Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
Therapeutic Targets for Ankylosing Spondylitis – Recent Insights and Future Prospects
Authors Perrotta FM, Scriffignano S, Ciccia F, Lubrano E
Received 6 January 2022
Accepted for publication 7 April 2022
Published 19 April 2022 Volume 2022:14 Pages 57—66
DOI https://doi.org/10.2147/OARRR.S295033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Fabio Massimo Perrotta,1 Silvia Scriffignano,2 Francesco Ciccia,2 Ennio Lubrano1
1Academic Rheumatology Unit, Department of Medicine and Health Sciences “Vincenzo Tiberio”, University of Molise, Campobasso, Italy; 2Department of Precision Medicine, University of Campania “Luigi Vanvitelli”, Caserta, Italy
Correspondence: Ennio Lubrano, Academic Rheumatology Unit, Department of Medicine and Health Sciences “Vincenzo Tiberio”, University of Molise, Campobasso, Italy, Tel/Fax +39 0874 404745, Email [email protected]
Abstract: Ankylosing spondylitis (AS) is a chronic inflammatory rheumatic disease belonging to the axial spondyloarthritis (axSpA), a group of diseases that affects the axial skeleton and causes severe pain and disability. An early diagnosis and appropriate treatment can reduce the severity of the disease and the risk of progression. TNF-α inhibitors demonstrated efficacy and effectiveness in axSpA patients by reducing disease activity, minimizing inflammation and improving the quality of life. More recently, new insights in pathogenesis of axSpA, including the discovery of the role of IL-23/IL-17 axis and intracellular pathways, led to the development of new biologics and small molecules that improve our therapeutic armamentarium. New alternatives are also being soon available. The aim of this paper is to narratively review the recent insights and future prospects in the treatment of AS and, more in general, axSpA.
Keywords: axial spondyloarthritis, ankylosing spondylitis, treatment, biologic drugs, JAK inhibitors
Introduction
Spondyloarthritis (SpA) represents a group of common chronic inflammatory diseases that share genetic, immunopathological, clinical, laboratory, and radiological features. Among SpA group, axial SpA (axSpA) is characterized by inflammation at sacroiliac joints and spine, with the possible presence of peripheral and extra-articular manifestations.1 In 2009, the Assessment of SpondyloArthritis international Society (ASAS) proposed and validated classification criteria for axSpA2 which included both non-radiographic axSpA (nr-axSpA) and radiographic axSpA. This latter entity resembles the classical Ankylosing Spondylitis (AS), in which the ankylosis in sacroiliac joints, in the final stages of disease, and the presence of new bone formation at spine, are the main features.2 The pharmacological treatment of axSpA has been revolutionized since the introduction of biologic drugs. Anti-Tumor Necrosis Factor (TNF) drugs were the first biologics approved for AS and, more recently, for the whole group of axSpA, showing efficacy and effectiveness in the reduction of spinal inflammation, pain and in the improvement of function and quality of life.3–5
The possibility to achieve a status of low disease activity and remission as a treatment target in axSpA became possible in most of the patients with the implementation of anti-TNF therapy.6–8
Furthermore, data coming from randomized controlled trials on certolizumab and golimumab seem to suggest a reduction in the progression of spinal damage.9,10 However, unmet clinical needs still persist for those patients which are intolerant or non-responder to anti-TNF treatment, who may experience chronic pain, loss of mobility and a severe reduction of quality of life,11 in which non-pharmacological approach and switching in the same drug class has been implemented.12,13
More recently, a deeper understanding of SpA pathogenesis with the discovery of the key role of Interleukin (IL)-23/IL-17 axis in the development of inflammation at entheseal site and at other district led to the development of new biologics, with the aim to further increase our therapeutic precision. Moreover, the discovery of key intracellular pathways leading to the lymphocyte activation and cytokines production provided the biological basis for the development of Janus Kinase (JAK) inhibitors, which will improve our therapeutic armamentarium.14–16
The aim of this narrative review was to discuss the novel therapeutic targets for AS and axSpA with a focus on the future possible treatment strategies.
Anti-IL-17 Drugs
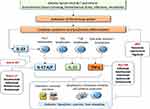
IL-23/IL-17 pathway plays a pivotal role in the pathogenesis of axSpA. IL-23 is the main cytokine involved in the activation of Th17-type immune response, that is one of the major immunological axes expressed in axSpA patients. Th17 cells are present in AS patients, and are the main source of IL-17 production that has been shown to drive synovial and entheseal inflammation and joint remodeling in both animal models and clinical studies.17 Moreover, recently, elegant studies demonstrated the presence of tissue resident populations of γδT-cells in enthesis that have transcript expression related to tissue repair and immunomodulation, and identifies a subset (Vδ1) able to produce IL-17 independently of IL-23 stimulation18 (Figure 1). On this basis, inhibition of this key cytokine has been studied in axSpA and different anti-IL-17 agents were developed or are currently in Phase II and III trials for the treatment of rheumatic and inflammatory diseases.
Secukinumab
Secukinumab is a monoclonal antibody that targets free IL-17A. Anti-IL-17A secukinumab was evaluated in phase II and III trials in patients with AS and nr-axSpA in the MEASURE 1–2–3 and PREVENT trials programme. In MEASURE 1 study, a total of 371 AS patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 819 while in the MEASURE 2 study 219 patients with AS received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline, at weeks 1, 2, and 3 and then every 4 weeks starting at week 4.20
In the MEASURE 3, the patients were randomized to receive secukinumab at 150 or 300 mg or placebo and followed up for 3 years.21
In the MEASURE 1 study, ASAS20 response at 16 weeks was seen in 76 (61%), 74 (60%), and 35 (29%) patients for secukinumab at doses of 150 mg, 75 mg and for placebo, respectively (P < 0.001 for both comparisons with placebo).19 In MEASURE 2 study ASAS20 response was achieved by 44 (61%), 30 (41%), and 21 (28%) patients for subcutaneous secukinumab at doses of 150 mg, 75 mg and for placebo, respectively (P < 0.001 for the 150-mg dose and P = 0.10 for the 75-mg dose).20 In the MEASURE 3, ASAS20 response rates at week 52 was 68.4% (52/76 patients) and 58.1% (43/74 patients) with secukinumab 300 mg and 150 mg respectively, and ASAS40 was 53.9% and 40.5%, respectively.21 Data from MEASURE 3 demonstrated the efficacy of secukinumab 300 mg in reducing disease activity: in the overall population, ASAS partial remission was achieved by 21.1% of patients receiving secukinumab 300 mg, compared with 9.5% of patients receiving secukinumab 150 mg at week 16. An increased response in the 300 versus 150 mg arm was observed through 3 years (28.3% versus 15.9%). These data support the current recommendation for dose escalation from secukinumab 150–300 mg in patients with axSpA who demonstrate an inadequate clinical response with the lower dose.21,22
Secukinumab also showed efficacy in reducing Magnetic Resonance Imaging (MRI) bone marrow oedema at sacroiliac joints in AS patients and data coming from MEASURE 1 demonstrated a possible effect on radiographic progression, with approximately 80% of patients that received secukinumab showing no radiographic progression assessed with the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS change) from baseline to week 104. Furthermore, in the same time period, new syndesmophytes were found in only 5% of patients who were without syndesmophytes.23,24
Secukinumab also showed long-term benefits with sustained efficacy thought 5-year period25 and, moreover, it provides rapid and sustained relief of pain and fatigue over 2 years regardless the baseline C-Reactive Protein (CRP) levels and prior TNF inhibitors therapy.26 Finally, post-hoc analysis of randomized trials confirmed the efficacy of secukinumab in AS patients over different domains including pain, fatigue, spinal stiffness, articular function, laboratory serum markers of inflammation, work productivity and health-related quality of life (reviewed in).27
The PREVENT study assessed the efficacy and safety of secukinumab 150 mg in respect to placebo in nr-axSpA patients with sacroiliitis at MRI. ASAS40 was significantly higher in both group of patients receiving and not receiving a loading dose at week 16 and week 52, vs placebo.28 In the PREVENT sub-analysis the highest treatment differences between secukinumab and placebo were observed in patients with both elevated CRP and evidence of sacroiliitis on MRI. Of note, the difference in the efficacy between HLA-B27 positive and negative subgroups was very low. Male patients had higher relative responses than female patients.29
Ixekizumab
Ixekizumab is a IgG4 monoclonal antibody against IL-17A that was recently approved for the treatment of AS. The study program COAST-V, COAST-W and COAST-X assessed the efficacy and safety of ixekizumab in both AS and nr-axSpA.
In the COAST-V study, 341 patients with AS and inadequate response or intolerance to non-steroidal anti-inflammatory drugs (NSAIDs) were randomly assigned to 80 mg subcutaneous ixekizumab every two (Q2W) or four (Q4W) weeks, 40 mg adalimumab Q2W (active reference arm), or placebo. The primary endpoint, ASAS40 response at week 16, was achieved by more patients receiving ixekizumab Q2W (N = 43, 52%, p<0.0001) and ixekizumab Q4W (N=39, 48%, p < 0.0001) than by those receiving placebo (N = 16, 18%). A numerically higher proportion of patients treated with ixekizumab achieved the ASAS40 response in respect to the active comparator adalimumab. Furthermore, 43% and 42% of AS patients treated respectively with ixekizumab Q2W and Q4W achieved the low disease activity defined as Ankylosing Spondylitis Disease Activity Score (ASDAS) <2.1. Both treatment regimens showed efficacy in the reduction of bone marrow oedema at spine and sacroiliac joints.30
Infections occurred at similar frequencies across all active treatment groups. There were no cases of opportunistic infection nor reactivation of latent tuberculosis in any treatment group. Moreover, no malignancies were reported. Injection site reactions were common and reported in about 5% of patients in the placebo group, in 13% of patients in the ixekizumab Q2W group, 4% of patients in the ixekizumab Q4W group, and 8% of patients in the adalimumab group.30
The COAST-W is a Phase III randomized, double‐blind, placebo‐controlled trial that assessed the efficacy and safety of ixekizumab in adult patients with an inadequate response to, or intolerance, at 1 or 2 TNF inhibitors and an established diagnosis of radiographic axSpA. Patients were treated with subcutaneous ixekizumab 80 mg Q2W, ixekizumab 80 mg Q4W, or matched placebo, with analysis of data at week 16. ASAS40 response was significantly higher among patients treated with ixekizumab Q2W (N = 30, 30.6%; P = 0.003) or Q4W (N = 29, 25.4%; P = 0.017) than among patients treated with placebo (N = 13, 12.5%), with a significant response observed as early as week 1 for both ixekizumab arms. No safety issues emerged from the study.31
The COAST-X was a 52-week, randomised, double-blind, placebo-controlled study in patients with nr-axSpA and objective signs of inflammation (assessed with MRI or CRP) naïve to biologic Disease Modifying Anti-Rheumatic Drugs (bDMARDs). Patients (N = 303) were randomly assigned to receive subcutaneous 80 mg ixekizumab Q4W or Q2W, or placebo. In this study, the efficacy of both regimens of ixekizumab was demonstrated at week 16, in which 34/96 (35%) of patients in the ixekizumab Q4W group (p = 0.0094 vs placebo), 41/102 (40%) in the ixekizumab Q2W group (p = 0.0016 vs placebo), and 20/105 (19%) of patients in the placebo group achieved the primary end point ASAS40. At week 52, 30% of patients in the ixekizumab Q4W group, 31% of patients in the ixekizumab Q2W group and 13% of patients in the placebo group achieved ASAS40 (p < 0.05 for all comparisons vs placebo). A significantly higher ASAS40 response rate was seen as early as week 1, demonstrating the rapid efficacy of both regimens in the treatment of disease symptoms and signs.32 Significant improvement of function and quality of life was also demonstrated in a post-hoc study.33
Finally, data coming from COAST-Y, in which axSpA patients that achieved remission were randomly assigned to continue ixekizumab Q4W, ixekizumab Q2W or withdraw to placebo, showed that patients who continued treatment with ixekizumab were significantly less likely to flare and had significantly delayed time-to-flare compared with patients who withdrew to placebo.34
Treatment with IL-17A inhibitors demonstrated reduction in inflammation as measured by MRI using the Spondyloarthritis Research Consortium of Canada Magnetic Resonance Imaging Index (SPARCC) and data from both MEASURE and COAST studies showed no or little progression of radiographic damage over 2 years, assessed with mSASSS, in up to 80% of patients treated with IL-17A inhibitors.24,35 However, no trials have tested this outcome versus a comparator group (either placebo or active drug). On the other hand, radiographic progression in axSpA is a slow process and it may be difficult to assess this outcome in respect to placebo. It would be more suitable to test the radiographic progression in respect to active drugs in future studies.
Bimekizumab
Bimekizumab is a monoclonal antibody which selectively inhibits both IL-17A and IL-17F. Bispecific antibodies target two different cytokines simultaneously, potentially offering a better disease control, considering that not only IL-17A but also IL-17F is increased in SpA.
In patients with active AS, the efficacy and safety of bimekizumab in different treatment regimens [16 mg (61 patients), 64 mg (61 patients), 160 mg (60 patients), 320 mg (61 patients) and placebo (60 patients)] was recently assessed in a phase IIb study.36
In this study, ASAS40, at week 12, was significantly higher in all bimekizumab-treated groups (29.5%, 42.6%, 46.7% and 45.9%, respectively, for bimekizumab 16 mg, 64 mg, 160 mg and 320 mg) compared with placebo (13.3%; p < 0.05 for all comparisons).36 Furthermore, bimekizumab demonstrated efficacy in the reduction of disease activity and in the achievement of ASDAS major improvement. Rapid reduction of CRP and bone marrow oedema at MRI were also observed in the different bimekizumab treatment regimens. Overall, bimekizumab was generally well tolerated. The safety profile in this study was consistent with that reported for other treatments targeting the IL-17 pathway, with 16 cases (5%) of oral candidiasis were reported in bimekizumab-treated patients after 48 weeks. Generally, safety profile was similar to that reported for phase III studies in psoriasis and psoriatic arthritis.20,36,37
Brodalumab
Brodalumab is a fully human anti-IL-17 receptor A monoclonal antibody. The efficacy and safety of brodalumab in patients with axSpA were evaluated in a multicentre, placebo-controlled Phase 3 study. Patients were randomised to receive subcutaneous brodalumab 210 mg (n = 80) or placebo (n = 79) during a 16-week double-blind period. The primary endpoint, ASAS40 response at week 16 was achieved in 35 patients with axSpA (43.8%) in the brodalumab group vs 19 patients (24.1%) in the placebo group. ASAS40 response was slightly higher among anti-TNF- naïve patients, in which was 45.3%, compared with 37.5% for anti-TNF-experienced patients at week 16. The ASAS40 response rate at week 16 was also higher in patients with HLA-B27 positivity than in those with HLA-B27 negativity.38
Among the group of patients classified as having nr-axSpA, ASAS40 response at week 16 was achieved by 35.3% of brodalumab-treated patients.38 Over 16 weeks, adverse events were reported in 55% of patients in the brodalumab group and in 57% of patients in the placebo group. No deaths were reported. In the brodalumab group, infections and infestations, gastrointestinal disorders and investigations were the most frequent adverse events reported. Previous studies on psoriasis reported suicidal ideation or suicidal behaviour in some patients treated with brodalumab and there is a boxed warning for brodalumab regarding suicidal ideation and behaviour in United States. However, no safety concerns emerging after 3 years of pharmacovigilance monitoring on patients with psoriasis treated with brodalumab39 and there were no suicidal ideation or suicidal behaviour in clinical trials for axSpA.38
JAK Inhibitors
JAKs are a family of non-receptor protein tyrosine kinases with four members: JAK1, JAK2, JAK3, and Tyrosine kinase 2 (Tyk2). Distinct combinations of homodimers of JAK proteins selectively associate with different cytokine receptors. Activation of JAK, after the interaction between cytokine and its receptor, lead to the phosphorylation of the signal transducer and activator of transcription (STAT) family proteins. Dimers of STATs translocate to the nucleus, where they regulate the expression of cytokine-responsive genes.40 Thus, the inhibition of each JAK member may impact on the signal transduction of different cytokines. Thus, JAK proteins are potential target for the treatment of several inflammatory diseases. Different cytokines signal using pairings of individual JAKs. IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 signal through the JAK1/JAK3 combination and have a pivotal role in the modulation of adaptive immune functions, including Th cell differentiation.41 In particular, in the pathogenesis of SpA, innate lymphoid cells seem to play a pivotal role and are dependent from IL-7 signalling.42 IFNγ and IL-12 are also dependent from JAK1/JAK2 and JAK2/TYK2 combinations and are critical for Th1 cell response which modulate the production of TNFα by macrophages.43 Importantly, given the role of the IL-23/IL-17 axis in SpA, JAKs influence signalling for several key cytokines involved in this pathway. In fact, JAK2/TYK2 combination is essential for the signalling of IL-23 produced by activated dendritic cells. As well as direct blockade of IL-23 signalling, an indirect consequence of JAK inhibition is downstream blockade of IL-17 production. Furthermore, signalling for IL-22 is mediated by the JAK1/TYK2 pairing.40
Different phase II and III studies demonstrated efficacy of JAK inhibitors including tofacitinib, upadacitinib, and filgotinib for the treatment of active AS despite treatment with NSAIDs. Improvements of multiple measures including ASAS20/40 response, pain and quality of life were seen in these studies.
Tofacitinib
Tofacitinib inhibits JAK1 and JAK3 and to a lesser extent, JAK2. Data from the phase 3 study of tofacitinib for the treatment of AS were recently published. In this study, adult patients with active AS were enrolled and randomised 1:1 to receive tofacitinib 5 mg two times per day, or placebo, for 16 weeks and then followed up (open-label phase) until week 48. In this study, the majority of the population was bDMARD-naïve, but approximately 20% had prior bDMARD use.44 The ASAS20 response rate was significantly (p < 0.0001) greater with tofacitinib (75 out 133; 56.4%) versus placebo (40 out 136; 29.4%) at week 16. Similarly, the ASAS40 response rate was significantly (p < 0.0001) greater with tofacitinib (40.6%) versus placebo (12.5%) at week 16. ASAS partial remission criteria and ASDAS inactive disease rate were significantly higher in the tofacitinib group in respect to placebo at week 16. Efficacy of tofacitinib was sustained up to the end of the open-label phase at week 48.44
Upadacitinib
Upadacitinib is selective JAK1 inhibitor, approved for the treatment of rheumatoid arthritis and recently studied in the SELECT-AXIS 1 clinical phase III trial in AS patients with inadequate response/intolerance or contraindication to NSAIDs. Patients were randomly assigned 1:1 to oral upadacitinib 15 mg once daily or oral placebo for the 14 weeks. Significantly more patients had an ASAS40 response in the upadacitinib group versus in the placebo group at week 14 [48 (52%) of 93 patients vs 24 (26%) of 94 patients; p = 0.0003); treatment difference 26% (95% CI; 13–40)]. Upadacitinib 15 mg once daily also showed sustained and consistent efficacy over 1 year.44 Upadacitinib 15 mg also showed efficacy in reducing the extent of inflammation at both spine and sacroiliac joints at week 14.45 No safety issue emerged, with no serious infections, herpes zoster, malignancy, venous thromboembolic events, or deaths reported during the study period.46 The SELECT-AXIS 2 is currently ongoing and is aimed to evaluate the efficacy and safety of upadacitinib in nr-axSpA.
Filgotinib
Filgotinib is a JAK1 selective inhibitor recently approved for the treatment of rheumatoid arthritis. A phase II study (TORTUGA) was performed to evaluate the efficacy and safety of filgotinib in active AS patients non responder to NSAIDs. Patients were randomly assigned (1:1) to receive filgotinib 200 mg or placebo orally once daily for 12 weeks. The mean ASDAS change from baseline to week 12 was −1.47 in the filgotinib group and −0.57 in the placebo group (p < 0.0001). At week 12, an ASAS20 response was achieved by 44 (76%) of 58 patients treated with filgotinib and by 23 (40%) of 58 patients that received the placebo (p < 0.0001). ASAS40 was achieved by 22 (38%) patients assigned to filgotinib and by 11 (19%) patients assigned to placebo (p = 0.019). There was a non-serious, grade 2 deep vein thrombosis in the calf musculature of a man aged 53 years who had a heterozygous factor V Leiden mutation. There were no malignancies (including lymphomas), opportunistic infections, cases of active tuberculosis, extra-articular manifestations (inflammatory bowel disease, psoriasis, or uveitis), or deaths reported in the study. Reports of any infection did not differ significantly between the groups.47 Table 1 summarizes the main clinical trial of new biologic and JAKi in the treatment of axSpA.
 |
Table 1 Main Clinical Trials of the New Biologic and Small Molecules for the Treatment of Both Ankylosing Spondylitis (AS) and Non-Radiographic Axial Spondyloarthritis (Nr-axSpA) |
The available evidence from a range of studies (mainly coming from rheumatoid arthritis and Inflammatory bowel diseases) suggests that JAK inhibitors are associated with an increased risk of thrombosis, but the risk is low and mostly confined to patients with additional risk factors. More data dealing with the new JAK inhibitors are needed to better assess the risk in axSpA patients. Malignancy is one of the major concerns for patients undergoing treatment with JAK inhibitors.48 A recently published meta-analysis which reviewed safety data from both interventional and observational studies of tofacitinib, filgotinib, upadacitinib, and baricitinib conducted in patients with different chronic inflammatory diseases found no increase in the incidence of malignancy in patients treated with JAK inhibitors in the controlled periods.49 Long-term safety data of tofacitinib for up to 9.5 years collected from rheumatoid arthritis patients showed a comparable incidence rate of malignancy in respect to patients treated with anti-TNF or other biologic DMARDs.50 However, recently, the post-marketing safety study raised some safety issues regarding this topic.51 In particular, a recent published safety trial on tofacitinib in rheumatoid arthritis showed some important results. In this trial comparing the combined tofacitinib doses with a TNF inhibitor in a cardiovascular risk-enriched population, major cardiovascular events (MACE) and cancers were higher with tofacitinib and did not meet non-inferiority criteria (3.4% and 4.2% of patients, respectively) than with a TNF inhibitor (2.5% and 2.9% of patients). The hazard ratios were 1.33 (95% confidence interval [CI], 0.91 to 1.94) for MACE and 1.48 (95% CI, 1.04 to 2.09) for cancers. Several other adverse events were more common with tofacitinib.52
Finally, concerns have been raised regarding the risk of venous thromboembolism (VTE) with JAK inhibitor therapy. In 2017, the Food and Drug Administration (FDA) added a black box warning to the Summary of Product Characteristics (SPC) for baricitinib, stating that it should be used with caution in patients at increased risk for VTE.3 This was followed by a similar warning from the FDA and the European Medicines Agency (EMA) in 2019 for tofacitinib 10 mg prescribed twice daily for the treatment of ulcerative colitis (UC). However, a recent meta-analysis of RCTs on JAK inhibitors does not provide evidence that support the current warnings of VTE risk for JAK inhibitors.53 Long-term studies and data from registries in axSpA should be taken.
Other Drugs with Different Mechanism of Action
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is an important cytokine with pleiotropic effect on myeloid cells including monocytes, macrophages, and dendritic cells. GM-CSF directly promotes the proliferation of different immune cells and also mediates their differentiation into a more inflammatory phenotype.
Data coming from animal models and clinical studies on rheumatoid arthritis showed that the inhibition of GM-CSF with monoclonal antibodies leads to a significantly reduction of pain and improvement in joint inflammation. Moreover, other evidences showed that overexpression of GM-CSF and its receptor have been found in the joints of axSpA patients where it could promote joint damage inducing the differentiation of granulocytes and macrophages into a more inflammatory phenotype.15,54,55 Thus, inhibition of GM-CSF could be a promising future treatment for patients with axSpA.
Namilumab
Namilumab is a human monoclonal antibody targeting GM-CSF. A Phase IIa proof of concept trial (NAMASTE) is ongoing to evaluate the efficacy of namilumab in axSpA patients.56 Forty-two paIJGM_A_363679tients have been randomized to receive the drug or placebo and the primary endpoint of ASAS20 is being assessed at week 12.56
In recent years, different drugs have been investigated for the treatment of axSpA; however, many of these failed to show a significant efficacy in the treatment of disease. Among the different drug classes, the anti-IL12/23 inhibitors ustekinumab and risankizumab, the T cell co-stimulation inhibitor abatacept, the IL-6 receptor inhibitors sarilumab and tocilizumab, the IL-1 receptor antagonist anakinra, the anti-CD20 antibody rituximab and the phosphodiesterase-4 inhibitor apremilast, were evaluated in clinical studies, but did not show sufficient efficacy in the treatment of axial disease.15 To date, no data were published on the efficacy of IL-23 inhibitor guselkumab in patients with axSpA, although promising results coming from studies on psoriatic arthritis patients with axial involvement in which a significantly reduction in disease activity measure was found in respect to placebo group.57
Conclusions
AxSpA are a group of chronic inflammatory diseases which can lead to disability and poor quality of life and increase the risk of cardiovascular diseases.58 Recent insights in the pathogenesis of axial inflammation through the discovery of key cytokines network allow to the development of new treatment strategies aimed to interfere to specific molecular targets and intracellular pathways. Beyond the inhibition of TNF, IL-17 inhibitors16 and JAK inhibitors showed efficacy and safety in the reduction of pain, stiffness, in the improvement of sacroiliac and spinal inflammation and in the improvement of patients reported outcomes, articular function and quality of life. Furthermore, together with non-pharmacological approaches59 new treatment strategies are currently on study with the aim to improve our therapeutic armamentarium. In terms of clinical practice, the access to a wide range of treatment options could allow, in the near future, to a more precise approach in management of patients and a more tailored treatment for each patient.
Disclosure
The authors declare that they have no conflicts of interest related to the work.
References
1. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73–84. doi:10.1016/S0140-6736(16)31591-4
2. Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–883. doi:10.1136/ard.2009.108233
3. Tański W, Świątoniowska-lonc N, Dudek K, Jankowska-Polańska B. Benefit of biological drugs for quality of life in patients with ankylosing spondylitis: a systematic review and meta-analysis of clinical trials. Adv Exp Med Biol. 2021;1335:63–78.
4. Li KJ, Jois R, Lichauco JJ, et al. A review on the effect of tumor necrosis factor inhibitors on structural progression in early axial spondyloarthritis using magnetic resonance imaging. Rheumatol Ther. 2019;6:139–163. doi:10.1007/s40744-019-0141-y
5. Rodrigues-Manica S, Silva J, Cruz-Machado R, et al. Biologic disease-modifying anti-rheumatic drugs and patient-reported outcomes in axial SpA: a systematic review and a call for action. Clin Rheumatol. 2021;40(1):33–41. doi:10.1007/s10067-020-05209-x
6. Lubrano E, Perrotta FM, Manara M, et al. The sex influence on response to tumor necrosis factor-α inhibitors and remission in axial spondyloarthritis. J Rheumatol. 2018;45(2):195–201. doi:10.3899/jrheum.17666
7. Lubrano E, Perrotta FM, Marchesoni A, et al. Remission in nonradiographic axial spondyloarthritis treated with anti-tumor necrosis factor-α drugs: an Italian multicenter study. J Rheumatol. 2015;42(2):258–263. doi:10.3899/jrheum.140811
8. Spadaro A, Lubrano E, Marchesoni A, et al. Remission in ankylosing spondylitis treated with anti-TNF-α drugs: a national multicentre study. Rheumatology. 2013;52(10):1914–1919. doi:10.1093/rheumatology/ket249
9. van der Heijde D, Baraliakos X, Hermann KA, et al. Limited radiographic progression and sustained reductions in MRI inflammation in patients with axial spondyloarthritis: 4-year imaging outcomes from the RAPID-axSpA phase III randomised trial. Ann Rheum Dis. 2018;77(5):699–705. doi:10.1136/annrheumdis-2017-212377
10. Braun J, Baraliakos X, Hermann KG, et al. The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial. Ann Rheum Dis. 2014;73(6):1107–1113. doi:10.1136/annrheumdis-2012-203075
11. Lubrano E, De Socio A, Perrotta FM. Unmet needs in axial Spondyloarthritis. Clin Rev Allergy Immunol. 2018;55(3):332–339. doi:10.1007/s12016-017-8637-0
12. Spadaro A, Punzi L, Marchesoni A, et al. Switching from infliximab or etanercept to Adalimumab in resistant or intolerant patients with spondyloarthritis: a 4-year study. Rheumatology. 2010;49(6):1107–1111. doi:10.1093/rheumatology/keq008
13. Lubrano E, Spadaro A, Amato G, et al. Tumour necrosis factor alpha inhibitor therapy and rehabilitation for the treatment of ankylosing spondylitis: a systematic review. Semin Arthritis Rheum. 2015;44(5):542–550. doi:10.1016/j.semarthrit.2014.09.012
14. Ceribelli A, Motta F, Vecellio M, Isailovic N, Ciccia F, Selmi C. Clinical trials supporting the role of the IL-17/IL-23 axis in axial spondyloarthritis. Front Immunol. 2021;12:622770. doi:10.3389/fimmu.2021.622770
15. Tahir H, Byravan S, Fardanesh A, Moorthy A. Promising treatment options for axial spondyloarthritis: an overview of experimental pharmacological agents. J Exp Pharmacol. 2021;13:627–635. doi:10.2147/JEP.S262340
16. Lubrano E, Perrotta FM. Secukinumab for ankylosing spondylitis and psoriatic arthritis. Ther Clin Risk Manag. 2016;12:1587–1592. doi:10.2147/TCRM.S100091
17. Sieper J, Poddubnyy D, Miossec P. The IL-23-IL-17 pathway as a therapeutic target in axial spondyloarthritis. Nat Rev Rheumatol. 2019;15(12):747–757. doi:10.1038/s41584-019-0294-7
18. Cuthbert RJ, Watad A, Fragkakis EM, et al. Evidence that tissue resident human enthesis γδT-cells can produce IL-17A independently of IL-23R transcript expression. Ann Rheum Dis. 2019;78(11):1559–1565. doi:10.1136/annrheumdis-2019-215210
19. Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9906):1705–1713. doi:10.1016/S0140-6736(13)61134-4
20. Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. doi:10.1056/NEJMoa1505066
21. Pavelka K, Kivitz A, Dokoupilova E, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19(1):285. doi:10.1186/s13075-017-1490-y
22. Pavelka K, Kivitz AJ, Dokoupilova E, et al. Secukinumab 150/300 mg provides sustained improvements in the signs and symptoms of active ankylosing spondylitis: 3-year results from the phase 3 MEASURE 3 study. ACR Open Rheumatol. 2020;2:119–127. doi:10.1002/acr2.11102
23. Braun J, Baraliakos X, Deodhar A, et al. Secukinumab shows sustained efficacy and low structural progression in ankylosing spondylitis: 4-year results from the MEASURE 1 study. Rheumatology. 2019;58:859–868. doi:10.1093/rheumatology/key375
24. Braun J, Baraliakos X, Deodhar A, et al.; MEASURE 1 study group. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 76;2017:1070–1077. doi:10.1136/annrheumdis-2016-209730
25. Baraliakos X, Braun J, Deodhar A, et al. Long-term efficacy and safety of secukinumab 150 mg in ankylosing spondylitis: 5-year results from the phase III MEASURE 1 extension study. RMD Open. 2019;5(2):e001005. doi:10.1136/rmdopen-2019-001005
26. Deodhar A, Conaghan PG, Kvien TK, et al.; MEASURE 2 study group. Secukinumab provides rapid and persistent relief in pain and fatigue symptoms in patients with ankylosing spondylitis irrespective of baseline C-reactive protein levels or prior tumour necrosis factor inhibitor therapy: 2-year data from the MEASURE 2 study. Clin Exp Rheumatol. 2019;37(2):260–269.
27. Braun J, Kiltz U, Bühring B, Baraliakos X. Secukinumab in axial spondyloarthritis: a narrative review of clinical evidence. Ther Adv Musculoskelet Dis. 2021;13:1759720X211041854. doi:10.1177/1759720X211041854
28. Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73(1):110–120. doi:10.1002/art.41477
29. Braun J, Blanco R, Marzo-Ortega H, et al. Secukinumab in non-radiographic axial spondyloarthritis: subgroup analysis based on key baseline characteristics from a randomized phase III study, PREVENT. Arthritis Res Ther. 2021;23(1):231. doi:10.1186/s13075-021-02613-9
30. van der Heijde D, Cheng-Chung Wei J, Dougados M, et al.; COAST-V study group. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet. 2018;392(10163):2441–2451. doi:10.1016/S0140-6736(18)31946-9
31. Deodhar A, Poddubnyy D, Pacheco-Tena C, et al.; COAST-W Study Group. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 2019;71(4):599–611. doi:10.1002/art.40753
32. Deodhar A, van der Heijde D, Gensler LS, et al.; COAST-X Study Group. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395(10217):53–64. doi:10.1016/S0140-6736(19)32971-X
33. Walsh JA, Magrey MN, Baraliakos X, et al. Ixekizumab improves functioning and health in the treatment of active non-radiographic axial spondyloarthritis: 52-week results, COAST-X trial. Arthritis Care Res. 2020. doi:10.1002/acr.24482
34. Landewé RB, Gensler LS, Poddubnyy D, et al. Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y). Ann Rheum Dis. 2021;80(8):1022–1030. doi:10.1136/annrheumdis-2020-219717
35. van der Heijde D, Østergaard M, Reveille JD, et al. Spinal radiographic progression and predictors of progression in patients with radiographic axial spondyloarthritis receiving ixekizumab over 2 years. J Rheumatol. 2021. doi:10.3899/jrheum.210471
36. van der Heijde D, Gensler LS, Deodhar A, et al. Dual neutralisation of interleukin-17A and interleukin-17F with bimekizumab in patients with active ankylosing spondylitis: results from a 48-week phase IIb, randomised, double-blind, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2020;79(5):595–604. doi:10.1136/annrheumdis-2020-216980
37. Schreiber S, Colombel J-F, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78:473–479. doi:10.1136/annrheumdis-2018-214273
38. Wei JC, Kim TH, Kishimoto M, Ogusu N, Jeong H, Kobayashi S; 4827-006 study group. Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann Rheum Dis. 2021;80(8):1014–1021. doi:10.1136/annrheumdis-2020-219406
39. Lebwohl M, Leonardi C, Armstrong A, et al. Three-year U.S. pharmacovigilance report of brodalumab. Dermatol Ther. 2021;34(6):e15105. doi:10.1111/dth.15105
40. Veale DJ, McGonagle D, McInnes IB, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology. 2019;58(2):197–205. doi:10.1093/rheumatology/key070
41. Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi:10.1111/j.1600-065X.2008.00754.x
42. Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17:758–764. doi:10.1038/ni.3482
43. Langrish CL, McKenzie BS, Wilson NJ, et al. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi:10.1111/j.0105-2896.2004.00214.x
44. Deodhar A, Sliwinska-Stanczyk P, Xu H, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80(8):1004–1013. doi:10.1136/annrheumdis-2020-219601
45. van der Heijde D, Song IH, Pangan AL, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, Phase 2/3 trial. Lancet. 2019;394(10214):2108–2117. doi:10.1016/S0140-6736(19)32534-6
46. Deodhar A, van der Heijde D, Sieper J, et al. Safety and efficacy of upadacitinib in patients with active ankylosing spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: one-year results of a double-blind, placebo-controlled study and open-label extension. Arthritis Rheumatol. 2021. doi:10.1002/art.41911
47. van der Heijde D, Baraliakos X, Gensler LS, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10162):2378–2387. doi:10.1016/S0140-6736(18)32463-2
48. Akkoc N, Khan MA. JAK inhibitors for axial spondyloarthritis: what does the future hold? Curr Rheumatol Rep. 2021;23(6):34. doi:10.1007/s11926-021-01001-1
49. Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–1573. doi:10.1053/j.gastro.2020.01.001
50. Wollenhaupt J, Lee EB, Curtis JR, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89. doi:10.1186/s13075-019-1866-2
51. Pfizer shares co-primary endpoint results from post-marketing required safety study of XELJANZ® (tofacitinib) in subjects with rheumatoid arthritis (RA). Available from: https://www.pfizer.com/news/pressrelease/press-release-detail/pfizer-shares-co-primary-endpointresults-post-marketing.
52. Ytterberg SR, Bhatt DL, Mikuls TR, et al.; ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi:10.1056/NEJMoa2109927
53. Yates M, Mootoo A, Adas M, et al. Venous thromboembolism risk with JAK inhibitors: a meta-analysis. Arthritis Rheumatol. 2021;73(5):779–788. doi:10.1002/art.41580
54. Hamilton JA. GM-CSF as a target in inflammatory/autoimmune disease: current evidence and future therapeutic potential. Expert Rev Clin Immunol. 2015;11(4):457–465. doi:10.1586/1744666X.2015.1024110
55. Taylor PC, Saurigny D, Vencovsky J, et al.; NEXUS Study Group. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: a randomized, controlled trial. Arthritis Res Ther. 2019;21(1):101. doi:10.1186/s13075-019-1879-x
56. Izana Bioscience Ltd. Efficacy and safety of namilumab for moderate-to-severe axial spondyloarthritis (NAMASTE); 2021. Available from: https://www.clinicaltrials.gov/ct2/show/study/.
57. Mease P, Helliwell P, Gladman D, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet. 2021;3(10):715–723.
58. Navarini L, Caso F, Costa L, et al. Cardiovascular risk prediction in ankylosing spondylitis: from traditional scores to machine learning assessment. Rheumatol Ther. 2020;7(4):867–882. doi:10.1007/s40744-020-00233-4
59. Perrotta FM, Musto A, Lubrano E. New insights in physical therapy and rehabilitation in axial spondyloarthritis: a review. Rheumatol Ther. 2019;6(4):479–486. doi:10.1007/s40744-019-00170-x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.