Back to Journals » Journal of Inflammation Research » Volume 15
Therapeutic Plasma Exchanges in Combination with High-Dose Steroid-Induced Immunosuppression as an Ultima Ratio Therapy in Severe Coronavirus Disease 2019 (COVID-19): A Case-Series Report
Authors Janikowska A , Soukup J, Pliquett RU , Abdel-Rahim R
Received 12 October 2021
Accepted for publication 7 January 2022
Published 3 February 2022 Volume 2022:15 Pages 715—722
DOI https://doi.org/10.2147/JIR.S344028
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Monika Sharma
Agata Janikowska,1 Jens Soukup,2 Rainer U Pliquett,1 Rabah Abdel-Rahim1
1Department of Nephrology and Diabetology, Carl-Thiem Hospital, Cottbus, Germany; 2Clinic for Anesthesiology, Intensive Therapy and Palliative Medicine, Carl-Thiem Hospital, Cottbus, Germany
Correspondence: Agata Janikowska, Tel +49 355 46 79592
, Fax +49 355 46 2240
, Email [email protected]
Abstract: We present 2 cases of severe Covid-19 with comorbidities (arterial hypertension, obesity, diabetes mellitus) treated with membrane-based therapeutic plasma exchanges in combination with a short-term high-dose immunosuppressive therapy. The therapy has been initiated in an attempt to alleviate the prevalent cytokine storm and to prevent intubation and invasive mechanical ventilation, when a long-term nasal oxygen therapy with a maximum flow rate of 8L/min was insufficient to achieve an adequate oxygenation. Even though patient 2 had to be intubated after the 4th cycle of plasmapheresis due to the exhaustion of the respiratory muscles and the subsequent acquired sepsis with a microbiological evidence of a mixed bacterial-fungal infection, both patients showed a good response to treatment, including improvement of laboratory and radiological findings. To our knowledge, this combination of therapeutic plasma exchange with a high-dose steroid therapy has not been reported previously.
Keywords: Covid-19, plasmapheresis, immunosuppression, cytokine storm
Introduction
The worldwide spread of the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) continues producing new variants at a very disturbing pace.
Many therapeutic strategies, including dexamethasone,1 anticoagulants,2 antibiotics for treatment and prevention of possible bacterial coinfections,3 high-flow oxygen therapy4 and, finally, invasive mechanical ventilation, have been shown to be effective in the supportive treatment of Covid-19. However, there are still many Covid-19 patients with comorbidities that put them at an increased risk of a critical illness course with a much higher mortality rate than in the general population.5 An overall mortality of about 3.4% (over 4.3 million absolute fatal cases) forced physicians and scientists to redirect their focus towards finding an appropriate curative therapy.
Taking into consideration recent reports,6,7 it seems reasonable that the serious complications of COVID-19 can be treated in a similar fashion as a vasculitis is being treated. Specifically, the removal of plasma constituents by therapeutic plasma exchanges in combination with a short-term high-dose immunosuppressive therapy lowers excessive plasma levels of cytokines and of complement and coagulation factors. This approach is currently an unproven but intensively tested therapy for severe COVID-19.8,9
There have been several reports published describing the use of therapeutic plasma exchanges in Covid-19.7,10–13 None of them, however, described it in combination with a high-dose immunosuppressive therapy.
In this report, we describe two cases of COVID-19 patients treated with plasmapheresis and high-dose corticosteroids as an off-label therapy to prevent intubation and invasive mechanical ventilation.
Case Presentation
Patient 1
A 59-year-old woman presented to the emergency department with dyspnea at rest, myalgia and increased body temperature.
Seven days prior to hospital admission, she was positively tested for COVID-19 in ambulatory settings. Back then, she was in an asymptomatic condition. The test has been conducted due to a close contact with an infected and symptomatic family member.
Upon admission, she had tachycardia and slight tachypnea. Blood pressure was within normal range. She denied any change in smell (anosmia) or taste.
Patient 2
A 64-year-old man, complaining of worsening shortness of breath accompanied by a dry cough, which started about 1 week prior to hospital admission. The patient also did not notice any smell or taste disturbance.
Despite normal blood pressure and heart rate, the clinical condition was greatly reduced with tachypnea, fever, and reduced transcutaneous oxygen saturation.
Both patients suffered from arterial hypertension and obesity. In addition, within the framework of metabolic syndrome, Patient 2 had type-2 diabetes treated with insulin.
Laboratory Findings and Initial Treatment
A positive SARS-CoV-2 PCR results from a nasopharyngeal swab confirmed the earlier established diagnosis in both patients. Both patients showed signs of acute hypoxic respiratory insufficiency upon admission, which could be confirmed in earlobe arterialized capillary blood analysis with coexisting evidence of hyperventilation.
An initiated long-term nasal oxygen therapy with a flow rate of 2L/min was sufficient to achieve an adequate oxygenation for Patient 1, whereas Patient 2 required an increase in oxygen supply to the available maximum flow rate of 8 L/min, still however, not yielding a full compensation of the inadequate gas exchange.
C-reactive protein was slightly increased in both cases with quite low plasma levels of procalcitonin and leukocytes within the normal range. As the bacterial co-infection could not be ruled out at this stage, an antibiotic therapy with azithromycin and ampicillin/sulbactam was administered.
Furthermore, a chest X-rays showed patchy opacities with basal and peripheral predominance.
Following the anticoagulation recommendations for COVID-19, both received anticoagulation therapy with subcutaneously applied enoxaparin since admission, even though only Patient 2 presented with abnormal D-dimer levels.
In addition, a low dose dexamethasone therapy (6 mg/day) was applied for its anti-inflammatory and immunosuppressant effects since admission in both patients. All initial laboratory findings are shown in Table 1.
 |
Table 1 Initial Laboratory and Clinical Findings |
Course of Disease
Due to the rapid progression of respiratory failure within 3 days after admission, patient 1 was transferred to the intensive-care unit.
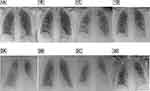
The chest X-ray findings have progressed, showing consolidated infiltrates in the right middle pulmonary lobe and newly occurring opacities in the left lower pulmonary lobe (Figure 1: 1A–C).
Subsequently, a heated humidified high-flow therapy with a flow of 50L/min and oxygen concentration (FiO2) of 100% as well as an early prone positioning therapy were applied.
In Patient 2, the initial chest X-ray findings showed severe spotty bilateral opacities, progressing in both middle and basal pulmonary lobes, being consistent with pneumonic consolidated infiltrations accompanied by alveolar pulmonary edema (see Figure 1: 2A). Likewise for Patient 1, Patient 2 has been immediately transferred to an intensive care unit, where the same therapeutic measures have been applied.
Plasmapheresis in COVID-19 Patients
Considering that both patients were in a critical condition, had worsening global respiratory insufficiency and suffered from relevant comorbidities, an off-label use of therapeutic plasma exchanges with albumin replacement has been initiated to avoid an endotracheal intubation. The main reason for this decision was that those comorbidities are considered to be independent risk factors for endotracheal intubation and, consecutively, incur a high likelihood of death in hospital.13
For each patient, after obtaining informed consent, a temporary, uncuffed dialysis catheter was placed into a jugular internal vein, 5–6 membrane-based therapeutic plasma exchanges were performed over 8 days using the Octa Nova apheresis system by Diamed Medizintechnik (Cologne, Germany). Blood flow was 100–120 mL/min. As a replacement fluid, a 5% albumin solution (3 liters or 86–107% of estimated plasma volume. Plasma volume was estimated by Nadler’s formula for blood volume14 and multiplied by (1-hematocrit) yielding an estimated plasma volume of 2.8 L in patient 1 and 3.5 L in patient 2.
As for anticoagulation, heparin-based systemic anticoagulation was started for 2 therapeutic plasma-exchanges in Patient 2. As a complication, patient 2 developed a type-2 heparin-induced thrombocytopenia proven by positive antibodies. The platelet count was 80–109 Giga particles/L. Except for the 2 initial plasmapheresis sessions in patient 2, both patients received a regional citrate-based anticoagulation until the end of the scheduled 5–6 sessions. Calcium was reinfused until free plasma calcium was within normal range. Membrane-based plasmapheresis using regional anticoagulation with citrate was adopted and reported previously by our group.15
In addition, a 4/5-day course of methylprednisolone (100 mg per day) was applied. After the pulse therapy, a gradual taper-off over 5 days was performed concurrently to therapeutic plasma exchanges. The total methylprednisolone doses applied were 675 mg to Patient 1 and 750 mg to Patient 2. Throughout methylprednisolone taper, we continued the dexamethasone-therapy (6 mg/day) over 10 days in total.
In both subjects, during the therapy, a swift decrease of C-reactive protein, procalcitonin and lactate dehydrogenase was observed, as well as concurrent reduced oxygen demand as shown in Table 2 and an improved radiological findings (Figure 1: 1D and 2B).
 |
Table 2 Laboratory Controls Under Plasmapheresis |
In Patient 1, after the fifth session of therapeutic plasma exchange, anti-SARS-CoV-2-Virus IgG was detectable. The patient reported an improvement in symptoms and general condition – she could be transferred to a regular ward and participate in an intensified physiotherapy program. After 22 days, Patient 1 was discharged from hospital with a long-term oxygen therapy with a flow of 0.5 l/min at rest and 2L/min on exertion.
Patient 2 initially showed a good response to treatment, including improvements in radiological findings as well. However, the patient’s condition suddenly deteriorated as a result of exhaustion of his respiratory muscles, despite high-flow oxygen therapy. He had to be intubated after the fourth plasma exchange. D-dimer levels were found to be increased. After intubation, two more plasma exchanges were applied resulting in both clinical and laboratory improvements.
Two days after the completion of the therapy described above, inflammatory parameters rose (Procalcitonin: 62 µg/L, C-reactive protein: 363 mg/L and IL-6:80 ng/L). Proteus mirabilis, Staphylococcus epidermidis, and fungal antigen were detected in serial blood cultures. Since an antibiotic therapy consisting of azithromycin and ampicillin/sulbactam has already been discontinued, a new course of antibiotics with meropenem, linezolid and caspofungin for 10, 9 and 12 days, respectively, was started. A chest X-ray control confirmed the progression of pneumonia (Figure 1: 2C). After a prolonged treatment, the patient could be extubated and was in a stable condition. In chest X-ray controls, a regression of pulmonary infiltrates was found (Figure 1: 2D). The patient was transferred to a regular ward to take part in an intensive physiotherapy program. He was discharged after 57 days of hospitalization and underwent further physiotherapy in an inpatient rehabilitation facility.
Discussion
Recent reports suggest that COVID-19 cannot be treated by eliminating the virus itself, but it can possibly be treated by an early and effective management of the underlying immune dysregulation in terms of overly elevated serum cytokine levels known as a cytokine storm. Immune mediators (eg IL-1, IL-2, IL-6, GM-CSF, IFN- γ, TNF) are known to interfere with vascular hemostasis through at least two mechanisms: firstly, they cause an endothelial-cell damage followed by hypercoagulation as an innate pathway to limit blood loss, and secondly, they enhance the activity of the complement cascade.16
In addition, as part of the cytokine storm, interleukin-6 stimulation was shown to impair endothelial-cell-barrier function.17 A virally mediated reduced endothelial-cell permeability is known to promote vascular leakage and extravasation of fluid into the alveolar space.18
The indication for plasmapheresis has been discussed for quite some time by different groups.8,9,19 Without randomized clinical trials, the use of plasmapheresis is considered to be an ultima ratio therapy driven by considerations of comorbidities, the need for intubation and evidence of cytokine storm based on elevated interleukin-6 plasma concentrations. Clearly, the use of plasmapheresis in the 2 patients presented here was based on those grounds and on preliminary data published recently,6 where 4 out of 6 critically ill COVID-19 patients undergoing plasmapheresis survived. Here, a short-time course of a high-dose methylprednisolone given intravenously was performed concurrently to plasmapheresis. In fact, this combination therapy has not been reported previously. Based on the experience of these 2 patients who both survived, this combination therapy appears to be superior to either therapy option alone. However, in the presence of bacterial infections like in Patient 2, the high-dose steroid therapy should be withheld. Nevertheless, the cytokine storm appears to be at the highest peril for seriously ill COVID-19 patients. From our experience, many ICU patients suffering from COVID-19 were initially deemed to be in a stable condition, thus remaining off bounds for an ultima ratio plasmapheresis therapy. However, they presented evidence for a cytokine storm and died shortly after the initial assessment.
We therefore urge the medical community to plan a randomized clinical trial assessing therapeutic plasma exchanges in combination with a short-term steroid pulse in severely ill COVID-19 patients presenting evidence for a cytokine storm.
Conclusion
In summary, in 2 cases of severely ill COVID-19 patients doomed for endotracheal intubation due to respiratory global insufficiency, a combination of therapeutic plasma exchanges and short-term steroid pulse therapy stabilized the clinical condition, improved the radiological lung findings and attenuated the laboratory findings associated with the cytokine storm. Based on comorbidities, both patients were deemed to have a poor prognosis, yet they recovered within 18 and 56 days, respectively, after the start of this combination therapy. On the down-side, the short-term steroid pulse therapy has the potential for bacterial and/or fungal superinfections. Based on these 2 cases and on previous small studies, we encourage the initiation of a clinically randomized study investigating this combination therapy as ultima ratio in severely ill COVID-19 patients with evidence for cytokine storm.
Abbreviations
IL-6, interleukin 6; Ig, immunoglobulin; FiO2, fraction of inspired oxygen; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN- γ, interferon gamma; TNF, tumor necrosis factor.
Ethics and Consent
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Horby P, Lim WS, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi:10.1056/NEJMoa2021436
2. Hayıroğlu Mİ, Çınar T, Tekkeşin Aİ. Fibrinogen and D-dimer variances and anticoagulation recommendations in Covid-19: current literature review. Rev Assoc Med Bras. 2020;66(6):842–848. doi:10.1590/1806-9282.66.6.842
3. Westblade LF, Simon MS, Satlin MJ. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol. 2021;29(10):930–941. doi:10.1016/j.tim.2021.03.018
4. Gürün Kaya A, Öz M, Erol S, Çiftçi F, Çiledağ A, Kaya A. High flow nasal cannula in COVID-19: a literature review. COVID-19ʹda yüksekakımlınazalkanüloksijenkullanımı: literatürtaraması. TuberkToraks. 2020;68(2):168–174. doi:10.5578/tt.69807
5. World Health Organization. Report of the WHO-China joint mission on Coronavirus Disease 2019 (COVID-19) 16–24 February 2020 [Internet]. Geneva: World Health Organization; 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
6. Truong AD, Auld SC, Barker NA, et al. Therapeutic plasma exchange for COVID-19-associated hyperviscosity. Transfusion. 2021;61(4):1029–1034. doi:10.1111/trf.16218
7. Hashemian SM, Shafigh N, Afzal G, et al. Plasmapheresis reduces cytokine and immune cell levels in COVID-19 patients with acute respiratory distress syndrome (ARDS). Pulmonology. 2021;27(6):486–492. doi:10.1016/j.pulmoe.2020.10.017
8. Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi:10.1016/j.immuni.2020.06.017
9. Balagholi S, Dabbaghi R, Eshghi P, Mousavi SA, Heshmati F, Mohammadi S. Potential of therapeutic plasmapheresis in treatment of COVID-19 patients: immunopathogenesis and coagulopathy. Transfus Apher Sci. 2020;59(6):102993. doi:10.1016/j.transci.2020.102993
10. Turgutkaya A, Yavaşoğlu İ, Bolaman Z. Application of plasmapheresis for Covid-19 patients. Ther Apher Dial. 2021;25(2):248–249. doi:10.1111/1744-9987.13536
11. Dogan L, Kaya D, Sarikaya T, et al. Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. 2020;87:155–158. doi:10.1016/j.bbi.2020.05.022
12. Sedokani A, Feizollahzadeh S. Plasmapheresis, anti-ACE2 and anti-FcγRII monoclonal antibodies: a possible treatment for severe cases of COVID-19. Drug Des Devel Ther. 2020;14:2607–2611. doi:10.2147/DDDT.S262491
13. Pourahmad R, Moazzami B, Rezaei N. Efficacy of plasmapheresis and immunoglobulin replacement therapy (IVIG) on patients with COVID-19 [published online ahead of print, 2020 Jul 31]. SN Compr Clin Med. 2020;1–5. doi:10.1007/s42399-020-00438-2
14. Frank RC, Mendez SR, Stevenson EK, Guseh JS, Chung M, Silverman MG. Obesity and the risk of intubation or death in patients with coronavirus disease 2019. Crit Care Med. 2020;48(11):e1097–e1101. doi:10.1097/CCM.0000000000004553
15. Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232.
16. Shkodivskyi P, Dressel A, Handreka R, et al. A case of acute optic neuritis during pregnancy treated by membrane-based therapeutic plasma exchanges without systemic anticoagulation. Transfus Apher Sci. 2021;60(5):103178. doi:10.1016/j.transci.2021.103178
17. Alsaffar H, Martino N, Garrett JP, Adam AP. Interleukin-6 promotes a sustained loss of endothelial barrier function via Janus kinase-mediated STAT3 phosphorylation and de novo protein synthesis. Am J Physiol Cell Physiol. 2018;314(5):C589–C602. doi:10.1152/ajpcell.00235.2017
18. Ji W, Hu Q, Zhang M, et al. The disruption of the endothelial barrier contributes to acute lung injury induced by coxsackievirus A2 infection in mice. Int J Mol Sci. 2021;22(18):9895. doi:10.3390/ijms22189895
19. Tabibi S, Tabibi T, Conic RRZ, Banisaeed N, Streiff MB. Therapeutic plasma exchange: a potential management strategy for critically ill COVID-19 patients. J Intensive Care Med. 2020;35(9):827–835. doi:10.1177/0885066620940259
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.