Back to Journals » International Journal of Nanomedicine » Volume 12
Therapeutic effects of lornoxicam-loaded nanomicellar formula in experimental models of rheumatoid arthritis
Authors Helmy HS, El-Sahar AE, Sayed RH , Shamma RN , Salama AH, Elbaz EM
Received 30 July 2017
Accepted for publication 29 August 2017
Published 22 September 2017 Volume 2017:12 Pages 7015—7023
DOI https://doi.org/10.2147/IJN.S147738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Hebatullah Samy Helmy,1 Ayman E El-Sahar,2 Rabab H Sayed,2 Rehab Nabil Shamma,3 Alaa Hamed Salama,4 Eman Maher Elbaz1
1Department of Biochemistry, 2Department of Pharmacology and Toxicology, 3Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Cairo University, 4Department of Pharmaceutical Technology, National Research Center, Cairo, Egypt
Background: Rheumatoid arthritis (RA) is a chronic inflammatory disease treated by nonsteroidal anti-inflammatory drugs (NSAIDs) including lornoxicam (LX). Nanocarriers have been used to increase the efficacy and reduce the side effects of various drugs. The objective of the present study was to compare the therapeutic efficacy of systemic administration of lornoxicam-loaded nanomicellar formula (LX-NM) with that of free LX.
Materials and methods: The LX-loaded mixed polymeric nanomicellar formula was prepared by direct equilibrium technique. Two rat models were used in the study: carrageenan-induced acute edema and Freund’s complete adjuvant (FCA)-induced chronic arthritis.
Results: The inhibitory effect of LX-NM on carrageenan-induced edema was higher than free LX for the same dose (1.3 mg/kg, i.p.). LX-NM (0.325 mg/kg, i.p.) produced effects comparable to that of diclofenac, which served as a standard. In the FCA model, daily treatment with LX-NM (0.325 mg/kg, i.p.) starting on day 14 significantly reduced the percentage of edema and increased weight growth. However, the same dose of LX failed to confer any significant change. Additionally, LX-NM significantly attenuated the rise of tumor necrosis factor-α (TNF-α), interleukin-1β, prostaglandin E2, nuclear factor-κβ, malondialdehyde and nitric oxide serum levels. In contrast, LX failed to show any significant reduction in elevated serum biomarkers except for TNF-α.
Conclusion: LX-NM is an alternative delivery system that is simply prepared at low costs. It showed a superior therapeutic efficacy against RA compared to free LX. Thus, LX-NM can be considered as a promising candidate for treatment of RA and similar inflammatory disorders.
Keywords: lornoxicam, polymeric micelles, nano-carriers, rheumatoid arthritis, inflammatory mediators
Background
Rheumatoid arthritis (RA) is a chronic autoimmune condition that affects the joints, connective tissues, muscle, tendons and fibrous tissue. It is characterized by inflammation that can lead to impaired movement, progressive disability and systemic complications. RA affects ~0.3%–1% of people worldwide, especially women, smokers and the elderly.1
Main RA treatment goals include reduction of joint pain and swelling as well as prevention of deformity and joint erosions, thus improving patients’ productivity and quality of life.2 The first choice for the management of RA symptoms is non-steroidal anti-inflammatory drugs (NSAIDs). However, treatment protocols suggest using NSAIDs at the lowest effective dose for the shortest possible time due to their potential gastrointestinal, liver, cardiac and renal toxicities.3
One of the approaches adopted to avoid toxicity of NSAIDs and allow its long-term use is to load them on carrier systems.4 Among the different nanocarriers are micellar delivery systems. These systems are <200 nm and are designed to encapsulate lipophilic drugs in order to increase drug stability in plasma, enhance tissue penetration and targetability and avoid nonspecific distribution and toxicity.5
Lornoxicam (LX) is an NSAID of oxicam class. It is a yellow crystalline solid with an acid ionization constant (pKa) of 4.7. Its molecular weight is 371.8. It is highly ionized at physiological pH and has relatively low lipophilicity. Systemically, LX has analgesic, antipyretic and anti-inflammatory action because it inhibits the action of cyclooxygenase (COX) enzyme and consequently prevents prostaglandin biosynthesis. Prostaglandins are involved in all phases of inflammatory events including fever and pain reactions.6 Compared to other NSAIDs, LX is a strong anti-inflammatory drug. Its analgesic activity is comparable to that of opioids.7 Besides, LX was suggested to have protective effects against RA. It stimulates the synthesis of reparative proteoglycans and inhibits the release of superoxide radical and platelet-derived growth factor, both of which contribute to the pathogenesis of RA.8
Previous studies including ours demonstrated a considerable anti-inflammatory effect of the topical application of nano-formulas that contain LX.9,10 However, little work has addressed the effects of LX-loaded nano-systems when administered systemically. The objective of the present study was to assess the therapeutic efficacy of the systemic administration of lornoxicam-loaded nanomicellar formula (LX-NM) in rat models of acute or chronic inflammation. The fore-mentioned formula was prepared by our research colleagues who previously reported its effective ocular penetration with least adverse effects.9 To provide extra evidence on the effectiveness of the LX-NM formula as an alternative delivery system, we compared its possible anti-inflammatory and antioxidant effects to those shown by the free drug in experimental models of RA.
Materials and methods
Chemicals
Tetronic® 701 (T701), Synperonic® PE/P84 (P84), diclofenac sodium, carrageenan and Freund’s complete adjuvant (FCA) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). LX was kindly provided by Delta Pharma (10th of Ramadan City, Egypt).
Preparation of polymeric nanomicellar systems
Mixed polymeric nanomicellar systems were prepared by direct equilibrium technique using the method described previously.11 Briefly, the nanomicellar system was prepared by dissolving accurately weighted amounts (0.5 g) of each polymer (T701, P84) in 10 mL distilled water and left to equilibrate in a thermostatically controlled water bath adjusted at 37°C for 24 hours. Afterward, accurately weighted amount of LX (1 mg/mL) was dissolved in the prepared nanomicellar system to obtain LX-loaded nanomicelles.
LX-NM was prepared by dissolving LX (1 mg/mL) in a previously prepared polymeric nanomicellar system. The analysis method of the formula was ultraviolet (UV) analysis at 378 nm, as mentioned in our previous work.9
Determination of the size of the prepared mixed polymeric nanomicellar systems
The hydrodynamic diameter12 and the polydispersity index (PDI) of LX-loaded mixed polymeric nanomicellar systems were determined by photon correlation spectroscopy (PCS) that analyzes the fluctuations in light scattering owing to the Brownian movement of particles. The nanomicellar system was diluted (10 times) with bidistilled water and was placed into a quartz cuvette at 25°C±0.5°C, at 90° to the incident beam using a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcester-shireMalvern, UK). All measurements were performed, in triplicate.
Morphological examination of prepared mixed polymeric nanomicellar systems
The morphological appearance of the prepared LX-loaded micellar system was evaluated using transmission electron microscopy (TEM). One drop of the diluted vesicular dispersion was deposited on the surface of a carbon-coated copper grid, negatively stained with 2% phosphotungstic acid, and then allowed to dry at room temperature for 10 minutes for investigation by TEM.
Animals
Adult male Wistar rats, weighing 120–180 g, were obtained from the animal facility of Faculty of Pharmacy, Cairo University, Egypt. Animals were housed under controlled environmental conditions: constant temperature (25°C±2°C), humidity (60%±10%) and a 12/12-hour light/dark cycle. Standard chow diet and water were allowed ad libitum. The investigation was approved by the ethics committee of Animal Experimentation of Faculty of Pharmacy, Cairo University (Approval number: BC2001), and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85–23, revised 1996).
Carrageenan-induced hind paw acute edema
The experiments were conducted according to the method of Winter et al.13 In brief, 0.1 mL of carrageenan (1% w/v in saline) was injected into the subplantar region of the left hind paw. As a control, the contralateral paw (right hind paw) received 0.1 mL of saline. At hourly intervals for 4 hours after carrageenan administration, edema was measured by using Plethysmometer (Ugo Basile 7140; Ugo Basile, Varese, Italy). Edema is expressed in milliliter as the difference between the right and left paws. The animals were pretreated with diclofenac (3 mg/kg, i.p.),14 LX-NM (0.325, 0.65 and 1.3 mg/kg, i.p.) or free LX in saline solution (1.3 mg/kg, i.p.)15 1 hour before injection of carrageenan. Percent inhibition of edema is directly proportional to the anti-inflammatory activity.
FCA-induced chronic arthritis
Arthritis was induced in rats by subplantar injection of 400 μL of FCA in the right hind paw.16 As a control, the contralateral paw (left paw) received 400 μL of saline. After 14 days, rats were allocated to four groups of six animals each, treated as follows: 1) FCA control; 2) diclofenac group: received diclofenac as a reference drug in the dose of 3 mg/kg, i.p.; 3) LX group: received free LX in a dose of 0.325 mg/kg i.p. and 4) LX-NM group: received LX-NM in a dose of 0.325 mg/kg i.p. based on the results of carrageenan-induced hind paw edema. A separate group of animals was run concurrently as a normal control and received saline.
The anti-inflammatory activity was assessed by measuring edema on days 0, 2, 6, 10, 14, 18, 22, 24 and 28 following FCA injection using Plethysmometer (Ugo Basile 7140) and was expressed in milliliter as the difference between the right and left paws. The body weight of rats was measured every 7 days, and the changes in body weight are shown as weight growth (g).
After measuring paw volume of rats on day 28, blood was withdrawn from retro-orbital plexus under thiopental anesthesia before sacrificing them by cervical dislocation. The blood was used to separate serum for assessment of oxidative stress and inflammation biomarkers. Hind paws were removed from rats for histological examination.
Determination of oxidative stress biomarkers
Serum malondialdehyde (MDA) was determined as thiobarbituric reactive substances (TBARS) according to the method described by Mihara and Uchiyama17 and expressed as nanomoles per milliliter. Serum nitric oxide (NO) was determined as total nitrate/nitrite (NOx) spectrophotometrically at 540 nm using Griess reagent after reducing nitrate to nitrite by vanadium trichloride18 and was expressed as moles per milliliter.
Determination of inflammatory biomarkers
Serum levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), prostaglandin E2 (PGE2) and nuclear factor-κβ (NF-κβ) were measured using commercially available rat-specific enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Histopathological examination
Hematoxylin and eosin (H&E) staining of separated tissue specimens was performed, fixed in 10% neutral-buffered formalin and decalcified in EDTA for 30 days at 4°C. After procession for paraffin embedding, sections were cut at 4 μm thicknesses, stained with H&E and viewed under a light microscope.
Histopathological changes were scored using semiquantitative grading with five scores as follows: 0, normal; 1, minimal synovitis without cartilage/bone erosion; 2, synovitis with some marginal erosion but with joint architecture maintained; 3, moderate synovitis and erosion with a change in joint architecture and 4, severe synovitis and erosion with loss of normal joint architecture.19
Statistical analysis
The data are presented as mean ± standard error of the mean (SEM). Data were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test. The GraphPad Prism software (version 5; GraphPad Software, Inc., La Jolla, CA, USA) was used to perform the statistical analysis and create the graphical presentations. The level of significance was fixed at p≤0.05, with respect to all statistical tests.
Results
Characters of the prepared polymeric nanomicellar system
Polymeric micelles including LX showed a mean diameter of 169.45 nm, with narrow size distribution (PDI =0.243). TEM images of the optimized LX-loaded nanomicellar system confirmed that the particles were non-aggregated and spherical in shape with narrow size distribution (Figure 1). It was observed from different frames that only a monomodal size species was present throughout the investigated sample. The mean size of the particles observed in the TEM micrographs was in good agreement with the size obtained from the particle size analyzer.
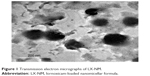  | Figure 1 Transmission electron micrographs of LX-NM. |
Effects of LX and LX-NM on carrageenan-induced hind paw acute edema in rats
The injection of carrageenan produced a gradual increase in edema volume over the 4 hours experimental period. Treatment with free LX (1.3 mg/kg, i.p.) and LX-NM (0.325, 0.65 and 1.3 mg/kg, i.p.) significantly decreased the edema volume at the third hour and fourth hour after induction of paw edema (Figure 2). The higher doses of LX-NM (0.65 and 1.3 mg/kg, i.p.) exerted more significant reduction in edema volume (p<0.001) than that exerted by the low dose of LX-NM (0.325 mg/kg, i.p.) (p<0.01) at the third hour and fourth hour after induction of paw edema (Figure 2). The inhibitory effect of LX-NM on the paw edema was higher than that of free LX for the same dose (1.3 mg/kg, i.p.) at the same time. Moreover, the low dose of LX-NM (0.325 mg/kg, i.p.) produced effects comparable to that of diclofenac (3 mg/kg, i.p.); accordingly, this dose was selected to evaluate the anti-inflammatory activity of LX-NM against chronic inflammation (Figure 2).
Effect of LX and LX-NM on percentage edema and weight growth in FCA-induced chronic arthritis in rats
Injection of FCA led to a marked peripheral edema of the injected paw in all treatment groups during the first 14 days. Daily treatment with LX-NM (0.325 mg/kg, i.p.) starting on day 14 significantly reduced the percentage of edema when compared with the FCA group and produced enhanced reduction than that of diclofenac (3 mg/kg, i.p.; Figure 3). The relationship between the extent of joint inflammation and the weight loss was also investigated. As shown in Figure 4, animals treated with FCA gained weight more slowly than the normal control group. Although the rate of weight gain in LX-NM- and diclofenac-treated rats was lower than that of the normal control group, it was still much higher than that of FCA-injected rats. However, free LX (0.325 mg/kg) failed to produce any significant effect in FCA-induced arthritic rats.
Effect of LX and LX-NM on inflammatory biomarkers in FCA-induced chronic arthritis in rats
The development of arthritis after 28 days was associated with a marked increase in the serum cytokines TNF-α and IL-1β to reach 15-fold and 20-fold of the normal control values, respectively (p<0.001). The serum levels of PGE2 and NF-κB were also increased to reach 10-fold and sixfold of the normal control values, respectively (p<0.001). Diclofenac (3 mg/kg, i.p.) and LX-NM (0.325 mg/kg, i.p.) nearly normalized the level of TNF-α and significantly attenuated the rise in IL-1β, PGE2 and NF-κB levels as compared with the FCA group (p<0.001). Free LX (0.325 mg/kg, i.p.) significantly decreased serum TNF-α in FCA rats (p<0.05) but failed to show any significant effect in serum IL-1β, PGE2 and NF-κB levels (Figure 5).
Effect of LX and LX-NM on oxidative stress biomarkers in FCA-induced arthritis in rats
MDA and NO levels were increased significantly in the serum of FCA-injected rats to reach sevenfold and fivefold of the normal control values, respectively (p<0.001). Treatment with diclofenac (3 mg/kg, i.p.) and LX-NM (0.325 mg/kg, i.p.) attenuated the rise in both parameters as compared with the FCA group (p<0.001). However, free LX (0.325 mg/kg, i.p.) failed to produce any significant change in serum MDA and NO levels (Figure 6).
Histopathological findings
The control group showed normal paw tissue histology (Figure 7A). While the FCA group showed massive inflammatory cell infiltration in the dermis of the skin, the subcutaneous tissues and periosteum which showed detectable osteodystrophy and osteoporosis (Figure 7B1) in addition to articular cartilaginous surface focal degeneration and cellular proliferation of the synovial membrane (Figure 7B2). LX administration (0.325 mg/kg, i.p.) did not improve the histological damage due to FCA injection (Figure 7C). However, the infiltration of inflammatory cells was significantly decreased with diclofenac (3 mg/kg, i.p.) or LX-NM (0.325 mg/kg, i.p.) treatments (Figure 7D1 and E1). Additionally, diclofenac group (Figure 7D2) showed intact cartilaginous, bone and synovial structures. Similarly, the articular cartilaginous surface and synovial membrane were histologically intact in the LX-NM group (Figure 7E2). Histological scores also indicated that only diclofenac and LX-NM managed to significantly reduce the histological changes in FCA-induced chronic arthritis (Figure 7F).
Discussion
RA is a chronic inflammatory disease often causing pain, disability and deformity in joints.1 First-line treatments include systemic NSAIDs such as LX. However, the available literature precludes using LX for long-term inflammatory control due to its significant side effects. LX-reported complications include hair loss,20 gastrointestinal bleeding, cardiovascular diseases21 and lung disorders.22 Thus, new delivery systems are needed to avoid LX toxicity and allow its long-term use. Nanocarriers such as liposomes and polymeric micelles have been used for drug delivery and targeting, thus increasing therapeutic efficacy and minimizing adverse effects at nontarget sites.5
In the present study, polymeric micelles including LX were fabricated using a simple method and showed a mean diameter of 169.45 nm, with narrow size distribution (PDI =0.243). Furthermore, we studied the therapeutic efficacy of the prepared formula, LX-NM in two classical RA rat models. The first was carrageenan-induced acute edema. The other was FCA-induced arthritis, which is a model of chronic polyarthritis with features that resemble RA. FCA-induced arthritis produces an immune reaction that involves inflammatory destruction of cartilage and joints associated with the swelling of surrounding tissues. Both models have been widely used for the evaluation of different NSAIDs including LX.6,23 We used the acute inflammatory model to select the dose of LX-NM that gives anti-inflammatory activity comparable to that of diclofenac, which served as a standard.24 The chronic model was further used to compare the effects of the selected dose of LX-NM to those shown by the same dose of the free drug. It is worth mentioning that the used dose for LX-NM is a subtherapeutic dose and represents one-fourth of the median effective dose (1.3 mg/kg) of LX for the anti-inflammatory effect.15
In the present study, we observed that LX-NM exerted a significantly greater inhibitory effect on circulating inflammatory mediators than that shown by free LX. Mediators of inflammation include reactive oxygen species and cytokines. TNF-α and IL-1β are considered to be the major proinflammatory cytokines that mediate the pathogenesis of RA.25 Systemically, they activate NF-κβ signaling pathway. NF-κβ is a pivotal regulator of inflammatory response in RA. It induces the expression of several genes, including those of inducible nitric oxide synthase, and COX-2 with subsequent production of NO and PGE2.26 PGE2 can elicit and amplify inflammatory symptoms such as edema and hyperalgesia. Additionally, it has catabolic activity mediated by metalloproteinases, resulting in cartilage and bone erosions.27 NO also has several effects related to the pathogenesis of joint inflammation and tissue damage in RA, including stimulating blood flow, modulating the immune response, inducing bone resorption and mediating apoptosis.28
Our results showed that LX-NM significantly reduced serum levels of NO and PGE2. These findings were consistent with those of previous studies of Futaki et al29 and Berg et al30 who related it to the ability of LX to inhibit the synthesizing enzymes iNOS and COX1-2. We also observed a significant ameliorating effect of LX-NM on TNF-α, IL-1β and NF-κβ. However, this was not in agreement with Berg et al30 This may go to their use of a cell line that does not involve production of prostaglandins. LX can indirectly reduce release of cytokines via inhibiting synthesis of prostaglandins. Prostaglandins, besides their own inflammatory effects, promote the formation of other substances such as proinflammatory cytokines, histamine and NO creating an inflammatory loop.31,32
TNF-α and IL-1β are classified as sarcoactive cytokines; they stimulate protein catabolism and whole-body protein loss.33 This can account for the observed weight loss in the rats of the FCA group. However, LX-NM managed to maintain normal weight growth and improve arthritic symptoms via suppressing serum TNF-α, IL-1β and NF-κβ levels.
Inflammatory diseases are characterized by increased oxidative stress. MDA is considered an important marker of lipid peroxidation in RA, reviewed by a previous study.34 In addition to its anti-inflammatory effect, LX-NM showed a potent antioxidant activity evidenced by its ability to significantly reduce elevated serum levels of MDA. Our results are supported by those reported by Ayan et al.35
The superior anti-inflammatory efficacy of LX-NM at a subtherapeutic dose can be related to the incorporation of the drug into nanomicelles. Compared to the other micelles, polymeric ones have lower critical micellar concentration, higher ability to withstand dilution and greater drug solubilizing and stabilizing potential. It has been reported that poloxamine-based nanomicellar systems, similar to that used in our study, exhibit an enhanced adsorption pattern to plasma proteins. Such fore-mentioned properties of polymeric nanomicellar carriers lead to reduced reticuloendothelial uptake and prolonged circulation time of the loaded drugs and thus their promoted systemic efficacy.5
Besides its systemic effects, LX-NM possesses superior local action. Our results showed that LX-NM, not free LX, significantly inhibited FCA-induced long-term joint swelling. Furthermore, the histopathological observations were consistent with and supported our laboratory findings. A considerable explanation is that RA microenvironment probably allows site-specific delivery of LX-NM. Inflammation is characterized by plasma leakage into injured tissues due to the presence of pores in the endothelial cell lining. Moreover, overexpressed inflammatory mediators such as TNF-α and IL-1β trigger vasodilatation and induce neutrophil and monocyte recruitment from circulation. This develops additional endothelial gaps,36 which facilitates the passive targeting by nanosized drug carriers.37 In addition to increased permeability of inflamed tissues, they are characterized by relatively low pH.38 This property can mediate higher release of LX from polymeric nanomicelles at arthritic sites. A previous study stated that at low pH, polymeric chains undergo conformational changes creating stress and further distortion of the micellar core, which causes the leakage of the entrapped drug.39
Conclusion
LX-NM that was prepared by a simple and cost-effective technique showed a superior therapeutic efficacy in experimentally induced RA compared to free LX. This effect can be achieved by systemic suppression of inflammatory mediators and selective targeting to inflamed sites. Our results suggest LX-NM as a promising candidate for RA treatment. Further clinical studies concerning LX-NM potency against various inflammatory disorders and its potential to improve the adverse effects of the free drug are needed.
Disclosure
The authors report no conflicts of interest in this work.
References
Chaudhari K, Rizvi S, Syed BA. Rheumatoid arthritis: current and future trends. Nat Rev Drug Discov. 2016;15(5):305–306. | ||
Wasserman AM. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2011;84(11):1245–1252. | ||
Deighton C, O’Mahony R, Tosh J, Turner C, Rudolf M. Management of rheumatoid arthritis: summary of NICE guidance. BMJ. 2009;338:b702. | ||
Couvreur P, Barratt G, Fattal E, Legrand P, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carrier Syst. 2002;19(2):99–134. | ||
Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond). 2010;5(3):485–505. | ||
Pruss TP, Stroissnig H, Radhofer-Welte S, et al. Overview of the pharmacological properties, pharmacokinetics and animal safety assessment of lornoxicam. Postgrad Med J. 1990;66(suppl 4):S18–S21. | ||
Radhofer-Welte S, Rabasseda X. Lornoxicam, a new potent NSAID with an improved tolerability profile. Drugs Today (Barc). 2000;36(1):55–76. | ||
Tayal S. The role of lornoxicam in pain and inflammation: a review. Curr Res Pharm Sci. 2012;1:1–4. | ||
Salama AH, Shamma RN. Tri/tetra-block co-polymeric nanocarriers as a potential ocular delivery system of lornoxicam: in-vitro characterization, and in-vivo estimation of corneal permeation. Int J Pharm. 2015;492(1–2):28–39. | ||
Narahari N, Das M. Lornoxicam loaded solid lipid nanoparticles for topical delivery: ex vivo assessment and pharmacodynamics activity. World J Pharm Sci. 2005;3(5):862–871. | ||
Chen F, Rice KC, Liu XM, Reinhardt RA, Bayles KW, Wang D. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm Res. 2010;27(11):2356–2364. | ||
Kulthe SS, Inamdar NN, Choudhari YM, Shirolikar SM, Borde LC, Mourya VK. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: implications for controlled and targeted drug delivery. Colloids Surf B Biointerfaces. 2011;88(2):691–696. | ||
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. | ||
el-Ghazaly M, Kenawy S, Khayyal MT, Roushdy H, Saleh S. Effect of exposure to radiation on the inflammatory process and its influence by diclofenac. Br J Pharmacol. 1985;85(1):45–50. | ||
Bianchi M, Panerai AE. Effects of lornoxicam, piroxicam, and meloxicam in a model of thermal hindpaw hyperalgesia induced by formalin injection in rat tail. Pharmacol Res. 2002;45(2):101–105. | ||
Pearson CM. Experimental models in rheumatoid disease. Arthritis Rheum. 1964;7:80–86. | ||
Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278. | ||
Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. | ||
Zhang ZC, Zhang SJ, Jin B, et al. Ciclamilast ameliorates adjuvant-induced arthritis in a rat model. Biomed Res Int. 2015;2015:786104. | ||
Keny MS, Ghodge RR, Bandekar SM. Lornoxicam-induced hair loss: an unusual case. J Postgrad Med. 2013;59(3):218–219. | ||
Hillstrom C, Jakobsson JG. Lornoxicam: pharmacology and usefulness to treat acute postoperative and musculoskeletal pain a narrative review. Expert Opin Pharmacother. 2013;14(12):1679–1694. | ||
Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis. 2014;6(suppl 1):S78–S98. | ||
Whiteley PE, Dalrymple SA. Models of Inflammation: Carrageenan- Induced Paw Edema in the Rat. In: Current Protocols in Pharmacology. Hoboken, NJ, USA: John Wiley and Sons, Inc.; 2001. | ||
Bhattacharya A, Agrawal D, Sahu P, Kumar S, Mishra S, Patnaik S. Analgesic effect of ethanolic leaf extract of Moringa oleifera on albino mice. Indian J Pain. 2014;28(2):89–94. | ||
Feldmann M, Brennan FM, Foxwell BM, Maini RN. The role of TNF alpha and IL-1 in rheumatoid arthritis. Curr Dir Autoimmun. 2001;3:188–199. | ||
Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis NF-κB and its relevance to arthritis and inflammation. Rheumatology. 2008;47(5):584–590. | ||
Fattahi MJ, Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis. 2012;2012:7. | ||
van’t Hof RJ, Hocking L, Wright PK, Ralston SH. Nitric oxide is a mediator of apoptosis in the rheumatoid joint. Rheumatology. 2000;39(9):1004–1008. | ||
Futaki N, Harada M, Sugimoto M, et al. The importance of brain PGE2 inhibition versus paw PGE2 inhibition as a mechanism for the separation of analgesic and antipyretic effects of lornoxicam in rats with paw inflammation. J Pharm Pharmacol. 2009;61(5):607–614. | ||
Berg J, Fellier H, Christoph T, Grarup J, Stimmeder D. The analgesic NSAID lornoxicam inhibits cyclooxygenase (COX)-1/-2, inducible nitric oxide synthase (iNOS), and the formation of interleukin (IL)-6 in vitro. Inflamm Res. 1999;48(7):369–379. | ||
Davies P, Bailey PJ, Goldenberg MM, Ford-Hutchinson AW. The role of arachidonic acid oxygenation products in pain and inflammation. Annu Rev Immunol. 1984;2:335–357. | ||
Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990;2(3):205–210. | ||
Rall LC, Roubenoff R. Rheumatoid cachexia: metabolic abnormalities, mechanisms and interventions. Rheumatology (Oxford). 2004;43(10):1219–1223. | ||
Quiñonez-Flores CM, González-Chávez SA, Del Río Nájera D, Pacheco-Tena C. Oxidative stress relevance in the pathogenesis of the rheumatoid arthritis: a systematic review. Biomed Res Int. 2016;2016:6097417. | ||
Ayan E, Bayram Kaplan M, Koksel O, et al. Efficiency of lornoxicam in lung and trachea injury caused by peroxynitrite. Pulm Pharmacol Ther. 2008;21(1):201–207. | ||
Ochoa CD, Stevens T. Studies on the cell biology of interendothelial cell gaps. Am J Physiol Lung Cell Mol Physiol. 2012;302(3):L275–L286. | ||
Chiong HS, Yong YK, Ahmad Z, et al. Cytoprotective and enhanced anti-inflammatory activities of liposomal piroxicam formulation in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int J Nanomedicine. 2013;8:1245–1255. | ||
Andersson SE, Lexmuller K, Johansson A, Ekstrom GM. Tissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the rat. J Rheumatol. 1999;26(9):2018–2024. | ||
Wei L, Cai C, Lin J, Chen T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials. 2009;30(13):2606–2613. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.