Back to Journals » OncoTargets and Therapy » Volume 12
Therapeutic Effect Of First-Line EGFR-TKIs Combined With Concurrent Cranial Radiotherapy On NSCLC Patients With EGFR Activating Mutation And Brain Metastasis: A Retrospective Study
Authors An N, Wang H, Li J, Zhai X, Jing W, Jia W, Kong L, Zhu H, Yu J
Received 15 July 2019
Accepted for publication 20 September 2019
Published 8 October 2019 Volume 2019:12 Pages 8311—8318
DOI https://doi.org/10.2147/OTT.S223216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Ning An,1 Haoyi Wang,2 Ji Li,3 Xiaoyang Zhai,3 Wang Jing,3 Wenxiao Jia,3 Li Kong,3 Hui Zhu,3 Jinming Yu3
1Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong University, Jinan, People’s Republic of China; 2Department of Hematology, Qilu Hospital, Shandong University, Jinan, People’s Republic of China; 3Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, People’s Republic of China
Correspondence: Hui Zhu; Jinming Yu
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, People’s Republic of China
Tel/fax +86 531 6762 6782; +86 531 6762 6947
Email [email protected]; [email protected]
Purpose: Non-small cell lung cancer (NSCLC) patients with EGFR mutation are suffering from a high incidence of brain metastasis (BM). It is still controversial whether cranial radiotherapy could be delayed when the EGFR-tyrosine kinase inhibitors (TKIs) used as first-line therapy for EGFR-positive patients with BM. This study aims to investigate the therapeutic effect of TKIs combined with concurrent cranial radiotherapy on BM.
Patients and methods: NSCLC patients with EGFR mutation and BM were retrospectively analyzed from January 2013 to December 2016 in Shandong Cancer Hospital. Identified cases were treated with first-line EGFR-TKIs with or without concurrent cranial radiation.
Results: A total of 64 eligible patients were enrolled in this study, while 35 patients received first-line EGFR-TKIs plus cranial radiotherapy (RT+TKI group) and 29 patients received first-line EGFR-TKIs only (TKI alone group). The intracranial progression-free survival (PFS) of the RT+TKI group was significantly longer than the TKI alone group (25 vs 16 months; p=0.019), but no significant differences were observed between the two groups on extracranial PFS (20 vs 17 months, p=0.660). The median overall survival was also longer in the RT+TKI group (31 vs 24 months, p=0.019).
Conclusion: Our retrospective data suggest that first-line TKIs plus concurrent cranial radiotherapy is a promising therapeutic strategy that led to remarkable intracranial PFS improvement and survival benefits for EGFR-mutant NSCLC with BM. Hence, it should be considered as a crucial treatment method during clinical management.
Keywords: non-small cell lung cancer, EGFR mutation, brain metastasis, radiotherapy, tyrosine kinase inhibitor
Introduction
Lung cancer continues to be the most common cancer (11.6% of all cases) and the leading cause of mortality (18.4% of the total cancer deaths).1 The 5-year survival rate of lung cancer is less than 10% in patients with advanced disease.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers.2 EGFR positive mutations are key drivers of NSCLC, especially in adenocarcinoma, which occurred in 47.9% Asian patients and 19.2% Western patients.2,3 With the development of EGFR-tyrosine kinase inhibitors (TKIs), patients tend to have survival benefits with EGFR mutation. However, NSCLC patients with EGFR mutations suffering a higher incidence of brain metastasis (BM), with a range of 44% to 63%.4
Cranial radiotherapy, including whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS) or WBRT with simultaneous integrated boost (WBRT-SIB), is the cornerstone treatment for patients with BM. The choice of radiation regimen was dependent on the tumor characteristics, such as the site of lesions, the number and size of tumors.5,6 However, cranial radiotherapy, especially WBRT, causes central nervous system (CNS) toxicity like general weakness and lymphocytopenia. It also causes long-term effects such as neurocognitive function impaired, which mainly occur in elderly patients.7 A lower quality of life after WBRT may trouble the patients. SRS has also been used to treat BM, but is only applicable to the situation of limited number and size of brain lesions.8
Considering the good effect of EGFR-TKIs in the treatment of BM, it is notionally rational to treat BM of EGFR-mutant NSCLC patients with EGFR-TKIs. The clinical efficacy of EGFR-TKIs in NSCLC with BM has been reported.9,10 In a Phase II trial, EGFR-mutant NSCLC patients with BM had a longer intracranial progression-free survival (PFS) than those without EGFR mutation (median PFS, 15.2 vs 4.4 months, p<0.02) when treated with EGFR-TKIs.9 And in a prospective study, EGFR-TKIs reached an intracranial disease control rate of 93% (83% of patients getting a partial response, 11% getting stable disease).10
Preclinical data reported that EGFR-TKIs might have synergistic effect with radiation therapy on tumor control.11,12 And some studies have pointed out that EGFR-TKIs can be applied concurrently with cranial radiotherapy and gain good curative effect.13 However, whether the concurrent combination of cranial radiotherapy and TKIs could bring curative benefits is controversial. A recent Phase II study failed to demonstrate any benefits of intracranial PFS and overall survival (OS) when concurrent WBRT and erlotinib compared with WBRT alone in EGFR-mutant NSCLC patients with multiple BM.14 The therapeutic effects of combination therapy may relate to multiple factors, such as the applying time of cranial radiotherapy. It has been discussed whether the time of cranial radiotherapy could be delayed after TKI administration for EGFR-mutant NSCLC patients with newly developed BM to avoid the serious neurological side effects.15,16 However, no Phase III clinical trial has been published to show compelling evidence.
The optimal management of BM in patients with EGFR-mutant NSCLC is controversial. Aiming at finding the therapeutic effect of concurrent cranial radiotherapy combined with EGFR-TKIs, we compared the intracranial PFS and OS following first-line TKIs treatments with and without concurrent cranial radiotherapy in newly diagnosed EGFR-mutant NSCLC patients with BM.
Materials And Methods
Patients
We reviewed the clinical records of patients diagnosed with lung adenocarcinoma and BM at Shandong Cancer Hospital (Shandong, People’s Republic of China) between January 2013 to December 2016. The clinical records of these patients included their demographic data, clinical characteristics, tumor‐related features, treatment modalities, and clinical outcomes. Clinical characteristics included age, gender, smoking status, and KPS at diagnosis. Tumor‐related features comprised NSCLC histological types, EGFR/ALK mutation status, treatments (including EGFR-TKIs and cranial radiotherapy), gadolinium‐enhanced magnetic resonance imaging (MRI) findings and date of death or last follow‐up. The inclusion criteria were: 1) diagnosed with lung adenocarcinoma histopathologically or cytologically; 2) BM identified by MRI; 3) patients with sensitive EGFR mutations (19del and 21L858R); 4) first-line treatment of EGFR-TKIs; and 5) cranial radiotherapy was conducted at the initial of TKI administration. According to the treatment modality, all enrolled patients were divided into 2 groups: first-line TKIs therapy combined with concurrent cranial radiotherapy treatment group (RT +TKI group) or first-line TKIs therapy alone group (TKI alone group).
Treatment
EGFR-TKIs including erlotinib 150 mg daily, gefitinib 250 mg daily, and icotinib 125 mg 3 times daily were provided orally at recommended doses. Cranial radiotherapy, which should be conducted before progression, included WBRT, WBRT-SIB, and SRS. The choice of WBRT, WBRT-SIB, or SRS for brain RT was the decision of the multidisciplinary team ground on number of BM, patient’s age, performance status, and other individual factors.
Clinical follow-up examinations were performed every 4 to 8 weeks, including image examination, physical examination, and routine laboratory tests. PFS was defined as the time from the initiation of EGFR-TKIs treatment until the time of progression or last follow-up visit (September 18, 2018). OS was defined as the time from the initiation of EGFR-TKIs treatment until the time of death or last follow-up visit.
Statistical Analysis
PFS and OS curve were calculated by Kaplan–Meier curve. Differences in intracranial progression between TKI alone group and TKI+RT group were compared using a Fine and Gray model (competing risks model).17 Extracranial progression was regarded as competing risk events in the intracranial progression of patients. Differences in OS were compared by log-rank test. Independent factors associated with OS or PFS were calculated by Cox proportional hazards regression. Statistical significance was defined as p<0.05 with 2 sides. SPSS 20.0 (Armonk, NY, USA) and R statistical software were used to conduct statistical analyses (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Characteristics
According to the inclusion criteria, 64 eligible newly diagnosed EGFR-mutant NSCLC patients with BM treated with first-line EGFR-TKIs were enrolled in the retrospective study. The median age of patients was 53 years (range 36–81 years), 65.6% of them were female. Forty-seven patients (72.3%) were never smokers. Thirty-three patients (51.6%) had extracranial metastasis at the time of diagnosis. All the patients had 19del (30, 46.9%) or 21L858R (34, 53.1%) mutation. Determination of EGFR mutation was performed via a fragment analysis using PCR and the real-time quantitative PCR techniques. The patient characters are detailed in Table 1. Of the 64 EGFR-mutated oligometastatic NSCLC patients, 35 received TKIs plus concurrent cranial radiotherapy (10 with WBRT, 13 with WBRT-SIB, and 12 with SRS), while the other 29 cases received first-line TKI alone. The patient’s characteristics were balanced between the two groups (Table 1).
 |
Table 1 Baseline Demographic Data Of 64 Patients And Comparison Of Clinical Characteristics Between 2 Groups |
All patients were stage IV lung adenocarcinoma with BM when diagnosed. The choice of the dose-fractionation regimen was made by the treating radiotherapist, with curative intent when possible. Accepted definitive radiotherapy included SRS (30 to 45Gy in 5 to 10-fraction), WBRT (30 Gy in 3-Gy fractions), and WBRT-SIB (30Gy for the whole brain and 50–60Gy for BM).
Survival Outcomes
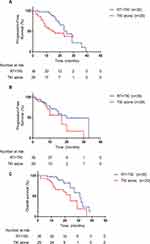
The median follow-up duration time was 30.5 months. Of the 64 patients, 21 patients (32.8%) were alive without evidence of disease progression, and 25 (39.1%) were dead due to disease progression, intracranial progression was detected in 32 patients (50.0%). The median intracranial PFS in RT+TKI group was 25 months and 16 months in TKIs alone group (p=0.019, Gray test, Figure 1A). No significant differences were observed between the two groups regarding extracranial PFS (20 vs 17 months, p=0.660, Gray test, Figure 1B). In univariate analysis of all patients included, cranial radiation therapy (HR=0.465; 95% CI: 0.224–0.965; p=0.040) is the only factor that significantly associated with longer intracranial PFS (Table 2). And multivariate analysis showed that cranial radiation therapy (HR=0.433; 95% CI: 0.207–0.905; p=0.026, Table 2) is an independent prognostic factor for PFS.
 |
Table 2 Univariate And Multivariate Analysis Of Intracranial Progression-Free Survival In Patients Treated With TKI Alone Or With Cranial Radiotherapy, According To The Cox Regression Model |
For the whole group, the median OS is 27 months. The median OS was longer in the RT+TKIs group (31 vs 24 months, p=0.019, Figure 1C) than that in TKIs alone group. 70.1% of patients in RT+TKIs group were alive 2 years later from the initial of the therapy. However, the 2-year survival rate of TKIs alone group is only 38.1%. Univariate analysis showed cranial radiotherapy plus TKI (HR=0.381, 95% CI: 0.164–0.887 P=0.025, Table 3) conduce to significantly longer OS than TKIs alone. OS was significantly related to cranial radiotherapy in multivariate analyses (HR=0.397, 95% CI: 0.169–0.932, p=0.034, Table 3). No statistic difference of whether the number of brain metastases has influence on survival was observed in this study (HR=0.481, 95% CI: 0.185–1.246, p=0.132, Table 3).
 |
Table 3 Univariate And Multivariate Analysis Of Overall Survival In Patients Treated With TKI Alone Or With Cranial Radiotherapy, According To The Cox Regression Model |
Discussion
Patients with EGFR-positive mutation were reported to have more risk suffering from BM, which may cause poor prognosis compared with other sites of metastasis.4 For the NSCLC patients with BM, radiotherapy has been considered the standard treatment. However, CNS toxicity effects such as neurocognitive dysfunction and memory loss often restrain patients from receiving adequate irradiation dose and further therapy.12 With the impressive effect that TKIs therapy achieved in EGFR-positive NSCLC patients, the status and the applying time of cranial radiotherapy have attracted a lot of debate in this cohort of patients. In this study, we observed whether the concurrent cranial radiotherapy could have a better efficiency when combined with first-line TKIs. Clinical benefits of patients were observed in both intracranial PFS and OS when treated with concurrent cranial radiotherapy and TKIs therapies.
Our results are in conformity to previous studies that reported the cranial radiotherapy to BM of NSCLC patients could prolong intracranial PFS.18,19 Consistent with the existing studies,19,20 the intracranial median PFS of patients with BM in the TKI alone group of our study was 16 months while the median PFS of RT+TKI group reached 25 months. The blood–brain barrier is the main interface between circulating blood and CNS, and it selectively limits many substances, including TKIs drugs, from entering the brain.4 It has also been reported that TKI increases the radiosensitivity in EGFR-positive tumor cells.11,21 This may explain the benefit of radiotherapy combined with TKI in patients with BM of this study.
The survival benefits of cranial radiotherapy plus TKIs are controversial. Chen et al compared 79 patients received TKIs alone therapy with 53 received TKIs plus cranial radiotherapy.19 The intracranial PFS was significantly improved in TKIs plus cranial radiotherapy (media, 24.7 vs 18.2 months, p=0.004). However, they failed to observe significant benefits in OS (median, 48.0 vs 41.1 months; p=0.740).19 Jiang’s study also reported that cranial radiotherapy plus TKIs did not result in a survival benefit compared with TKIs alone in EGFR-mutant NSCLC patients with BM.22 However, there were also other studies reported concurrent radiotherapy of BM plus TKIs for NSCLC produced longer OS.13,23 In our study, we also observed survival benefits in cranial RT+TKI group (31 vs 24 months, p=0.019, Figure 1). The prognosis of patients with BM related to multiple factors, such as the metastatic site, whether it results in symptoms, the number and diameter of the metastasis and so on.24 In this study, we observed the simple PFS with several studies but opposite results of survival. The main difference between our study and this study is that the regimen of cranial radiotherapy. Most of the patients received SRS and WBRT-SIB in our study, which could reach a therapeutic dose without distinct neurotoxicity.
In addition, the timing of cranial radiotherapy applied in the treatment of EGER-positive NSCLC with BM gets debated. Several meta-analyses discussed that for EGFR-mutated NSCLC patients with newly developed BMs, whether the time of cranial RT could be delayed after TKIs administration alone to avoid the serious neurological side effects.15,16 However, no Phase III clinical trials has been published to show compelling evidence. Due to the limitation of clinical data, the meta-analysis did not provide a definite conclusion. Ground on the currently available evidence, we can only draw a conclusion that EGFR-mutant NSCLC patients with BM may have better OS and PFS when they receive up-front cranial radiotherapy plus TKIs than TKIs alone.15,16 In our study, cranial radiotherapy was applied concurrently with the first-line TKI therapy before progression. This therapeutic regimen prolonged both intracranial PFS and OS significantly, which provides compelling evidence for the benefits of early applying of cranial radiotherapy in EGFR-mutated NSCLC patients with newly developed BMs.
We have the following limitations in this study. Firstly, the enrolled patients in our study were relatively small and they were all from a single institution. Secondly, the study was a retrospective study and some data are not available, which might cause selection bias.
Conclusion
Our retrospective data suggest that first-line TKIs plus early cranial radiotherapy is a promising therapeutic strategy that led to remarkable intracranial PFS improvement and survival benefits for EGFR-mutant NSCLC with BM. Hence, it should be considered as an important medical treatment during clinical management.
Ethical Approval
Our study was approved by the Ethics Committee of Shandong Cancer Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration.
Informed Consent
Verbal informed consent was obtained from all individual participants included in this study; for this type of study, written informed consent is not required.
Acknowledgment
The authors would such as to express their great thanks to the Innovation Project of the National Key R&D Program of China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Dearden S, Stevens J, Wu Y-L, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24(9):2371–2376. doi:10.1093/annonc/mdt205
3. Yatabe Y, Kerr KM, Utomo A, et al. EGFR mutation testing practices within the Asia Pacific region: results of a multicenter diagnostic survey. J Thoracic Oncol. 2015;10(3):438–445. doi:10.1097/JTO.0000000000000422
4. Soria J-C, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
5. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis (es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210–225. doi:10.1016/j.prro.2011.12.004
6. Scoccianti S, Ricardi U. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol. 2012;102(2):168–179. doi:10.1016/j.radonc.2011.08.041
7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665–1672. doi:10.1016/S0140-6736(04)16250-8
8. Won YK, Lee JY, Kang YN, et al. Stereotactic radiosurgery for brain metastasis in non-small cell lung cancer. Radiat Oncol J. 2015;33(3):207. doi:10.3857/roj.2015.33.3.207
9. Wu Y-L, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG–0803). Ann Oncol. 2012;24(4):993–999. doi:10.1093/annonc/mds529
10. Park S, Kim H, Lee D, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556–560. doi:10.1016/j.lungcan.2012.05.092
11. Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 2005;65(8):3328–3335. doi:10.1158/0008-5472.CAN-04-3547
12. Doherty MK, Korpanty GJ, Tomasini P, et al. Treatment options for patients with brain metastases from EGFR/ALK-driven lung cancer. Radiother Oncol. 2017;123(2):195–202. doi:10.1016/j.radonc.2017.03.007
13. Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non–small-cell lung cancer. J Clin Oncol. 2013;31(7):895. doi:10.1200/JCO.2013.49.0219
14. Lee SM, Lewanski CR, Counsell N, et al. Randomized trial of erlotinib plus whole-brain radiotherapy for NSCLC patients with multiple brain metastases. J Natl Cancer Inst. 2014;106(7). doi:10.1093/jnci/dju151
15. Du X-J, Pan S-M, Lai S-Z, et al. Upfront cranial radiotherapy vs. EGFR tyrosine kinase inhibitors alone for the treatment of brain metastases from non-small-cell lung cancer: a meta-analysis of 1465 patients. Front Oncol. 2018;8. doi:10.3389/fonc.2018.00603
16. Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up-front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: A meta-analysis. Lung Cancer. 2018;122:94–99. doi:10.1016/j.lungcan.2018.05.014
17. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144
18. Gerber NK, Yamada Y, Rimner A, et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys. 2014;89(2):322–329. doi:10.1016/j.ijrobp.2014.02.022
19. Chen Y, Yang J, Li X, et al. First-line epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinoma. Cancer Sci. 2016;107(12):1800–1805. doi:10.1111/cas.13079
20. Byeon S, Ham JS, Sun J-M, et al. Analysis of the benefit of sequential cranial radiotherapy in patients with EGFR mutant non-small cell lung cancer and brain metastasis. Med Oncol. 2016;33(8):97. doi:10.1007/s12032-016-0811-3
21. Kriegs M, Gurtner K, Can Y, et al. Radiosensitization of NSCLC cells by EGFR inhibition is the result of an enhanced p53-dependent G1 arrest. Radiother Oncol. 2015;115(1):120–127. doi:10.1016/j.radonc.2015.02.018
22. Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thoracic Oncol. 2016;11(10):1718–1728. doi:10.1016/j.jtho.2016.05.013
23. Shukuya T, Takahashi T, Naito T, et al. Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure. Lung Cancer. 2011;74(3):457–461. doi:10.1016/j.lungcan.2011.04.007
24. Baykara M, Kurt G, Buyukberber S, et al. Management of brain metastases from non-small cell lung cancer. J Cancer Res Ther. 2014;10(4):915. doi:10.4103/0973-1482.137939
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.