Back to Journals » International Journal of Nanomedicine » Volume 15
Therapeutic Potential of Zinc Oxide-Loaded Syringic Acid Against in vitro and in vivo Model of Lung Cancer
Authors Yang N, Qiu F, Zhu F, Qi L
Received 3 August 2020
Accepted for publication 29 September 2020
Published 27 October 2020 Volume 2020:15 Pages 8249—8260
DOI https://doi.org/10.2147/IJN.S272997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Linlin Sun
Ning Yang,1 Feng Qiu,2 Feng Zhu,3 Lei Qi4
1Tumor Research and Therapy Center, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong 250021, People’s Republic of China; 2Department of Pain Management, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, China; 3Department of Thoracic Surgery, Shandong Provincial Chest Hospital, Jinan, Shandong 250013, People’s Republic of China; 4Department of Thoracic Surgery, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong Province 250012, People’s Republic of China
Correspondence: Lei Qi
Department of Thoracic Surgery, Qilu Hospital, Cheeloo College of Medicine, Shandong University, No. 107 Wenhuaxi Road, Jinan, Shandong Province 250012, People’s Republic of China
Email [email protected]
Introduction: Lung cancer is one of the most aggressive forms of cancer that leads to a high mortality rate amongst several cancer types and it is a widely recurrent cancer globally. The use of zinc oxide nanoparticles (ZnONPs) in the formulation of sun cream, food flavors, and colorings due to its varied biological properties. The extensive significance of nanoparticles encourages their production but the approaches are a common challenge in concluding the direct beneficial effect for the disease treatment. Hence, in the present study, zinc oxide-loaded syringic acid (ZnO-SYR) phytochemical was used to elucidate the therapeutic effect against lung cancer.
Methods: The ZnO-SYR nanoparticles were synthesized and characterized by UV-visible spectroscopy, X-ray diffraction, dynamic light scattering, and FT-IR analysis. The characterized ZnO-SYR was tested on in vivo mouse model of lung cancer (benzo(a)pyrene (BAP)) and in vitro A549 cells.
Results: The results demonstrated the significant restoration of body weight with attenuated serum marker enzymes compared to BAP-treated animals. In addition, cytokine estimation revealed ameliorated levels of TNF-α, interleukins, IL-6, IL-1β with evidenced histological observations in ZnO-SYR-treated mice compared to BAP-induced lung cancer mice.
Discussion: Furthermore, cytotoxicity analysis demonstrated the altered mitochondrial membrane potential (MMP), with a profound increase in reactive oxygen species (ROS) levels, and apoptosis mechanism by ZnO-SYR compared to control cells. The conclusions of the present study put forward an evident confirmation of the protective and beneficial effects of zincoxide-loaded syringic acid against the BAP-induced lung cancer model.
Keywords: zinc oxide nanoparticles, syringic acid, lung cancer, apoptosis, A549
Introduction
Lung cancer is one of the aggressive forms of cancer that leads to a higher mortality rate up to 15–17% of all cancer types.1 As stated by the World Health Organization, the estimates of lung cancer were found to be 2.09 million cases (as of 2018) with increasing new incidences and death rates surpass that of the prostate, colon, and breast cancers collectively.2 It is generally diagnosed at a stage, which is not treatable as the surgical treatment is risky accompanied by the side effects due to radiation and chemotherapy. Tobacco usage in the form of smoking is the foremost reason for the incidence of lung cancer and accounts for roughly 90% of entire cancer victims.3 As mentioned above, the treatments such as surgical removal, radiation, and chemotherapy not only abolish cancer cells in the pathway of the beam but the healthy cells too to some extent. At some point of time, these treatments in turn stimulate further metastasis that makes the condition worse. The chemotherapy uses drugs to terminate cancer cells, typically through hindering the proliferation of cancer cells. This treatment procedure has been beneficial and improves the lifetime of individuals with lung cancer at all stages.4,5
Recent therapies including CART cells, stem cells, targeted therapy, viral vectors and nanomaterials are in the attractive stage at the research level. Among the scientific evidence on the use of nanoparticles, combined nanomedicine with traditional plant-based-treatment method fits in the arena of nanotechnology which exploits fabricated nanoparticles (NPs) with plant metabolites shows promising results against cancer treatment. This treatment has the effective competence to target and hinder cancer at its initial phase. Nanomedicine is extremely consistent due to its effective aiming, excellent solubility, bio-accessibility, and numerous assets over outdated techniques of existing cancer treatments.6,7 Among several nanoparticles accessible, zinc oxide nanoparticles (ZnONPs) have enhanced consideration currently for its powerful and effective anticancer, antibacterial, and antifungal possessions.8 At present, ZnONPs are primarily cast-off in managing cancer and it is initiated to display cytotoxicity in numerous cancer cells.9–13 ZnONP’s make a substantial contribution to the expansion of approaches in the drug delivery in cancer. It is also evident to be extremely effectual, soluble, steady, cost-effective, and toxic less that makes it simple to influence the directed locations of the deed. The effectual exploit of ZnONP’s fallouts in deprivation of mitochondria which leads to augmented reactive oxygen species, lipid peroxidations, and impairment to DNA is well studied.14–19
Consequently, consideration has moved to both nutritive and non-nutritious phytoconstituents that exist in the natural plant-related nourishment as probable chemotherapeutic agents. Preceding outcomes estimate that more than a thousand dissimilar phytochemicals hold beneficial and therapeutic activities.20 Recent scientific attention in managing cancer with the exploitation of existing phytoconstituents for chemotherapy is the need of the hour.21 In current years, cumulative devotion has been engrossed on plant and food resultant phytoconstituents as possible anti-cancer drugs. Roughly, 70% of entire medicines utilized currently for the action against cancers are obtained from herbal sources.22 Amongst, syringic acid (4-hydroxy-3,5-dimethoxybenzoic acid) is a polyphenolic compound present in grapes, pumpkin, honey, olives, red wine, dates, and acai palm. Syringic acid also exists in high percentage in plants, namely Hemidesmus indicus, Annona squamosa, Raphanus sativus, Tagetes erecta, Linn flower, and Catunaregam. Syringic acid has formerly reported possessing solid antioxidant properties as well as anticancer, anti-angiogenic, hepatoprotective, neuroprotective, anti-osteoporotic, and anti-steatotic effects. Numerous extracts and several plants that contain syringic acid have established anti-inflammatory activities.23–30 Scientific literature suggests that syringic acid has a possible therapeutic effect on A549 lung cancer cells.31 Hence, in the present study, the protective effect of zinc oxide-loaded syringic acid (ZnO-SYR) was illuminated against in vitro A549 cells and in vivo mouse model of benzo(a)pyrene (BAP)-induced lung cancer.
Materials and Methods
Chemicals
Syringic acid, benzo(a)pyrene (BAP), bovine serum albumin, were procured from Sigma Aldrich, United States of America. All other chemicals utilized in the present work were of diagnostic grade.
UV-Visible Spectroscopy Examination
The formulated ZnO-SYR nanoparticles were confirmed via UV-Visible Spectrophotometer (Agilent Scientific Instruments, Shimadzu Model) with an absorbance wavelength from 380nm to 700nm.32
X-Ray Diffraction (XRD) Analysis
To capture the appearance and dimensions of formulated zincoxide nanoparticles (ZnO-SYR), XRD was employed. Samples were solubilized in ultrapure water and then washed thrice, subsequently centrifugation. The resultant pellet was gathered and permitted to dry. Samples were placed on the XRD grid and the images were captured at 40kV and 30mA with beta and alpha radiation using XRD (Microprocessor controlled 1710 Philips Model). The diluted powers were established from 25°C to 85°C at 2θ. The ZnO-SYR was envisioned from the array of XRD peak, via the formula (Debye-Scherrer).33 The photoluminescence capacity of the fabricated ZnO-SYR was inspected via spectrofluorimeter at the wavelength of 340 to 800nm (PerkinElmer, Waltham, USA).
Fourier Transform Infrared (FTIR) Analysis
FTIR has been widely used to identify functional clusters, metal subdivisions, and organic fragments or molecules. FTIR bands were long-established at 1cm−1 wave by FTIR spectrophotometer (IRTracer 100 Shimadzu, Japan) using the Potassium Bromide technique.34 The occurrence range was captured as a wave or movement typically in the range of 4500–4000 cm−1.
Dynamic Light Scattering (DLS) Analysis
To regulate the magnitude or measurement of the particle via DLS, we executed the standardized approach established by (Kumar et al 2005).35 In this method, 0.01gm of polymer precipitate comprised of 12mmol silver nitrate extracted in 20mL distilled solutions.
Experimental in vivo Lung Cancer Model
Healthy male adult swiss albino mice (18–25g) were utilized in the current investigation. Animals were acquired from the Shanghai Laboratory Animal Center (Shanghai, China). All the animals used in this study complied with animal care strategies by the National Institutes of Health, United States. The studies were permitted by the Institutional Animal Ethics committee, Shandong University, Jinan, Shandong Province, 250,012, China (IAEC No:2020SDPH2819). Mice were acclimatized in a 12-hr light/dark cycle with a continuous proportional humidity of 60% and a temperature of 25°C. Before experimentations, all mice were shifted to their new experimental condition for at least one week and fed with regular food and water ad libitum.
Experimental Procedure
Mice were grouped into four with six mice in each. Group-I mice were administered with corn oil as vehicle control. Group-II mice challenged with BAP (50mg/kg b.wt) via orally two times a week, for four following weeks from the second to sixth week. Group-III mice treated with ZnO-SYR (20mg/kg b.wt, liquified in corn oil) commencing from the twelfth week of the investigation and with BAP until the completion of the experimental schedule. Group-IV animals were administered with ZnO-SYR alone for 18 weeks.
Body Weight and Biochemical Analysis
The lung weight and body weight of normal and experimental mice were calculated. Also, the estimation of protein in serum and lung tissues was performed as described previously.36 Serum marker enzymes, AHH (aryl hydrocarbon hydroxylase),37 GGT (γ-glutamyl transpeptidase),38 5′-NT (5 ′-nucleotidase),39 and LDH (lactate dehydrogenase)40 were examined as described earlier.
Evaluation of Cytokine Level and Carcinoembryonic Antigen (CEA)
Cytokines such as IL-1β, TNF-α, IL-6 and CEA were estimated using an enzyme-linked immunosorbent assay (ELISA) kit as per protocols of manufacturer instruction (Thermo Fischer Scientific, USA).
Histological Observations
For histopathological examinations, a portion of lung tissue was fixed in formalin and embedded in paraffin, sectioned as 5µm sections and stained using with H&E stain and examined using optical microscope.4
MTT Assay
A549 cells were cultured using DMEM media supplemented with 10% fetal bovine serum and allowed to reach confluent before the experiment. The cytotoxicity of ZnO-SYR was tested in cells using MTT dye.41 Briefly, cells were seeded in a microtitre plate with various concentrations of ZnONP’s syringic acid (1 to12.5µM). After 24hr of exposure to ZnO-SYR, MTT (50µL) was added to the cells and then kept in the darkroom at room temperature for 3hr. Subsequently, the growth medium was eliminated, and resultant formazan crystals were dissolved in dimethyl sulfoxide (200µL) and the absorbance was read at 540nm. The percentage of cell viability was calculated and compared between groups. In addition, trypan blue (1%) was added t the cells to get a visual identification of cell viability.
Determination of Intracellular ROS
The status of ROS release was detected using 2,7-dichlorofluorescein diacetate (DCFDA; Sigma, Aldrich, USA) dye.41 ZnO-SYR (5µM and 7.5µM) were added to the microtitre plate cultured with A549 cells and incubated for 24hr. Following the exposure, the plates were washed thrice with saline and incubated with DCFDA dye (20µM) for 30min at 37°C. Then, corresponding cells resuspended in saline (200µL) and fluorescence were recorded in a microplate reader (Winooski, Vermont, USA) at an excitation and emission wavelengths of 538nm and 495nm. The amount of ROS release was done utilizing a fluorescence microscope (Leica Biosystems, Singapore).
Mitochondrial Membrane Potential (MMP)
MMP status was illuminated using 5,5ʹ,6,6ʹ-tetrachloro-1,1ʹ,3,3ʹ-tetraethylbenzimidazolcarbocyanine iodide.42 Cells exposed to ZnO-SYR (5µM and 7.5µM) were subjected to trypsinization and washed twice with phosphate buffer saline. Then, ZnO-SYR treated cells were sustained with MMP dye, JC-1 (10µM) for 10min at 37°C were washed with PBS. The cells were picturized for red and green fluorescence under a fluorescence microscope (Leica microsystems).
Acridine Orange/Ethidium Bromide (AO/EB) Staining
Cells were loaded in 12-well plates and exposed to ZnO-SYR (5µM and 7.5µM) for 24hr. Following this, the plates were washed with saline43 and stained with 100μg/mL AO and 100μg/mL ethidium bromide and examined using a fluorescence microscope (Thermo Fischer Scientific, USA).
Cell Adhesion Assay
Cultured A549 cells exposed to ZnO-SYR (5µM and 7.5µM) for 24hr were fixed with paraformaldehyde and to the cells, crystal violet dye was added and incubated for 20min. After 20min, the excess crystal violet was washed with PBS and observed under light microscope.44
Statistics
Data were given as mean±SEM and statistics were assessed through the one-way ANOVA with Dunnett post hoc test to accomplish significance relative to control using SPSS v. 16.0 software (SPSS Incorporation, USA). p ˂ 0.05 was regarded as significant.
Results
Characterization of UV-Visible Spectroscopy and DLS
The formation of zinc oxide-loaded syringic acid nanoparticles was inspected via UV-Vis spectroscopy. UV-Vis spectra of NPs were illustrated in (Figure 1). UV-Vis absorption of ZnO NPs had the highest peak at 367 nm that is comparable to the absorbance of ZnONPs. After incubation, the color of the nanoparticles changed as seen in the excitation of surface plasmon environment. The reduction of zinc was confirmed through the inspection through UV-Vis spectroscopy. The spectra of ZnO NPs have a peak at 289nm, and the expansion of peak stipulates that the essential ingredients are detached. The consistency and extent of the surface plasmon absorption rely on the capacity and method of the metallic NPs and also on the dielectric consistent of metals and the adjacent intermediary.45 Syringic acid and zinc nitrate altered the ZnONP’s color. The maximum concentration at 367nm was investigational that designates the complete deterioration of zinc ions. UV-Vis spectra of ZnONPs exhibited absorbance at 367nm that supports an estimated reliable mass of the NPs.
DLS is a technique utilized for the measurement, size distribution, delineation, and polydispersity pointer of the fabricated NPs. Figure 2 displays the particle dimensions of the nanosheets illustrations. After examining the statistics, it was inferred that ZnO loaded syringic size was in 120nm. The highest portion of ZnO loaded syringic acid present in the solution was of 50.50nm.
 |
Figure 2 Intensity Distribution of zincoxide-loaded syringic acid nanoparticles by DLS. DLS study of zincoxide-loaded syringic acid contents 0.2 mol/L. |
Photoluminescence and X-Ray Diffraction Analysis
Figure 3A exhibits the photo-luminescent spectra of the formulated ZnONPs. The photoluminescence spectra confirmed the distinctive and highest peak in a wavelength at 644.64nm. It was proved the photo-luminescent asset of the fabricated ZnONPs. Figure 3B reveals that the diverse peaks in a XRD that proving the existence of crystalline ZnONPs with a hexagonal shape. XRD patterns exhibited the divergent peaks in a band of 2θ from 25° to 75° angles. The fabricated ZnO NPs were indexed as 100, 002, 101, 102, 110, 103, 200, 112, 201, and 004. Zinc was excited and indexed at 101 and 100. Additionally, no other peaks were noted in the sample and distinguished the fabrication of ZnONPs in a great transparency. Biological formulation of ZnONPs displayed sturdy diffraction peaks that coincided with the JCPDS folder No: 36,145. The maximum pureness and crystalline structures of formulated ZnONPs are evidenced.
Results of FT-IR Analysis
FT-IR investigation was utilized to distinguish the functional groups that were liable for the deteriorating of metallic ions into ZnONPs in the existence of syringic acid (Figure 4). The phytonutrient instigated syringic acid was implicated for the confirmation of a variability of NPs. FT-IR of syringic acid exhibited frequently integrated peaks extended from 3434cm−1 to 444cm−1. Figure 4 displays the illustrative FTIR spectrum attained from syringic acid. The frequency from 3434cm−1 peaks represents the OH extending tremor, and the appearance of amino acids and carbohydrates. The 2923cm−1 peaks signified the carbon and hydrogen stretching especially, lipid molecule. The occurrence of peaks from 1625cm−1 peaks is Amide I: C=O extending, i.e. protein molecules. Frequency of 1383 cm−1 peak denotes, sulfur complexes. Frequency series from 1121 cm−1 peaks demonstrate the C-N stretching of amino acids.
Outcome of Body, Lung Weight and Tumor Morphology of Zinc Oxide-Loaded Syringic Acid-Treated Animals
Table 1 displays the outcome of BAP induction and ZnO-SYR on lung and body weight, and tumor occurrence. Results showed decreased body (P < 0.05), augmented lung weight, and increased tumor induction in BAP alone treated group-II mice compared to control. While post-initiation with ZnO-SYR (20mg/kg b.wt) along with BAP demonstrated the improved body weight and decreased lung weight in cancer-bearing animals (group III). While ZnO-SYR (20mg/kg b.wt) alone treatment in group IV animals markedly improved the bodyweight and abrogated the tumor occurrence.
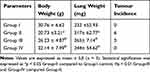 |
Table 1 Bodyweight, Lung Weight and Tumor Incidence of Zinc Oxide-Loaded Syringic Acid-Treated Mice |
Effect of ZnO-SYR on Xenobiotic and Liver Dysfunction Marker Enzymes
Figure 5 portrays the outcome of BAP induction and ZnO-SYR on marker enzymes. A noteworthy (p<0.05) increase in xenobiotic indicator enzymes AHH, GGT, 5′-NT, and LDH were found in BAP challenged animals (Group-II) compared to vehicle control. ZnO-SYR post-initiation treatment suggestively (P < 0.05) reinstated the status of xenobiotic marker enzymes to near-normal levels.
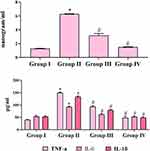
Effect of ZnO-SYR on CEA and Pro-Inflammatory Markers
Figure 6 represents the effect of BAP induction and ZnO-SYR on pro-inflammatory cytokines, TNF-α, IL-6, IL-1β, and CEA levels. A striking upregulation in the IL-1β, TNF-α, IL-6, and CEA levels was demonstrated in the BAP challenged animals (Group-II) while, post-initiation supplementation of ZnO-SYR (P < 0.05) restored the status of IL-1β, TNF-α, IL-6, and CEA levels to the normal and drug only administered animals displayed no significant changes associated with it.
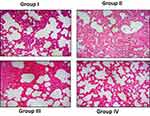
Zinc Oxide-Loaded Syringic Acid Effect on Lung Histology Tissues
Figure 7 exhibits the histological alterations of the lung segment of normal and investigational mice. Normal mice displayed typical architecture. While the benzo(a)pyrene-induced animals (group-II) displayed the diminished tissue architecture with slanted alveoli and augmented quantity of hyperchromatic nuclei in the lung tissues. ZnO-SYR -treated animals possessed the nearly usual architecture representing the non-toxic feature of syringic acid. Pre-supplementation with syringic acid distinctly abridged the alveolar impairment in group-IV mice thus protecting the near-usual structures.
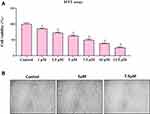
Cytotoxicity Results of ZnO-SYR
The cytotoxicity of ZnO-SYR was assessed by MTT assay. Figure 8 displays the cell viability in a dose-dependent manner (1,2.5,5,7.5,10 and 12.5μM) in that the diminished viability was observed at 7.5μM/mL when compared to 5μM/mL. The MTT test outcomes revealed the dose-reliant cytotoxicity was induced by ZnO-SYR and the LD50 level of ZnO NPs at 24hr was 7.5µM.
Effect of ZnO-SYR on the Intracellular ROS Status
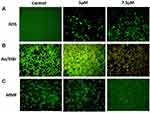
Cells with ZnO-SYR (5μM and 7.5μM) treatment for 24hr demonstrated a notable increase in the ROS release in a dose-dependent manner. The DCF fluorescence estimated qualitatively and quantitatively demonstrated that the ZnO-SYR indued ROS might have induced the cell death in A549 cancer cells (Figure 9).
Effect of ZnO-SYR on AO/EB Staining
The ZnO-SYR caused dosage-dependent deterioration in the total amount of live cells and also expands the early and late apoptotic, and necrotic cells which was evidenced from AO/EB staining (Figure 9). Control cells displayed normal cellular architecture, A549 cells treated with zinc-loaded syringic acid (5μM and 7.5μM concentration) displayed augmentation in the early apoptotic cells to late apoptotic stage compared to control.
Effect of Zinc-Loaded Syringic Acid on MMP Status
The apoptosis is mediated via the alteration in the MMP status during the commencement and that was inspected using JC-1 staining in the present study (Figure 9). The control group demonstrated a maximum amount of green fluorescence that signifies good MMP status (Figure 9B). At the same time, ZnO-SYR (5μM and 7.5μM concentration) demonstrated much alterations of ΔΨM (mitochondrial membrane potential) that had reduced green fluorescence illustrates in Figure 9B.
Effect of Zinc Oxide-Loaded Syringic Acid on Cell Adhesion Studies
Trypan blue staining procedure was executed to distinguish the viability and non-viability of cells by the infinitesimal investigation. Figure 10 shows the trypan blue assay which discloses the disruption of cell membrane integrity. Treatment of ZnO-SYR (5µM and 7.5µM) presented the degradation of organelles, which was visualized by ultra-structural observation as depicted in Figure.
 |
Figure 10 Cell adhesion assay using 0.1% crystal. The experimental details were given in the methodology section. Cells with ZnO-SYR (5μM and 7.5μM) treatment for 24hr. Magnification: 20×. |
Discussion
Lung cancer is presently the foremost reason for increased rates of mortality globally. In current decades, substantial consideration has been specified to augmented dietary consumption of phytoconstituents, meanwhile several epidemiological, and investigational researches, provided an optimistic association amongst the cancer risks and consumption of phytoconstituents.46 Syringic acid is an imperative and abundant phenolic compound that exists in olives, dates, grapes, spices, honey, red wine, pumpkin, acai palm, and other numerous herbs. Phenolic compounds have established their actions in cancer deterrence and handling that exists mostly because of their capability to regulate the production of ROS, which persuades cell death, wedging signaling pathways, and prevent cell propagation.47,48 Syringic acid displays beneficial assets in pharmaceutical or biomedical settings like anti-microbial, anti-oxidant, antidiabetic, anti-inflammation, anti-cancer, preventing cardiovascular and brain disorders.49–51 Preceding results displayed in vitro cytotoxic activities where the herbal Cuphea extract had a cytotoxic upshot against human lung cancer cells.
The cytotoxic action may be because of the existence of dissimilar phenolic compounds available in the plants. Phenolic compounds are supposed to employ its anticancer actions via improving the release of free radicals and lipids that could eliminate proteins, enzymes and reduce ATP in cancer cells, therefore promoting cell death.52 In the current study, the zinc-loaded syringic acid when used in a dosage-reliant mode induces moderate ROS release, disruption of MMP, morphological alteration as seen by dual staining, and prevents cell adhesion. The challenges of drug targetting demand the preparation and customizing of ecological nanoparticles with improved effectiveness, accessibility, rapid distribution, and quicker action in the lowest dose. Particle size is an imperative limitation as it can disturb physical steadiness, cellular commitment, and drug discharge from the nanoparticles which can distress its bio-availability.53
Incidentally, the providence of the drug-loaded nanoparticles principally depends on its physicochemical properties. Present findings recommended that zinc-loaded syringic acid was effectively synthesized with comparatively good encapsulation, yield, and in the expected size range as discerned through the characterization techniques, e.g. UV-Visible spectroscopy, dynamic light scattering, X-ray diffraction studies, and also by FTIR studies. The dispersion pattern in zinc-loaded nanoparticles also displayed a one mode particle dimensions dispersal, as confirmed by the afore-mentioned characterization studies. The significant diminution in weight of cancer-possessing animals may be due to the malabsorption or cancer cachexia that subsidizes liberal degradation especially in adipose tissue and skeletal muscle.54 In this current exploration, noteworthy augmentation in tumorigenesis in the lungs of cancer-possessing animals may because of the unrestrained propagation of tumor cells but the augmented bodyweight upon the management of syringic acid could be due to the shielding efficiency of this phenolic compound. BAP, a well-recognized carcinogen that is identified to accumulate massive volumes of free radicals are extremely sensitive, lethal, and mutagenic.55
These poisonous radicals are tangled in interceding tissue lipid peroxidation. The tissue harm due to lipid peroxidation is the profound aspect in tumorous situations and any corrosion or obliteration of the membrane could results in the seepage of these enzymes from tissues.46 AHH renovates polycyclic hydrocarbons to quinines, epoxides, phenols, and dihydrodiols. AHH extremely inducible in animal skins and also in many mammalian cells is associated with vulnerability to BAP cytotoxicity which leads to tumorigenesis.56 Elevated AHH status was found in respiratory cells and serum of BAP challenged animals.57 The transmission of γ-glutamyl groups from peptide presenters to peptide receptors and amino acids is the catalytic purpose of GGT. GGT is valuable in the analysis and also has analytical significance in disorders related to lung cancer.58
5′-NTs enzymes unbind the nucleotides from the phosphate group on the 5th carbon atom of ribose. It is believed to be extensively dispersed in tumor cells and amplified production in leukemia victims had previously been stated.59 LDH is documented as a probable tumor indication enzyme in evaluating the propagation of tumor cells and augmented serum and lung LDH action was observed in lung cancer.56 Tumor cells display an augmented amount of glucose metabolism that eventually results in raised LDH production. In our current investigation, momentous raise in all the above-mentioned tissue, and serum marker enzymes were found in BAP-induced mice. Zinc-loaded syringic acid supplementation revert back the status of those marker enzymes comparable to the control animals thus signifying its anti-cancer efficacy.
Carcinoembryonic antigen (CEA) is a general name of a glycoprotein group with the same antigenic determinant and with a molecular weight of about 180 kDa. Carcinoembryonic antigen is among the earliest elucidated tumor markers and is highly specific to many adenocarcinomas, particularly in the lung, breast, colon, liver, and gastric cancers.60 CEA has a comparatively high prevalence in lung cancer with the highest serum absorption in adenocarcinoma and lung cancer. Roughly 40% of individuals with lung adenocarcinoma display augmented carcinoembryonic levels. In this current investigation, the status of carcinoembryonic was determined and found that the increase in CEA; however, the decrease in CEA during ZnO-SYR treatment demonstrates its effectiveness in reducing the tumor burden.61 Thus, the present study results conclude that the use of nanoformulation in the combination of plant-based supplementation can lead to the novel strategy for the development of cancer drugs with reduced side-effects while improving the normal physiological functions.
Conclusion
Ultimately, our present study confirms that zinc-loaded nanoparticles of syringic acid play a very essential defensive function against lung carcinogenesis and have the capability to act as an effective phenolic compound against BAP-provoked lung carcinogenesis animal model. In the current exploration, the zinc-loaded syringic acid on a concentration-dependent manner induces moderate ROS generation, disrupts mitochondrial membrane potential, morphological modification by dual staining and viability and non-viability by cell adhesion assay.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Sikdar S, Mukherjee A, Khuda-Bukhsh AR. Ethanolic extract of Marsdenia condurango ameliorates Benzo[α]pyrene-induced lung cancer of rats-condurango ameliorates BaP-induced lung cancer in rats. J Pharmacopunct. 2014;17:7–17. doi:10.3831/KPI.2014.17.011
2. Khan N, Afaq F, Khusro FH, Adhami VM, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130:1695–1705. doi:10.1002/ijc.26178
3. Ravenel JG. Lung Cancer Imaging. New York, USA: Springer; 2013:23–38.
4. El-Kott AF. Anti-angiogenic effectiveness of the pomegranate against Benzo(a)Pyrene induced lung carcinoma in mice. Inte J Cancer Res. 2015;11(4):164–174. doi:10.3923/ijcr.2015.164.174
5. Bergman B, Aaronson NK, Ahmedzai S, Kaasa S, Sullivan M. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC study group on quality of life. Eur J Cancer. 1994;30:635–642. doi:10.1016/0959-8049(94)90535-5
6. Wang R, Billone PS, Mullett WM. Nanomedicine in action: an overview of cancer nanomedicine on the market and in clinical trials. J Nanomater. 2013;2013:12.
7. McNeil SE. Nanoparticle therapeutics: a personal perspective, Wiley Interdiscip. Rev Nanomed Nanobiotechnol. 2009;1(3):264–271. doi:10.1002/wnan.6
8. Ates T, Tatar C, Yakuphanoglu F. Preparation of semiconductor ZnO powders by sol-gel method: humidity sensors. Sens Actuator A. 2013;190:150e160. doi:10.1016/j.sna.2012.11.031
9. Akhtar MJ, Ahamed M, Majeed Khan MA. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine. 2012;7:845e857.
10. Wahab R, Siddiqui MA, Saquib Q, et al. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf B. 2014;117:267e276. doi:10.1016/j.colsurfb.2014.02.038
11. Pujalte I, Passagne B, Brouillaud M, Treguer E, Durand C, Ohayon-Courtès C, l’Azou B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells, Part. Fibre Toxicol. 2011;3:8e10.
12. Guan R, Kang T, Lu F, Zhang Z, Shen H, Liu M. Cytotoxicity, oxidative stress, and genotoxicity in human hepatocyte and embryonic kidney cells exposed to ZnO nanoparticles. Nanoscale Res Lett. 2012;7:602e610. doi:10.1186/1556-276X-7-602
13. Kang T, Guan R, Chen X, Song Y, Jiang H, Zhao J. In vitro toxicity of different sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res Lett. 2013;8:496e501. doi:10.1186/1556-276X-8-496
14. Chahal A, Saini AK, Kumar A, Saini RV. Natural antioxidants as defense against cancer. Asian J Pharm Res. 2018;11:38–44.
15. Ibsen S, Zahavy E, Wrasdilo W, et al. A novel doxorubicin prodrug with controllable photolysis activation for cancer chemotherapy. Pharm Res. 2010;27:1848–1860. doi:10.1007/s11095-010-0183-x
16. Yousefpour P, Atyabi F, Farahani EV, Sakhtianchi R, Dinarvand R. Polyanionic carbohydrate doxorubicin-dextran nanocomplex as a delivery system for anticancer drugs: in vitro analysis and evaluations. Int J Nanomedicine. 2011;6:1487–1496.
17. Amornpitoksuk P, Suwanboon S, Sangkanu S, et al. Synthesis, photocatalytic and antibacterial activities of ZnO particles modified by diblock copolymer. Powder Technol. 2011;212:432–438. doi:10.1016/j.powtec.2011.06.028
18. Selvakumari D, Deepa R, Mahalakshmi V, Subhashini P, Lakshminarayan N. Anticancer activity of ZnO nanoparticles on MCF7 (breast cancer cell) and A549 (cancer cell). ARPN, J Eng Appl Sci. 2015;10:5418–5421.
19. Liong M, Lu J, Kovochich M, et al. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano. 2008;2:889–896. doi:10.1021/nn800072t
20. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi:10.1038/nrc1189
21. Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi:10.1016/j.bcp.2006.02.009
22. Ashokkumar P, Sudhandiran G. Luteolin inhibits cell proliferation during azoxymethane-induced experimental colon carcinogenesis via Wnt/β-catenin pathway. Invest New Drugs. 2011;29:273–284. doi:10.1007/s10637-009-9359-9
23. Srinivasulu C, Ramgopal M, Ramanjaneyulu G, Anuradha CM, Kumar CS. Syringic acid (SA)‒a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed Pharmacother. 2018;108:547–557. doi:10.1016/j.biopha.2018.09.069
24. Sevgi K, Tepe B, Sarikurkcu C. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem Toxicol. 2015;77:12–21. doi:10.1016/j.fct.2014.12.006
25. Jeong JH, Seung TW, Park SK, Park CH, Jin DE, Heo HJ. Learning and memory effect of syringic acid on amyloid-β-induced neurotoxicity in ICR mice. Agric Life Sci. 2015;49:233–244. doi:10.14397/jals.2015.49.4.233
26. Itoh A, Isoda K, Kondoh M, et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull. 2009;32:1215–1219. doi:10.1248/bpb.32.1215
27. Itoh A, Isoda K, Kondoh M, et al. Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biol Pharm Bull. 2010;33:983–987. doi:10.1248/bpb.33.983
28. Karthik G, Angappan M, Vijaya Kumar A, Natarajapillai S. Syringic acid exerts antiangiogenic activity by downregulation of VEGF in zebrafish embryos. Biomed Prev Nutr. 2014;4:203–208. doi:10.1016/j.bionut.2014.01.007
29. Ham JR, Lee HI, Choi RY, Sim MO, Seo KI, Lee MK. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat-diet-induced obese mice. Food Funct. 2016;7:689–697. doi:10.1039/C5FO01329A
30. Tanaka T, Kawaguchi N, Zaima N, Moriyama T, Fukuta Y, Shirasaka N. Anti-osteoporotic activity of a syringic acid diet in ovariectomized mice. J Nat Med. 2017;71:632–641. doi:10.1007/s11418-017-1105-6
31. Karthick G, Vijayakumar AR, Natarajapillai SU. Preliminary study on salubrious effect of syringic acid on apoptosis in human lung carcinoma A549 cells and in silico analysis through docking studies. Asian J Pharm Clin Res. 2014;7:46–49.
32. Bartzatt R, Bartlett M, Handler N. Detection and quantitative analysis for 2-thiobarbituric acid utilizing Uv-visible spectrophotometer. Am J Pharmacol Sci. 2013;1(1):10–14. doi:10.12691/ajps-1-1-3
33. Tensingh Baliah N, Lega Priyatharsini S. Biosynthesis and characterization of zinc oxide nanoparticles using onion bulb extract. Int J Trend Sci Res Dev. 2018;2(2):36–43. doi:10.31142/ijtsrd8305
34. Marouani E, Benzina NK, Ziadi N, Bouslimi B, Abouda A, Koubaa A. Deinking sludge compost stability and maturity assessment using fourier transform infrared spectroscopy and thermal analysis. Waste Manag Res. 2019;37(10):1043–1057. doi:10.1177/0734242X19864638
35. Kumar R, Mu¨nstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005;26(14):2081–2088. doi:10.1016/j.biomaterials.2004.05.030
36. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275.
37. Mildred K, Richerd L, Joseph G, Alexander W, Conney A. Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally accruing flavonoids. Cancer Res. 1981;41:67.
38. Orlowski M, Meister A. Isolation of γ-glutamyl transpeptidase from hog kidney. J Biol Chem. 1965;240:338–347.
39. Hardonk MJ, de Boer HGA. HG BDB. 5′ -nucleotidase. 3. Determinations of 5′-nucleotidase isoenzymes in tissues of rat and mouse. Histochemie. 1968;12(1):29–41. doi:10.1007/BF00306345
40. King C. The transferases-alanine and aspartate transaminases. In: Van D, editor. Practical Clinical Enzymology. London: Nostrand Company Ltd.; 1965:121–138.
41. Gunaseelan S, Balupillai A, Govindasamy K, et al. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS One. 2017;12(5):e0176699. doi:10.1371/journal.pone.0176699
42. Srilatha Sakamuru MS, Attene-Ramos MS, Xia M. Mitochondrial membrane potential assay. Methods Mol Biol. 2016;1473:17–22.
43. Ribble D, Goldstein NB, Norris DA, Shellman YG. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005;5:12. doi:10.1186/1472-6750-5-12
44. Ruan JS, Liu YP, Zhang L, et al. Luteolin reduces the invasive potential of malignant melanoma cells by targeting beta- 3-integrin and the epithelial-mesenchymal transition. Acta Pharmacol Sin. 2012;33:1325–1331. doi:10.1038/aps.2012.93
45. Melvin Joe M, Jayochitra J, Vijayapriaya M. Antimicrobial activity of some common spices against certain human pathogens. J Med Plants Res. 2009;3:1134–1136.
46. Ramakrishnan G, Augustine TA, Jagan S, Vinodhkumar R, Devaki T. Effect of silymarin on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Exp Oncol. 2007;29:39–44.
47. Oke GO, Abiodun AA, Imafidon CE, Monsi BF. Zingiber officinale (Roscoe) mitigates CCl4-induced liver histopathology and biochemical derangements through antioxidant, membrane-stabilizing and tissue-regenerating potentials. Toxicol Rep. 2019;6:416–425. doi:10.1016/j.toxrep.2019.05.001
48. Parathodi Illam S, Hussain A, Elizabeth A, Narayanankutty A, Raghava Menon AC. Natural combination of phenolic glycosides from fruits resists pro-oxidant insults to colon cells and enhances intrinsic antioxidant status in mice. Toxicol Rep. 2019;6:703–711. doi:10.1016/j.toxrep.2019.07.005
49. Pezzuto JM. Grapes and human health: a perspective. J Agric Food Chem. 2008;56(16):6777–6784. doi:10.1021/jf800898p
50. Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from açai (Euterpe oleracea Mart.). J Agric Food Chem. 2008;56(12):4631–4636. doi:10.1021/jf800161u
51. Kiran P, Denni M, Daniel M. Antidiabetic principles, phospholipids and fixed oil of kodo millet (Paspalum scrobiculatum Linn.). Ind J Appl Res. 2014;4:13–15. doi:10.15373/2249555X/FEB2014/17
52. Ma C, Song M, Zhang Y, Yan M, Zhang M, Bi H. Nickel nanowires induce cell cycle arrest and apoptosis by generation of reactive oxygen species in HeLa cells. Toxicol Rep. 2014;1:114–121. doi:10.1016/j.toxrep.2014.04.008
53. Rani D, Somasundaram VH, Nair S, Koyakutty M. Advances in cancer nanomedicine. J Indian Inst Sci. 2012;92(2):187–218.
54. Magesh V, Singh JPV, Selvendiran K, Ekambaram G, Sakthisekaran D. Antitumour activity of crocetin in accordance to tumor incidence, antioxidant status, drug metabolizing enzymes and histopathological studies. Mol Cell Biochem. 2006;287(1–2):127–135. doi:10.1007/s11010-005-9088-0
55. Selvendiran K, Senthilnathan P, Magesh V, Sakthisekaran D. Modulatory effect of piperine on mitochondrial antioxidant system in Benzo(a)pyrene-induced experimental lung carcinogenesis. Phytomedicine. 2004;11(1):85–89. doi:10.1078/0944-7113-00355
56. Kamaraj S, Vinodhkumar R, Anandakumar P, Jagan S, Ramakrishnan G, Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol Pharm Bull. 2007;30(12):2268–2273. doi:10.1248/bpb.30.2268
57. Hecht SS, Murphy SE, Carmella SG, et al. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:693. doi:10.1158/1055-9965.EPI-04-0542
58. Obrador E, Carretero J, Ortega A, et al. γ-Glutamyl transpeptidase overexpression increases metastatic growth of B16 melanoma cells in the mouse liver. Hepatology. 2002;35:74–81. doi:10.1053/jhep.2002.30277
59. Erdemli HK, Adam B, Bavbek N. Pyrimidine 5′nucleotidase I and II activities in acute leukaemias. Acta Medica (Hradec Kralove). 2004;47:129–131. doi:10.14712/18059694.2018.78
60. Wang W, Li Y, Zhang X, et al. Evaluating the significance of expression of CEA mRNA and levels of CEA and its related proteins in colorectal cancer patients. J Surg Oncol. 2014;109(5):
61. Iwasaki A, Shirakusa T, Yoshinaga Y, Enatsu S, Yamamoto M. Evaluation of the treatment of non‑small cell lung cancer with brain metastasis and the role of risk score as a survival predictor. Eur J Cardiothorac Surg. 2004;26:
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.