Back to Journals » Clinical and Experimental Gastroenterology » Volume 7
Therapeutic treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates murine colitis
Authors Gupta R, Chaudhary A, Shah B, Jadhav A, Zambad S , Gupta R, Deshpande S, Chauthaiwale V, Dutt C
Received 23 July 2013
Accepted for publication 27 September 2013
Published 24 January 2014 Volume 2014:7 Pages 13—23
DOI https://doi.org/10.2147/CEG.S51923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ram Gupta,1 Anita R Chaudhary,2 Binita N Shah,1 Avinash V Jadhav,3 Shitalkumar P Zambad,1 Ramesh Chandra Gupta,4 Shailesh Deshpande,4 Vijay Chauthaiwale,4 Chaitanya Dutt4
1Department of Pharmacology, 2Cellular and Molecular Biology, 3Preclinical Safety Evaluation, 4Discovery, Torrent Research Centre, Torrent Pharmaceuticals Ltd, Gandhinagar, Gujarat, India
Background and aim: Mucosal healing in inflammatory bowel disease (IBD) can be achieved by improvement of intestinal barrier protection. Activation of hypoxia-inducible factor (HIF) has been identified as a critical factor for barrier protection during mucosal insult and is linked with improvement in symptoms of colitis. Although prophylactic efficacy of HIF hydroxylase inhibitors in murine colitis have been established, its therapeutic efficacy in clinically relevant therapeutic settings have not been established. In the present study we aim to establish therapeutic efficacy of TRC160334, a novel HIF hydroxylase inhibitor, in animal models of colitis.
Methods: The efficacy of TRC160334 was evaluated in two different mouse models of colitis by oral route. A prophylactic efficacy study was performed in a 2,4,6-trinitrobenzene sulfonic acid-induced mouse model of colitis representing human Crohn's disease pathology. Additionally, a therapeutic efficacy study was performed in a dextran sulfate sodium-induced mouse model of colitis, a model simulating human ulcerative colitis.
Results: TRC160334 treatment resulted in significant improvement in disease end points in both models of colitis. TRC160334 treatment resulted into cytoprotective heatshock protein 70 induction in inflamed colon. TRC160334 successfully attenuated the rate of fall in body weight, disease activity index, and macroscopic and microscopic scores of colonic damage leading to overall improvement in study outcome.
Conclusion: Our findings are the first to demonstrate that therapeutic intervention with a HIF hydroxylase inhibitor ameliorates IBD in disease models. These findings highlight the potential of TRC160334 for its clinical application in the treatment of IBD.
Keywords: IBD, hypoxia-inducible factor, HIF activation
Introduction
Inflammatory bowel disease (IBD) is comprised of Crohn’s disease (CD) and ulcerative colitis (UC) and is a chronic gastrointestinal inflammation disorder with considerable morbidity and health care burden.1 Although the pathophysiological understanding of IBD has enhanced over time, its exact etiology is yet to be established and the disease remains incurable. IBD remains a complex multifactorial disease involving dysfunctional intestinal epithelial barrier, aberrant innate immune system responses, contribution by intestinal flora along with genetic predisposition2 and stress.3 However, recent research indicates that breach in intestinal barrier function is one of the early and crucial factors in the pathogenesis of IBD.4 Breach of intestinal integrity allows nonselective influx of luminal antigens which acts as a trigger for immune cells in the lamina propria, resulting in prolonged, chronic inflammation ultimately leading to clinical presentation of disease in the form of abdominal pain, intestinal bleeding, weight loss, fever, and diarrhea of different severity.5
Current therapeutic strategies for IBD involve multidisciplinary management targeting inflammation and immune components associated with the disease. However, several limitations exist with current therapeutic options such as inadequate response or nonresponsiveness, loss of response, and immunosupression.5 More recently, approval of anti-tumor necrosis factor (TNF) α antibodies for IBD have revolutionized the treatment approach. However, in addition to high cost, adverse effects such as immunogenicity, infusion site reaction, and the loss of efficacy have been observed for anti-TNFα antibodies.6 Recently, mucosal healing has emerged as a key treatment goal in IBD and as a predictor for sustained clinical remission and-resection free survival of patients. While current therapeutic strategies for IBD reduce disease activity and improve disease symptoms, these agents are inadequate in achieving mucosal healing.7 Indeed, despite therapeutic developments, the number of IBD patients ultimately requiring surgical intervention remain high.8 This highlights the significant unmet need which can be addressed by new therapeutics aimed at effective mucosal healing for IBD. Effective blockade of breach in intestinal barrier function is expected to bring about such mucosal healing in IBD.
Under physiological conditions, intestinal tissue presents a unique steep oxygen gradient with an oxygen rich vascular bed and almost anoxic luminal epithelial interface.9 However, when inflamed, a high degree of intestinal mucosal hypoxia is observed in the animal model for colitis, an observation that has been further confirmed in human IBD patients.10 Hypoxia-inducible factor (HIF) activation is one of the prominent adaptive mechanisms associated with hypoxia/ischemia. HIF activation induces transcription of genes in a concerted manner that performs multiple functions to adapt and to recover from hypoxic/ischemic conditions.11 Recently, we demonstrated that TRC160334, a novel HIF hydroxylase inhibitor, ameliorates ischemic acute kidney injury in rats.11 The potential of inhibiting HIF hydroxylase to bring about subsequent HIF activation has been identified for the treatment of diseases like anemia12 and IBD.13
In the context of IBD, HIF activation results in upregulation of a set of target genes linked with maintenance of intestinal barrier function (heatshock protein 70 [HSP70], multidrug resistant gene 1, intestinal trefoil factor).14 Karhausen et al14 have shown that transgenic mice with targeted mutation for HIF-1α in intestinal epithelial cells (inability to form HIF-1) demonstrate a more severe colitic phenotype. In addition, animals mutant for von Hippel-Lindau gene (forming constitutively active HIF-1) were protected from developing colitis. Pharmacological inhibition of HIF hydroxylase and subsequent HIF activation has resulted in protection of colitis in mice.13 However, this study employed only the prophylactic treatment of HIF hydroxylase inhibition and did not explore the therapeutic treatment regimen.13 In the present study, we have studied the potential of TRC160334 for treatment of colitis in clinically relevant animal models simulating human CD and UC disease conditions using prophylactic as well as therapeutic treatment regimens.
Methods
TRC160334
TRC160334 is a novel HIF hydroxylases inhibitor and its chemical name is [(2-hydroxy-4-oxo-6,7,8,9-tetrahydro-4H,5H-10-thia-1,4a-diaza-benzo[a]azulene-3-carbonyl)-amino]-acetic acid. The chemistry in terms of design, synthesis, and biological activity of HIF activation by TRC160334 have been described previously.11
In vivo studies
Animals
In-house bred BALB/c mice (7 to 11 week of age) were used for the study. Mice were housed in individually ventilated cages, maintained on 12-hour light–dark cycles, and had access to Harlan-2014c rodent chow (Harlan Laboratories, Inc., Madison, WI, USA) and water ad libitum. The individually ventilated cages were maintained under a controlled environment of temperature 22°C±3°C, relative humidity 30%–70%, and air exchange rate of 40–50 air changes per hour. Animal care and all experimental procedures were carried out in accordance with guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India.15 The study protocols were approved by the Institutional Animal Ethics Committee of Torrent Pharmaceuticals Limited at Gujarat, India.
2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced murine colitis model
Male BALB/c mice, based on their body weights, were randomized into two groups: group 1 consisted of TNBS colitis mice receiving vehicle (n=22) and group 2 consisted of TNBS colitis mice treated with TRC160334 (n=12). On day 0 under isofluorane anesthesia (Forane; Aesica Queenborough Ltd., Kent, UK), acute colitis was induced in 16 hour fasted mice by an intrarectal administration of 1.5 mg TNBS (Sigma-Aldrich Co, St Louis, MO, USA) in 0.1 mL 50% ethanol through a flexible catheter 4 cm proximal to the anus. To ensure proper distribution of TNBS within the colon and to prevent reflex leakage, mice were kept in a vertical head down position for 45 seconds after instillation. Six control mice underwent identical procedures but were instilled with 50% ethanol.
Prophylactic efficacy of TRC160334 in TNBS-induced murine colitis
Treatment with TRC160334, 2 mg/kg/day, per oral (po) was initiated a day prior to induction of colitis (day -1) and continued until study termination, ie, day 4 (Figure 1A). Deaths occurring within 24 hours of TNBS instillation were not included in study.14
Dextran sulfate sodium (DSS)-induced murine colitis model
Autoclaved drinking water with 5% dissolved DSS (molecular weight 36,000–50,000 Da; MP Biomedicals LLC, Solon, OH, USA) was provided ad libitum to female BALB/c mice for induction of colitis. Fresh DSS solution was provided every alternate day. DSS was provided to mice until study day 11, followed by three DSS free days, ie, until day 14, the study termination day. On day 5, mice were randomized into three groups based on their body weight and severity of disease activity index (DAI): group 1 consisted of DSS colitis mice receiving vehicle (n=9), and groups 2 and 3 were DSS colitis mice treated with TRC160334 at 2 mg/kg/day (n=9) or 5 mg/kg/day (n=9), respectively. Five control mice underwent identical procedures and received autoclaved drinking water throughout the study duration.
Therapeutic efficacy of TRC160334 in DSS-induced murine colitis
Treatment with TRC160334 at 2 and 5 mg/kg/day, po was initiated from day 5 and continued until study termination (Figure 1B). Only mice that demonstrated an initial response to DSS treatment were included in the study; this response was defined as a fall in mean body weight (day 1 to day 5) by ≥1%.
Assessment of inflammation and colitis severity
DAI for colitis
For assessment of clinical severity of colitis, DAI was calculated as reported earlier.16,17 DAI is a composite score derived on the basis of loss in body weight, stool consistency, and appearance of blood in feces. Briefly, body weights were monitored daily, whereas stool consistency and rectal bleeding were examined every alternate day. The loss in body weight was scored as: 0, no weight loss; 1, weight loss of 1% to 5%; 2, weight loss of 6% to 10%; 3, weight loss of 11% to 20%; and 4, weight loss >20%. The assessment of diarrhea (stool consistency) was scored as 0 for normally formed pellets; 2 for pasty and semiformed pellets; and 4 for liquid stools. Bleeding was scored using the standard Guaiac paper test (Hemospot; Crest Biosystems, Goa, India) as follows: 0, negative hemoccult; 2, positive hemoccult; and 4, visible blood/gross bleeding from the rectum. These scoring parameters were added together resulting in a total clinical score ranging from 0 (healthy) to 12 (maximal ill/activity of colitis). At the end of the experiment, the colons were harvested for further macroscopic/microscopic damage scoring and expression study.
Macroscopic assessment of colonic damage
The assessment of the macroscopic colonic damage was performed using a slightly modified Morris score.18 The colon was excised, opened longitudinally, and washed in cold phosphate buffered saline. Features of macroscopic damage were graded as follows: 0, no ulcer and no inflammation; 1, focal hyperemia, but no ulcer; 2, severe hyperemia; 3, ulceration and inflammation at one site only; 4, ulceration and inflammation at two or more sites; 5, ulceration extending 1 cm but less than 2 cm; 6 to 10, score increased by 1 point for increase of every 1 cm in lesion area up to maximum score of 10.
Histological evaluation of colonic damage
The colonic tissues were fixed in 10% neutral buffered formalin, processed, sectioned at 4 μm thickness, and stained with a routine hematoxylin and eosin method.19 Stained sections were evaluated in a blinded manner by a pathologist.
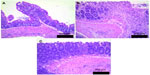
Sections from TNBS treated mice were semiquantified for severity of lesion using a score of 0 to 6: 0, normal crypt architecture; 1, focal to multifocal inflammatory cell infiltration or focal necrosis; 2, diffuse inflammatory cell infiltration or focal to multifocal erosions with necrosis extending up to muscularis mucosa; 3, multifocal erosions or ulceration with and without partial loss of crypts along with inflammatory cell infiltration; 4, diffuse erosions or focal to multifocal ulceration along with partial loss of crypts and inflammatory cell infiltration; 5, multifocal to diffuse ulceration with necrosis extending up to serosa, complete loss of crypts, inflammatory cell infiltration, and mineralization in mucosa and submucosa; 6, multifocal to diffuse ulceration with necrosis perforating serosa, complete loss of crypts, inflammatory cell infiltration, and mineralization in mucosa and submucosa.
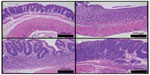
Sections from DSS treated mice were semiquantified for severity of lesion using a scoring of 0 to 4: 0, normal crypt architecture; 1, crypt shortening, loss of basal one third crypt, lamina propria prominent with minimal inflammatory changes; 2, crypt shortening, loss of basal two third crypt, thinning of epithelium with mild inflammatory changes; 3, loss of entire crypt with intact epithelial layer and moderate inflammatory changes; 4, loss of entire crypt along with the epithelial layer (erosion) with severe inflammatory changes.20,21
Evaluation of gene expression
Colon tissue of vehicle and TRC160334 treated animals were processed and total RNA was isolated. Expression of interleukin (IL)-10, TNF-α, and interferon (INF)-γ mRNA along with expression of 18S rRNA was monitored employing gene specific primer and probes (Applied Biosystems, Foster City, CA, USA) by quantitative real-time polymerase chain reaction on ABI 7900 HT (Applied Biosystems, Foster City, CA, USA). mRNA expression was normalized relative to the expression of 18S rRNA. The results were expressed as fold induction relative to DSS control.
Colon tissue of vehicle and TRC160334 treated animals were processed for whole cell extract preparation. Proteins were separated on sodium dodecyl sulfate polyacrylamide electrophoresis followed by immunoblotting employing HSP70 antibody (SPA-810; Stressgen, Farmingdale, NY, USA) and β-actin antibody (SC47778; Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA).
Statistical analysis
Unless mentioned, results are expressed as mean ± standard error of the mean. The difference between the means of two treatment groups was assessed using Student’s t-test. One-way analysis of variance with Dunnett’s post hoc test was used for comparing more than two treatment groups. Values exceeding 95% critical limits (P<0.05) were considered to be statistically significant. Statistical analysis has been performed using statistical analysis system (SAS, version 9.1; SAS Institute Inc, Cary, NC, USA) and Prism (version 3.0; GraphPad Software Inc, La Jolla, CA, USA).
Results
Prophylactic treatment with TRC160334 is protective in TNBS-induced colitis
Improvement of DAI
Induction of colitis caused hemoccult positive soft stools along with sustained body weight loss, an indicator of disease severity, whereas no such signs were observed in control group animals. Treatment with TRC160334 significantly reduced DAI, measured at day 2 and day 4 (9.9% and 33.6%, respectively) with respect to vehicle treated animals (Figure 2A).
Amelioration of colonic damage
Treatment with TRC160334 remarkably attenuated severity and extent of colonic damage in comparison to vehicle treated animals. This improvement is also reflected by significantly less colonic damage observed with TRC160334 treatment in comparison to vehicle treated animals when assessed in the form of macroscopic damage scores and histological scores (Figure 2B and C). Histopathological analysis of vehicle treated colitis mice revealed colonic lesions ranging from lymphocytic and/or polymorphonuclear cell infiltration, edema, partial to complete loss of crypts, and erosion to ulceration. Treatment with TRC160334 resulted in a significant reduction in severity of inflammatory and necrotic changes. A few mice in the TRC160334 treatment group also demonstrated regeneration of mucosal epithelium as the reparative response (Figure 3A–C).
Amelioration of body weight loss
Rectal TNBS caused approximately 10% loss in body weight within 24 hours of instillation. Treatment with TRC160334 significantly prevented loss in body weight from day 2 until study termination in comparison to vehicle treated animals (Figure S1). Percent reduction in body weights observed on day 4 was 13.1%±1.8% for TRC160334 treated animals as compared to 21.6%±1.6% in vehicle treated animals (Figure 2D).
Improvement in survival rate
The combination of effect produced by TRC160334 by virtue of its ability to improve DAI and reduce colonic damage has resulted in a significantly better survival rate. The survival rate after treatment with TRC160334 was 42% compared to 18% in vehicle treated animals.
Therapeutic treatment with TRC160334 is protective in DSS-induced colitis
Improvement of DAI
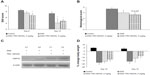
Induction of colitis with 5% DSS in drinking water resulted in a progressive increase in DAI score from day 2, reaching a maximum by day 10. The control group of animals showed no change in DAI score (score =0). In vehicle group animals receiving DSS, the score was maintained on day 12 followed by a slight reduction on day 14. Treatment with TRC160334, which was initiated from day 5, successfully prevented the increment in both the rate and extent of DAI score from as early as day 8 and this trend continued until study termination (Figures 4A and S2).
Reduction in colonic damage
All mice exposed to DSS developed colonic lesions characteristic of colitis which included loss of crypts, infiltration of inflammatory cells into the mucosa and submucosa, and erosion and ulceration of graded severity. Vehicle treated animals developed a moderate to severe degree of colitis compared to control animals (3.71±0.18 vs 0.0±0.0). Treatment with TRC160334 resulted in a dose related reduction in colitis score (Figure 4B). TRC160334 treated animals showed healthier epithelial lining when compared to vehicle treated animals. Moreover, regeneration and proliferation of basal epithelial cells as a reparative response to injury was also observed in the majority of TRC160334 treated animals (Figure 5A to D).
Induction of cytoprotective HSP70
Treatment with TRC160334 resulted in a pronounced induction of HSP70 protein in the colon when compared with the respective vehicle control (Figure 4C). Trends in reduction of mRNA expression in the proinflammatory cytokines, TNFα and INFγ, were observed after TRC160334 treatment. Additionally, elevation of expression in the anti-inflammatory cytokine, IL-10, was also observed after TRC160334 treatment (Figure S3).
Reduction in body weight loss
Animals started showing a trend in loss of body weight as early as day 1 after DSS treatment. This trend continued with vehicle treated animals until day 11. Vehicle treated animals showed some gain in body weight indicating initiation of a recovery phase from day 12 onwards (after withdrawing DSS). Treatment with TRC160334 retarded the rate of loss in body weight in comparison to vehicle treated animals in a dose dependent manner. This was evident from day 6 with the TRC160334 at 5 mg/kg/day group whereas the TRC160334 2 mg/kg/day group demonstrated this trend from day 12 (Figures 4D and S4).
Discussion
Recent advances in the pathophysiology of IBD have revealed that HIF activation may be beneficial in IBD.13,14 In the present study, we first demonstrated that prophylactic treatment with TRC160334, a novel HIF hydroxylase inhibitor, is protective in TNBS-induced colitis. Further, we demonstrated efficacy of TRC160334 in a clinically relevant therapeutic setting with the DSS-induced colitis model. The disease attenuating potential of TRC160334 in animal models of CD (TNBS-induced colitis)14,22 and UC (DSS-induced colitis)23,24 at remarkably low doses via an oral route makes TRC160334 an attractive prospective therapeutic for the treatment of IBD.
TNBS-induced colitis is a clinically relevant model of CD as it displays similar functional and anatomical microvascular abnormalities as observed in human CD.14,22 Treatment with TRC160334 results in amelioration of body weight loss, significant improvement in DAI, reduction in colon injury severity, and better survival rate in TNBS-induced colitis. However, prophylactic treatment has limited clinical applicability as in most of the cases of IBD, treatment is initiated upon clinical disease presentation and in such cases therapeutic intervention rather than a preventive treatment strategy is warranted. The therapeutic efficacy of HIF hydroxylase inhibitors in clinically relevant therapeutic settings for colitis has not been reported. Therefore, we explored the therapeutic intervention of TRC160334 in a DSS-induced colitis model.
In the present study, we demonstrated that therapeutic treatment with TRC160334 is protective in DSS-induced colitis. The DSS-induced colitis model is a clinically relevant model of UC. DSS induces intestinal barrier dysfunction, stimulating local and systemic inflammation and thus reproduces many of the histopathologic and clinical features of human colitis.23,24
Treatment with TRC160334 was initiated on day 5 of DSS induction after ascertaining that DAI had reached a value of around 50% of its maximum attainable value. We continued DSS treatment for a duration of 11 days and then kept three DSS free days in our protocol. This ensured that the trigger which leads to an acute episode of colitis is still present while the treatment is initiated and continued, thus enabling us to explore the therapeutic potential of TRC160334 in the disease model. TRC160334 treatment resulted in improvement in body weight loss and DAI, along with improvement in colon histopathology in DSS-induced colitis. This finding is the first to demonstrate that therapeutic intervention with a HIF hydroxylase inhibitor is efficacious in a colitis model.
Therapeutic treatment with TRC160334 favorably impacted inflammation in the colitis model as trends of reduction in expression of the proinflammatory cytokines, TNFα and INFγ, and elevation in the expression of the anti-inflammatory cytokine, IL-10, were observed. Treatment with TRC160334 also resulted in a significant increase in HSP70 expression which is one HIF target protein. Interventions leading to enhanced HSP70 response have been suggested as a potential approach to improve outcomes of IBD.25 HSP70 transgenic mice demonstrated significantly milder colitis in response to DSS than wild type.26 HSP70 expression is found to be significantly reduced in actively inflamed mucosa from both human IBD biopsies and IL-10−/− mice with spontaneous colitis.27 Intestinal epithelial HSP70 plays an important role in protecting mucosal integrity and function by stabilizing the tight junctions between intestinal epithelial cells. Such intestinal epithelial protection is associated with restricted bacterial translocation and a reduction in inflammation.28 Conversely, compromised expression of HSP70 renders the mucosa highly susceptible to inflammatory and immune processes, and to potentially otherwise harmless enteric flora.27
An earlier report with another HIF hydroxylase inhibitor, FG-4497, has shown a beneficial effect of HIF activation by attenuating TNBS-induced colitis.13 However, benefits upon therapeutic treatment with FG-4497 are not known. Secondly, the prophylactic efficacy of FG-4497 was shown at higher doses (in a dose range of 20 to 40 mg/kg/day) and by an intraperitoneal route of administration,13 whereas TRC160334, as reported in current study, has shown both prophylactic and therapeutic benefits in relevant animal models of CD and UC, respectively. Additionally, comparatively lower doses and ease of administration by oral route is likely to improve compliance and convenience in clinical settings.
Our findings with TRC160334, a novel HIF hydroxylase inhibitor, confirms a prophylactic effect of such inhibitors for IBD. In addition, our findings are the first to demonstrate that therapeutic intervention with a HIF hydroxylase inhibitor can also ameliorate IBD. These findings highlight the potential of TRC160334 for its clinical application in the treatment of IBD.
Acknowledgments
The authors would like to thank Mr Prakash Dhamecha and Ms Kinjal Patel for assisting in the experimental work and Mr Ajay Shivalkar for statistical analysis.
Disclosure
The authors declare that no conflict of interest exists and they are all employees of Torrent Pharmaceuticals Ltd, India. The study is supported by Torrent Pharmaceuticals Ltd, India.
References
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. | |
Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361(21):2066–2078. | |
Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):14811–11491. | |
Hindryckx P, Laukens D, De Vos M. Boosting the hypoxia-induced adaptive response in inflammatory bowel disease: a novel concept of treatment. Inflamm Bowel Dis. 2011;17(9):2019–2022. | |
Cummins EP, Doherty GA, Taylor CT. Hydroxylases as therapeutic targets in inflammatory bowel disease. Lab Invest. 2013;93(4):378–383. | |
Shah B, Mayer L. Current status of monoclonal antibody therapy for the treatment of inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6(4):607–620. | |
Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61(11):1619–1635. | |
Wilson BS, Lönnfors S, Vermeire S, et al. The True Impact of IBD: A European Crohn’s and Ulcerative Colitis Patient Life. IMPACT Survey 2010–2011. Available from: http://efcca-solutions.net/media/jointhefight/ImpactReport.pdf. Accessed June 4, 2013. | |
Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7(5):281–287. | |
Glover LE, Colgan SP. Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology. 2011;140(6):1748–1755. | |
Jamadarkhana P, Chaudhary A, Chhipa L, et al. Treatment with a novel hypoxia-inducible factor hydroxylase inhibitor (TRC160334) ameliorates ischemic acute kidney injury. Am J Nephrol. 2012;36(3):208–218. | |
Bernhardt WM, Wiesener MS, Scigalla P, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21(3):2151–2156. | |
Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134(1):145–155. | |
Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114(8):1098–1106. | |
Committee for the Purpose of Control and Supervision on Experiments on Animals. CPCSEA Guidelines for Laboratory Animal Facility. Ind J Pharmacol. 2003;35(4):257–274. | |
Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324(1):23–33. | |
Zhang DK, Yu JJ, Li YM, et al. A Picrorhiza kurroa derivative, picroliv, attenuates the development of dextran-sulfate-sodium-induced colitis in mice. Mediators Inflamm. 2012;2012:751629. | |
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. | |
Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: McGraw-Hill; 1968. | |
Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim. 2000;49(1):9–15. | |
Murano N, Murano M, Ishida K, et al. Therapeutic effect of SHI-219, a novel water soluble prodrug of EG626 (phtalazinol), on mouse dextran sodium sulfate-induced colitis. Bull Osaka Med Coll. 2006;52(2):69–79. | |
Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19(1):51–62. | |
Dieleman LA, Palmen MJ, Akol H, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114(3):385–391. | |
Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. | |
Xue H, Sufit AJ, Wischmeyer PE. Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr. 2011;35(2):188–197. | |
Hu S, Zhu X, Triggs JR, et al. Inflammation-induced, 3′UTR-dependent translational inhibition of Hsp70 mRNA impairs intestinal homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1003–G1011. | |
Hu S, Ciancio MJ, Lahav M, et al. Translational inhibition of colonic epithelial heat shock proteins by IFN-gamma and TNF-alpha in intestinal inflammation. Gastroenterology. 2007;133(6):1893–1904. | |
Liedel JL, Guo Y, Yu Y, et al. Mother’s milk-induced Hsp70 expression preserves intestinal epithelial barrier function in an immature rat pup model. Pediatr Res. 2011;69(5 Pt 1):395–400. |
Supplementary figures
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.