was calculated using MedCalc version 14 (MedCalc Software, Ostend, Belgium) to detect area under the receiver-operating characteristic curve (AUC) of 0.8 with null hypothesis at AUC of 0.5. The incidence of ulnar nerve block failure in supraclavicular brachial plexus block ranged from 3% to 30%;13 therefore, we used a conservative approach in our calculations, considering an incidence of 10%. As a result, a minimum of 80 patients (with at least eight failed blocks) was required to achieve a study power of 80% and α error of 0.05.
Statistical Analysis
MedCalc and SPSS 26 for Microsoft Windows (IBM, NY, USA) were used for statistical analysis. Categorical data are presented as frequency (%). Continuous data were checked for normality using the Shapiro–Wilk test and are presented as means ± SD or median (quartiles) as appropriate. Unpaired continuous variables were compared using the Student’s t-test or Mann–Whitney U test as appropriate. Repeated-measures data were analyzed using the ANOVA. Adjustment for multiple measurement was done using the Bonferroni test. AUC analysis was performed to evaluate the ability of the temperature change at 5, 10, 15, and 20 min at each segment to predict failed block at the corresponding nerve. The best cutoff was determined from the AUC using the Youden index. Positive and negative predictive values were then calculated. AUCs at different time points were compared using the Hanley–McNeil test. P≤0.05 was considered significant.
Results
A total of 87 patients were screened for eligibility, and seven were excluded for not meeting the inclusion criteria. Eighty patients were included and analyzed. (Figure 1). The median (quartiles) age of the patients was 34 (28, 42) years, and 54/80 (68%) were male. The number of patients with ulnar, median, radial, and musculocutaneous nerve block failure was 13 of 80 (16%), 9 of 80 (11%), 9 of 80 (11%), and 15 of 80 (19%), respectively (Table 1). Most of these nerve block failures overlapped — details are illustrated in Figure 2). The cumulative incidence of patients with one or more segment block failures was 19 of 80 (24%), and 14 of 80 (18%) required conversion to general anesthesia (no rescue blockade was provided). Concurrent sensory loss and incomplete motor blockade of the same dermatome occurred in seven patients; however, conversion to general anesthesia was needed in four of them due to incomplete sensory loss in the other dermatomes. The other three cases did not require further intervention.
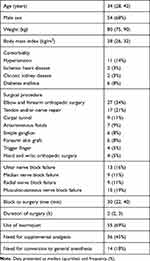 |
Table 1 Demographic, surgical, and anesthetic data |
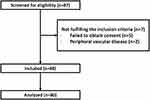 |
Figure 1 Patient enrolment. |
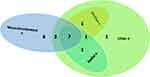 |
Figure 2 Details of nerve block failure. Abbreviation: n, nerve. |
Skin temperature increased in successful block segments in comparison to the baseline measurement, while it was lower or comparable to the baseline measurement at the failed block segments and contralateral side to the block (Figures 3 and 4). Temperature changes at each site in the ipsilateral limb to the block are shown in Figure 4. Details of temperature changes at the ipsi- and contralateral limbs to the block are presented in Supplementary Tables 1 and 2. The AUC (95% CI) for the ability of temperature change at 5 min to predict failed ulnar, median, and radial nerve block was 0.79 (0.68–0.87), 0.77 (0.67–0.86), and 0.79 (0.69–0.88), respectively. AUC (95% CI) increased progressively with subsequent readings and reached maximum values at 15 min (ulnar nerve 0.98 [0.92–1.00], median nerve 0.97 [0.90–0.99], radial nerve 0.96 [0.89–0.99]; Table 2).
 |
Table 2 AUC analysis of ability of temperature change at each site to predict segmental block failure of the corresponding nerve |
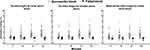 |
Figure 4 Temperature change in each patient at the corresponding dermatomal supply of each nerve in successful and failed block. |
Isolated musculocutaneous nerve sparing occurred in six patients. Temperature change at the tip of the little finger, tip of the dorsal index, and base of the dorsal index fingers were compared among patients with failed and successful musculocutaneous block (65 cases after removal of overlapping failure with other segments). The temperature change at the three sites did not differ between failed and successful musculocutaneous nerve block, except for that at the tip of the little finger at 5 and 10 min after the block. The temperature increased at the tip of the little finger in cases of failed musculocutaneous nerve block compared to successful block (Supplementary Table 3). The AUC analysis for these two measurements to predict musculocutaneous nerve sparing is presented in Supplementary Table 4.
Discussion
We evaluated the ability of thermal imaging to predict the failure of supraclavicular brachial plexus block at different nerve segments and found that it can accurately rule out patients with failed block. At 10 min after the block, thermal imaging showed excellent sensitivity and negative predictive value to rule out block failure (98%–100%). At 15 min, specificity approached >90%, denoting increased positive predictive value and perfect negative predictive value. Despite the high specificity in all segments after 15 min, positive predictive value increased in the ulnar segments rather than other segments, most probably due to the larger number of patients with ulnar sparing; therefore, thermal imaging can be used to confirm block success rather than failure. Therefore, any increase in skin temperature would confirm block success at the corresponding segment, while failure of skin temperature to increase would not reject block success with the same accuracy. Peripheral block success is usually associated with vasodilatation in the corresponding dermatomes, as the sympathetic nerve supply is segmentally distributed in the upper limb.11,14 This explains the increased temperature in the segments at which the block was successful.
No studies to the best of our knowledge have evaluated the ability of skin temperature using infrared thermography to predict supraclavicular brachial plexus block success. Few studies have evaluated other approaches to upper-extremity block, and their results were inconsistent in terms of the general accuracy of skin temperature or the magnitude of temperature change after successful block. The difference in the available literature highlights the complex input of the sympathetic nervous system in the upper limb and the different thermal responses to brachial plexus block when performed at different levels.
Two previous studies evaluated skin temperature after infraclavicular brachial plexus block using different types of thermometers, and both showed that increased skin temperature can be an indicator of block success. However, there are several differences in the design and results between our study and those. The first study was conducted by Minville et al evaluating 30 patients (with five failed blocks) using a simple infrared thermometer, and demonstrated that increased skin temperature can be an indicator of block success.6 Our study differed in the type of block (supraclavicular approach), measurement device (thermal camera), number of patients (80), segmental evaluation, and determination of cutoffs using AUC analysis. The second study was conducted by Asghar et al, who used infrared thermography to detect successful lateral infraclavicular block in 40 patients and found that increased temperature can detect successful block;9 however, they found larger changes in temperature than our values. This difference might be due to the use of cooled packs (5°C) before the block, which might have augmented the block effect on skin temperature in their study. Another important difference between our study and Asghar et al is the larger sample, the evaluation of different segments, and constructing receiver-operating characteristic curves for each segment.
Lange et al evaluated the thermal response to individual nerve blockade in the upper extremity and found that successful block of ulnar and median nerves is associated with increased skin temperature in their dermatomes. However, they did not find a similar increase in temperature in the radial or musculocutaneous segments.11 Many differences between our study and Lange et al’s might explain the difference between the results. Our study used the usual supraclavicular approach for brachial plexus block and followed the effect of the block on each segment, while Lange et al performed a single nerve block in each patient. Therefore, we believe that our study is closer to daily practice. Furthermore, the larger sample in our study compared to Lange et al (80 vs 42 patients) enabled the performance of AUC analysis and defining the positive and negative predictive values for thermal imaging in predicting block success.
In this study, the incidence of conversion to general anesthesia was 18%, which is within the range (0–37%) of that previously reported in supraclavicular brachial plexus block.13,15 Ulnar nerve block failure occurred in 16% despite using the double-injection technique including corner-pocket injection; however, this incidence is within the previously reported range for ulnar nerve sparing in supraclavicular brachial plexus block.13 Furthermore, previous data have shown that ulnar nerve sparing can occur despite corner-pocket injection.16–18
The volume and components of the local anesthetic mixture used in this study are the usual practice in our institution and were reported in previous studies of brachial plexus blocks.13,15 The practice of using different local anesthetic mixtures is controversial and data regarding its efficacy are conflicting;19,20 however, it is still a common practice used by many anesthetists. The use of fixed volume instead of weight-based volume is also common in brachial plexus blocks,13,15 and it has been reported that the effective volume of local anesthetic does not increase with increased body mass index.21 We avoided using a larger volume of local aesthetic to reduce the risk of complications.22
According to our findings and previous reports, we suggest that thermal imaging provides an easy, objective, noninvasive tool for evaluation of upper-limb block success. The cutoff for predicting block success is individual for each approach. Thermal imaging has many other advantages, such as avoidance of close contact with the patients, which is desirable in the peripandemic era. It is also considered economical, because the camera can be reused for many patients without expensive consumables. Thermal imaging can improve patient satisfaction compared to traditional subjective evaluation of block success, since it minimizes the need for pinpricking. It can also be used in sedated and anesthetized patients. Furthermore, our data suggest that the value of thermal imaging is apparent 15 min after block performance, which could be compromised by the fact that the clinical assessment would be able to detect block success by then; however, complete sensory block is not commonly reached by this time, and it is the usual practice to wait for 30 min to judge block success.
There is increased interest in peripheral nerve blocks due to their their excellent analgesic profile, which minimizes the need for systemic analgesic drugs. Upper-limb blocks can also be used solely for anesthesia. However, block failure results in undesirable stress for patients and physicians. A recent report showed that regional anesthesia for upper-limb procedures was associated with less patient satisfaction than general anesthesia, with insufficient block being the most common cause of low patient satisfaction.1 Therefore, it is desirable to predict block failure early to enable timely initiation of rescue techniques (eg, block supplementation or switching to general anesthesia).
Our study has many advantages in terms of having the largest sample, the first to perform AUC analysis for each segment, and the reasonable number of positive and negative cases. Room temperature and patient factors could have affected the results of thermal imaging; however, several measures were taken to avoid these issues, such as the room temperature being standardized, the patient being allowed to acclimatize to the surrounding temperature before initiating the thermal scan, the thermal camera being calibrated for each patient, and temperature change from baseline being used to avoid the effect of baseline temperature variability between patients.
There are some limitations, such as being performed in a single center. The skin temperature at the musculocutaneous nerve territory was not assessed, since the incidence of isolated musculocutaneous nerve sparing is low in supraclavicular block, unlike ulnar nerve sparing (which was our primary outcome).13 Lange et al11 performed isolated blocking of the musculocutaneous nerve on seven patients and reported that successful blockade was not associated with increased temperature in its territory. Therefore, to reduce examination time, specific temperature measurement for the musculocutaneous nerve was omitted and temperature change at the other sites was used to evaluate the blockade of this nerve. We found that the temperature increased at the skin point corresponding to the ulnar nerve in cases of isolated musculocutaneous nerve sparing. This might be related to preferential injection of a larger volume of local anesthetic in these cases at the pocket between the subclavian artery and the first rib (ulnar nerve origin) for fear of ulnar nerve sparing. This preferential injection might result in denser block of the ulnar nerve at the expense of the musculocutaneous nerve. Further studies are needed to confirm this observation. Patient and surgeon satisfaction at the end of the procedure was not recorded; however, proper anesthetic management was provided in cases of incomplete/failed block to ensure patient comfort.
In conclusion, evaluation of the temperature of different skin segments using a thermal camera is an accurate tool for excluding failed supraclavicular brachial plexus block. Increased skin temperature at each segment in the hand can exclude block failure in the corresponding nerve with an accuracy of 100%.
Data Sharing
The data that support the findings of this study are available from the authors upon reasonable request after gaining permission from Cairo University.
Ethics Approval and Informed Consent
We conducted this study after obtaining Research Ethics Committee of Cairo University approval (MD-376-2020). Written informed consent was obtained from all patients before enrollment. Our study complies with the Declaration of Helsinki.
Funding
There is no funding to report.
Disclosure
This paper or the abstract of this paper has not been presented at a conference or published.
References
1. Droog W, Hoeks SE, van Aggelen GP, et al. Regional anaesthesia is associated with less patient satisfaction compared to general anaesthesia following distal upper extremity surgery: a prospective double centred observational study. BMC Anesthesiol. 2019;19(1):115. doi:10.1186/s12871-019-0789-4
2. Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Fisher D. Sensory assessment of regional analgesia in humans: a review of methods and applications. Anesthesiology. 2000;93(6):1517–1530. doi:10.1097/00000542-200012000-00025
3. Smith GB, Wilson GR, Curry CH, et al. Predicting successful brachial plexus block using changes in skin electrical resistance. Br J Anaesth. 1988;60(6):703–708. doi:10.1093/bja/60.6.703
4. Sørensen J, Bengtsson M, Malmqvist EL, Nilsson G, Sjöberg F. Laser Doppler perfusion imager (LDPI)--for the assessment of skin blood flow changes following sympathetic blocks. Acta Anaesthesiol Scand. 1996;40(9):1145–1148. doi:10.1111/j.1399-6576.1996.tb05578.x
5. Galvin EM, Niehof S, Medina HJ, et al. Thermographic temperature measurement compared with pinprick and cold sensation in predicting the effectiveness of regional blocks. Anesth Analg. 2006;102(2):598–604. doi:10.1213/01.ane.0000189556.49429.16
6. Minville V, Gendre A, Hirsch J, et al. The efficacy of skin temperature for block assessment after infraclavicular brachial plexus block. Anesth Analg. 2009;108(3):1034–1036. doi:10.1213/ane.0b013e318195bf94
7. Bruins AA, Kistemaker KRJ, Boom A, Klaessens JHGM, Verdaasdonk RM, Boer C. Thermographic skin temperature measurement compared with cold sensation in predicting the efficacy and distribution of epidural anesthesia. J Clin Monit Comput. 2018;32(2):335–341. doi:10.1007/s10877-017-0026-y
8. Możański M, Rustecki B, Kalicki B, Jung A. Thermal imaging evaluation of paravertebral block for mastectomy in high risk patient: case report. J Clin Monit Comput. 2015;29(2):297–299. doi:10.1007/s10877-014-9599-x
9. Asghar S, Lundstrøm LH, Bjerregaard LS, Lange KHW. Ultrasound-guided lateral infraclavicular block evaluated by infrared thermography and distal skin temperature. Acta Anaesthesiol Scand. 2014;58(7):867–874. doi:10.1111/aas.12351
10. Hermanns H, Braun S, Werdehausen R, Werner A, Lipfert P, Stevens MF. Skin temperature after interscalene brachial plexus blockade. Reg Anesth Pain Med. 2007;32(6):481–487. doi:10.1016/j.rapm.2007.06.392
11. Lange KHW, Jansen T, Asghar S, Kristensen PL, Skjønnemand M, Nørgaard P. Skin temperature measured by infrared thermography after specific ultrasound-guided blocking of the musculocutaneous, radial, ulnar, and median nerves in the upper extremity. Br J Anaesth. 2011;106(6):887–895. doi:10.1093/bja/aer085
12. Franco CD, Vieira ZE. 1001 subclavian perivascular brachial plexus blocks: success with a nerve stimulator. Reg Anesth Pain Med. 2000;25(1):41–46. doi:10.1016/s1098-7339(00)80009-7
13. Park SK, Lee SY, Kim WH, Park HS, Lim YJ, Bahk JH. Comparison of supraclavicular and infraclavicular brachial plexus block: a systemic review of randomized controlled trials. Anesth Analg. 2017;124(2):636–644. doi:10.1213/ANE.0000000000001713
14. Campero M, Verdugo RJ, Ochoa JL. Vasomotor innervation of the skin of the hand: a contribution to the study of human anatomy. J Anat. 1993;182:361–368.
15. Albrecht E, Mermoud J, Fournier N, Kern C, Kirkham KR. A systematic review of ultrasound-guided methods for brachial plexus blockade. Anaesthesia. 2016;71(2):213–227. doi:10.1111/anae.13347
16. Luo Q, Yao W, Shu H, Zhong M. Double-injection technique assisted by a nerve stimulator for ultrasound-guided supraclavicular brachial plexus block results in better distal sensory-motor block. Eur J Anaesthesiol. 2017;34(3):127–134. doi:10.1097/EJA.0000000000000542
17. Roy M, Nadeau MJ, Côté D, et al. Comparison of a single- or double-injection technique for ultrasound-guided supraclavicular block: a prospective, randomized, blinded controlled study. Reg Anesth Pain Med. 2012;37(1):55–59. doi:10.1097/AAP.0b013e3182367b97
18. Fredrickson MJ, Patel A, Young S, Chinchanwala S. Speed of onset of “corner pocket supraclavicular” and infraclavicular ultrasound guided brachial plexus block: a randomised observer-blinded comparison. Anaesthesia. 2009;64(7):738–744. doi:10.1111/j.1365-2044.2009.05918.x
19. Cuvillon P, Nouvellon E, Ripart J, et al. A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: a double-blind randomized study. Anesth Analg. 2009;108(2):641–649. doi:10.1213/ane.0b013e31819237f8
20. Gadsden J, Hadzic A, Gandhi K, et al. The effect of mixing 1.5% mepivacaine and 0.5% bupivacaine on duration of analgesia and latency of block onset in ultrasound-guided interscalene block. Anesth Analg. 2011;112(2):471–476. doi:10.1213/ANE.0b013e3182042f7f
21. Gupta PK, Pace NL, Hopkins PM. Effect of body mass index on the ED50 volume of bupivacaine 0.5% for supraclavicular brachial plexus block. Br J Anaesth. 2010;104(4):490–495. doi:10.1093/bja/aeq017
22. Bao X, Huang J, Feng H, et al. Effect of local anesthetic volume (20 mL vs 30 mL ropivacaine) on electromyography of the diaphragm and pulmonary function after ultrasound-guided supraclavicular brachial plexus block: a randomized controlled trial. Reg Anesth Pain Med. 2019;44(1):69–75. doi:10.1136/rapm-2018-000014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

