Back to Journals » Cancer Management and Research » Volume 12
The Vascular Index of Superb Microvascular Imaging Can Improve the Diagnostic Accuracy for Breast Imaging Reporting and Data System Category 4 Breast Lesions
Authors Cai SM, Wang HY, Zhang XY, Zhang L, Zhu QL, Li JC, Sun Q, Jiang YX
Received 12 December 2019
Accepted for publication 27 February 2020
Published 11 March 2020 Volume 2020:12 Pages 1819—1826
DOI https://doi.org/10.2147/CMAR.S242101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Si-Man Cai,1 Hong-Yan Wang,1 Xiao-Yan Zhang,1 Li Zhang,1 Qing-Li Zhu,1 Jian-Chu Li,1 Qiang Sun,2 Yu-Xin Jiang1
1Department of Medical Ultrasound, Peking Union Medical College Hospital and Chinese Academy Medical Sciences, Beijing 100730, People’s Republic of China; 2Department of Breast Surgery, Peking Union Medical College Hospital and Chinese Academy Medical Sciences, Beijing 100730, People’s Republic of China
Correspondence: Hong-Yan Wang
Department of Diagnostic Ultrasound, Peking Union Medical College Hospital, Chinese Academy Medical Sciences, N0.1 Shuai Fu Yuan, Dong Cheng District, Beijing 100730, People’s Republic of China
Tel/Fax +86 10-69155494
Email [email protected]
Purpose: To investigate whether the vascular index (VI) of superb microvascular imaging (SMI) could improve the diagnostic efficiency for BI-RADS 4 breast lesions and reduce the number of unnecessary biopsies.
Patients and Methods: For this study, we selected 222 consecutive BI-RADS 4 breast lesions detected by ultrasound and confirmed by pathology from January 2016 to October 2018. A VI of 4.0 was set as the cutoff value to degrade BI-RADS classification. We calculated the accuracy, sensitivity and PPV of a BI-RADS diagnosis alone and the combination of BI-RADS and the VI.
Results: Pathologically, of the 222 lesions, 129 were confirmed to be benign, and 93 were found to be malignant. A VI of 4.0 was set as the cutoff value; when the VI≤ 4.0, those BI-RADS 4 masses were downgraded one level (4C-4B, 4B-4A, 4A-3) to an integral BI-RADS grade, while the others maintained the conventional grade. A total of 54 BI-RADS 4 lesions were degraded to BI-RADS 3, including 53 benign lesions and 1 malignant lesion. The diagnostic accuracy (65.3% vs 41.9%) and PPV (54.8% vs 41.9%) were significantly improved. The sensitivity decreased slightly (98.9% vs 100%) because 1 of the 54 downgraded BI-RADS 4 lesions, which had a pathological type of invasive ductal carcinoma, was incorrectly downgraded.
Conclusion: SMI is a noninvasive tool for visualizing the vascular structure with high-resolution microvascular images. As a quantitative index, the VI can be used to appropriately downgrade benign lesions classified as BI-RADS 4, which can improve the diagnostic accuracy and PPV and reduce unnecessary biopsies.
Keywords: superb microvascular imaging, breast neoplasms, ultrasonography, diagnostic imaging
Introduction
Breast cancer is the second most common malignant tumor worldwide. It is also the most common malignant tumor in women and the most common cause of death in women.1,2 China accounts for 12% of the newly diagnosed breast cancer every year in the world. Breast cancer, as the most common female cancer, is a serious threat to the health of women.3,4 Because of a combination of improved imaging tools that enable earlier detection, more effective treatments, and better supportive care, the five-year net survival continues to increase in most countries.5
The current (fifth edition) US Breast Imaging Reporting and Data System6 (BI-RADS) 4 lesions are a kind of masses with certain malignant signs, which is enough to be recommended for intervention. However, it covers a wide range of malignancies, ranging from 2% to 95%. The BI-RADS classifies the malignant risk possibility of BI-RADS 4 nodules as follows: BI-RADS 4A, >2% to ≤10% likelihood of malignancy(low suspicious); BI-RADS 4B, >10% to ≤50% likelihood of malignancy (moderate suspicious); BI-RADS 4C, >50% to <95% likelihood of malignancy(high suspicious). The US subcategories for BI-RADS 4 fail to accurately distinguish the benign and malignant lesions, so quantities of benign lesions are included in the nodules recommended for biopsy and surgery.7 Studies have shown that BI-RADS 4A lesions account for about half of BI-RADS 4 lesions, but only 7.6% of those lesions yielded malignant results.8,9 Thus, BI-RADS 4A lesions are appropriate to be reduced in rank to surveillance by providing more information about mass in more ways. A desirable specificity, accuracy, positive predictive value for the assessment of BI-RADS 4 lesions can thus reduce unnecessary biopsies and surgeries.
Neovascularization and micro-vessels are always the focus of breast cancer research, which are closely related to tumor invasion and metastasis. Malignant breast lesions have higher microvascular density than the benign significantly.10 The hypervascularity of breast lesions suggests malignancy progression.11 Superb microvascular imaging (SMI) is an advanced ultrasound technology that filters the different frequency spectrum signals generated by tissue motion artifacts and displays microvascular flow through an adaptive algorithm that removes clutter dramatically.12 Some studies13,14 have shown that SMI is more likely to detect microvascular flow than color Doppler flow imaging (CDFI) in the evaluation of malignant tumors. Vascular index (VI) represents the ratio between pixels of the Doppler signal and those of the total lesion, which can be calculated automatically by delineating the lesion boundary on SMI image with the most abundant blood flow to determine the region of interest (ROI). VI can be used for quantitative evaluation of blood flow richness in breast lesions. Adding functional information to original BI-RADS classification may help the radiologist better differentiate benign from malignant masses, potentially reducing false-positive findings, particularly in patients with BI-RADS 4A lesions. Our study aims to evaluate whether the VI contributed to the degradation of BI-RADS 4 assessments assigned with BI-RADS of benign masses. Specifically, this study assesses whether benign masses categorized as BI-RADS 4A can be downgraded to BI-RADS 3 with the VI. The correct downgrading of BI-RADS 4 masses can effectively reduce unnecessary biopsies and surgeries for benign masses.
Materials and Methods
Patients
The ethics committee of Peking union medical college hospital approved this study, all patients provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki. From January 2016 to October 2018, in total, 498 consecutive female patients with 502 breast lesions underwent US examinations before biopsy or surgery (Figure 1). Ten patients were excluded for they had received radiation therapy or chemotherapy, 6 patients were excluded for they had received biopsy or surgery previous for the same lesion, 6 patients were excluded for unqualified images, 4 patients were excluded for the diameter of the breast lesions larger than the probe, 3 patients were excluded for pregnancy or lactation. A total of 251 breast lesions were excluded for not being classified as BI-RADS 4. Ultimately, 222 lesions were included in the study.
 |
Figure 1 Study flow diagram. |
US Analysis
The US Aplio500 (Canon Medical Systems, Tokyo, Japan) equipped with SMI software which was used for ultrasonic examination by high frequency (14 MHz) linear array probe. Ultrasound examinations were conducted by a registered ultrasonic doctor with more than 15 years of working experience in breast imaging and 2 months of working experience in SMI. Once a breast lesion was detected, first evaluated and rated by BI-RADS according to the ultrasonic characteristics of the lesion. The examination mode then switched to SMI, settings were as follows: frame rate 50–60 fps; velocity range 1.2–1.6 cm/s; frame rate, 25–30/s; pulse repetition frequency, 15.4–20.2 kHz. Choose an SMI plane according to qualitative SMI with the most abundant neovascularization. By setting the ROIs with no healthy tissue along the boundary of the lesion one this plane, the VI could be automatically calculated. The VI was obtained after a radiologist manually traced the lesion boundary on the SMI image three times and averaged the values. For BI-RADS, lesions are considered malignant when classified as above BI-RADS 3 and considered benign when classified as category 3. In a previous study, the sensitivity, specificity, PPV, NPV and accuracy of the VI (with 4.0 as the threshold) were 76.0%, 66.1%, 70.2%, 72.4% and 71.2% (P<0.05) respectively, showing good diagnostic efficacy,15 In our study, VI ≤4.0 was the standard for downgrading masses. BI-RADS 4 masses were downgraded one level (4A-3, 4B-A, 4C-4B) to an integral BI-RADS grade, while the others maintained the conventional grade.
Statistical Analysis
All lesions were examined by ultrasonography before the operation and confirmed by pathology after resection. For the evaluations, BI-RADS 3 lesions were considered “test negative”, and BI-RADS 4 lesions were considered “test positive”. The ROC curve of image data was analyzed and compared by MedCalc (version 15.2.2; Mariakerke, Belgium) and SPSS (version 20.0; IBM Corp, Chicago, IL, USA). The area under the ROC curve (AUC) indicates the accuracy of the diagnostic test. The larger the AUC value is, the greater the value of the diagnostic test is. When AUC is close to 0.5, the diagnosis loses its clinical significance. McNemar’s test was used to calculate and compare the sensitivity, specificity, accuracy and positive predictive value. Differences were considered statistically significant when the P-value was less than 0.05.
Results
Lesion Characteristics
A total of 222 BI-RADS 4 breast lesions were included and consisted of 99 BI-RADS 4A lesions, 64 BI-RADS 4B lesions, and 59 BI-RADS 4C lesions. The age of the patients was 47.1±11.7 (18–85) years old, and the maximum lesion diameter on B-mode imaging was 19.8±17.1 (3–79) mm. A total of 93 lesions (41.89%) were diagnosed as malignant, and 129 lesions (58.11%) were diagnosed as benign on pathology. The histopathological details are shown in Table 1. The age of the patients in the malignant group was 50.5±11.5 (30–75) years old, which was older than the age of 44.6±11.3 (18–85) years old in the benign group (t=−3.830, P<0.001). The maximum lesion diameter in the malignant group was 23.2±13.7 (4–74) mm, which was larger than the maximum lesion diameter of 17.3±12.0 (3–79) mm in the benign group (t=−3.303, P<0.001).
 |
Table 1 Histological Diagnosis of the Lesions Confirmed by Pathology |
Diagnostic Performance
For the BI-RADS 4C-4B and BI-RADS 4B-4A lesions, this downgrading did not affect clinical decisions regarding biopsy and surgery, and the lesions were always considered “test positive”. For the BI-RADS 4A-3 lesions, a change from “test positive” to “test negative” was considered, so we focused on these lesions primarily.
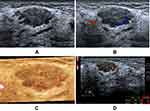
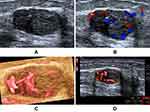
In total, 54 (24.3%) lesions were downgraded from BI-RADS 4A to BI-RADS 3. Table 2 shows the number of downgraded lesions and diagnostic performance. With the addition of the VI to the primary BI-RADS assessment, 53 (23.9%) benign BI-RADS 4A lesions (Figure 2) were successfully downgraded to BI-RADS 3, one (0.5%) BI-RADS 4A lesion was incorrectly downgraded to BI-RADS 3, 168 (75.7%) lesions (Figure 3) were BI-RADS 4 classification, which led to an improved accuracy (65.3% vs 41.9%) and PPV (54.8% vs 41.9%) for biopsy and surgery recommendations compared to the original BI-RADS assessment. The area under the ROC curve was 0.700 (95% CI: 0.635–0.760) and 0.500 (95% CI: 0.432–0.568) for diagnoses after and before downgrading, respectively. The difference in the AUC is 0.200, the Z statistic is 8.932, and P < 0.0001 (Table 3). The diagnostic performance of the BI-RADS system can be improved by the addition of the VI. One (0.5%) malignant lesion was mistakenly downgraded and had a pathological type of infiltrating ductal carcinoma.
 |
Table 2 Histological Diagnosis of the Lesions Downgraded to BI-RADS 3 |
 |
Table 3 Effect of Downgrading BI-RADS Category 4A Masses on Basis of Benign Vascular Parameters of SMI |
Discussion
As a classification system for breast lesions, the BI-RADS is conducive to the standardized management of breast lesions, which has been widely recognized and applied all over the world.16 The classification system exerts an enormous function on the diagnosis of breast lesions. At the same time to provide guidance for the scientific and effective management of breast lesions. However, it reported that more than half of the nodules that are biopsied and operated on are benign.17 There is, therefore, a great need to reduce unnecessary interventions for benign lesions by developing additional methods. Tumor angiogenesis is defined as the build-up of penetrating blood vessels of tumors that provide the necessary substances for tumor growth and metastasis.18,19 MVD is significantly higher in malignant breast lesions and may be associated with metastasis.20 Therefore, assessment of tumor angiogenesis is beneficial to diagnosis and prognosis prediction. SMI eliminates the clutter and presents more real blood flow information, which provides a more accurate vascular situation of the masses than CDFI.14,21 This method can be used as a quantitative guide by measuring VI for breast in the optimal SMI plane with the most abundant vessels. In our study, BI-RADS 4 lesions were downgraded one level (4A-3, 4B-A, 4C-4B) with 4.0 as the cutoff value of VI, the diagnostic accuracy (65.3% vs 41.9%), PPV (54.8% vs 41.9%) and AUC (0.700 vs 0.500, p < 0.001) were improved, highlighting a potential decrease in the number of biopsies and surgeries with negative findings. Compared with BI-RADS, the VI offers improved diagnostic efficiency. Park et al22 studied the diagnostic efficacy of degradation of BI-RADS 4A lesions with 8.9 as the cutoff value of VI. Twenty-six lesions were downgraded to BI-RADS 3 with improved PPV (56.9% vs 41.8%) and AUC (0.728 vs 0.500, p < 0.001) compared to the original BI-RADS assessment, consistent with our results. The addition of this index could potentially decrease false-positive findings and lessen the need for both biopsy and short-interval follow-up examinations by downgrading benign breast masses to BI-RADS 3.
We observed one false-negative finding in our study, which led to a slight decrease in sensitivity (98.9% vs 100.0%). The case involved a 57-year-old female patient with invasive ductal carcinoma, which had a maximum diameter of only 4 mm. The sensitivity of breast sonography is limited to small or non-palpable breast cancer. According to the relevant literature report, in the diagnosis of breast nodules, the accuracy of a mass diameter less than 1cm was lower than that of a mass diameter greater than 1cm.23 Among all sonographic features, irregular shape and non-circumscribed margin are significant ultrasonographic signs of small malignant breast tumors.23–25 Some malignant signs presented in large breast cancers, subcentimeter breast cancer may do not show, such as echogenic halo, hypoechogenicity. In this case, the breast lesion was small in size, had an unclear boundary, attenuated posterior echogenicity, and no blood flow signals were found on either CDFI or SMI. However, this false-negative finding would have not been missed or misdiagnosed because mammography displayed signs of malignancy, namely, speculated margins and microcalcifications. Therefore, the degradation of small breast tumors should be combined with mammography to ensure that no suspicious lesions are missed.26 Xiao et al confirmed that in evaluating subcentimeter breast masses, the specificity of CEUS was significantly higher, but the sensitivity was moderately lower than that of other imaging methods.27 A small tumor a few millimeters in size can grow without inducing substantial angiogenesis, but further expansion of the tumor cell population will require new capillary blood vessels.28 Therefore, for some subcentimeter breast cancers, the lack of internal blood flow to the nodule may be the reason for the false degradation of SMI. Although the sensitivity of detecting microvessels with imaging in small breast lesions is slightly reduced, the specificity is higher. Schmitz et al confirmed that as long as the number of blood vessels increases, regardless of tumor size, the risk of malignancy will increase.29 Overall, the features of small BI-RADS 4A masses, especially subcentimeter breast lesions, may not be similar to typical large lesions. For subcentimeter breast lesions, although negative findings on SMI have been revealed; however, there is a risk that malignant lesions could be incorrectly downgraded. Therefore, sonography and mammography should be used to thoroughly evaluate subcentimeter lesions and to facilitate clinical judgment.
There are some limitations in our study that need to be addressed. First, the number of participants was relatively small, a larger number of cases need to be included in the validation results of the study. Second, only registered ultrasonic doctors performed all the ultrasound examinations, without comparing the differences between operators.
Conclusion
Our findings show that SMI, as a tool to improve the evaluation of tumor vessels, facilitates the differential diagnosis of benign and malignant lesions. Among benign tumors classified as BI-RADS 4, 23.9% were accurately downgraded to BI-RADS 3, thus significantly improving the diagnostic accuracy and PPV of the BI-RADS system and potentially reducing both biopsies and surgeries with negative results. SMI can help reduce the amount of false-negative findings, and future analyses and studies on SMI may help unlock the full potential of this technology.
Acknowledgments
This study was supported by the National Natural Science Youth Foundation of China (81601517), Beijing Natural Science Foundation (7202156), Teaching Reform Project of Peking Union Medical College (10023201900113).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69(3):313–317. doi:10.1007/s13304-017-0424-1
2. Harbeck N, Gnant M. Breast cancer. The Lancet. 2017;389(10074):1134–1150. doi:10.1016/S0140-6736(16)31891-8
3. Fan L, Strasser Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi:10.1016/S1470-2045(13)70567-9
4. Azamjah N, Soltan Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: a 25-year study. Asian Pac J Cancer Prev. 2019;20(7):2015–2020. doi:10.31557/APJCP.2019.20.7.2015
5. Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–756. doi:10.1016/S1470-2045(08)70179-7
6. American College of Radiology. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System.
7. Spinelli Varella MA, Teixeira da Cruz J, Rauber A, Varella IS, Fleck JF, Moreira LF. Role of BI-RADS ultrasound subcategories 4A to 4C in predicting breast cancer. Clin Breast Cancer. 2018;18(4):e507–e511. doi:10.1016/j.clbc.2017.09.002
8. Elezaby M, Li G, Bhargavan Chatfield M, Burnside ES, DeMartini WB. ACR BI-RADS assessment category 4 subdivisions in diagnostic mammography: utilization and outcomes in the national mammography database. Radiology. 2018;287(2):416–422. doi:10.1148/radiol.2017170770
9. Zheng X, Huang Y, Wang Y, et al. Combination of different types of elastography in downgrading ultrasound breast imaging-reporting and data system category 4a breast lesions. Breast Cancer Res Treat. 2018;174(2):423–432. doi:10.1007/s10549-018-05072-0
10. Forsberg F, Kuruvilla B, Pascua MB, et al. Comparing contrast-enhanced color flow imaging and pathological measures of breast lesion vascularity. Ultrasound Med Biol. 2008;34(9):1365–1372. doi:10.1016/j.ultrasmedbio.2008.02.010
11. Park AY, Seo BK, Cha SH, Yeom SK, Lee SW, Chung HH. An innovative ultrasound technique for evaluation of tumor vascularity in breast cancers: superb micro-vascular imaging. J Breast Cancer. 2016;19:2. doi:10.4048/jbc.2016.19.2.210
12. Zhan J, Diao X-H, Jin J-M, Chen L, Chen Y. Superb microvascular imaging—A new vascular detecting ultrasonographic technique for avascular breast masses: a preliminary study. Eur J Radiol. 2016;85(5):915–921. doi:10.1016/j.ejrad.2015.12.011
13. Xiao X-Y, Chen X, Guan X-F, Wu H, Qin W, Luo B-M. Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol. 2016;89:1066. doi:10.1259/bjr.20160546
14. Ma Y, Li G, Li J, Ren W-D. The diagnostic value of Superb Microvascular Imaging (SMI) in detecting blood flow signals of breast lesions: a preliminary study comparing SMI to color doppler flow imaging. Medicine (Baltimore). 2015;94(36):e1502. doi:10.1097/MD.0000000000001502
15. Zhang XY, Zhang L, Li N, et al. Vascular index measured by smart 3-D superb microvascular imaging can help to differentiate malignant and benign breast lesion. Cancer Manag Res. 2019;11:5481–5487. doi:10.2147/CMAR.S203376
16. Elverici E, Barca AN, Aktas H, et al. Nonpalpable BI-RADS 4 breast lesions: sonographic findings and pathology correlation. Diagn Intervent Radiol. 2015;21(3):189–194. doi:10.5152/dir
17. Berg WA. Can optoacoustic imaging safely reduce benign breast biopsies? Radiology. 2018;287(2):413–415. doi:10.1148/radiol.2018180121
18. Yadav L. Tumour angiogenesis and angiogenic inhibitors: a review. J Clin Diagn Res. 2015. doi:10.7860/JCDR/2015/12016.6135
19. Park AY, Seo BK. Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography. 2018;37(2):98–106. doi:10.14366/usg.17043
20. Schneider BP, Miller KD. Angiogenesis of breast cancer. Comment J Clin Oncol. 2005;23(8):1614–1615. doi:10.1200/JCO.2005.01.016
21. Zhu YC, Zhang Y, Deng SH, Jiang Q. Diagnostic performance of Superb Microvascular Imaging (SMI) combined with shear-wave elastography in evaluating breast lesions. Med Sci Monit. 2018;24:5935–5942. doi:10.12659/MSM.910399
22. Park AY, Kwon M, Woo OH, et al. A prospective study on the value of ultrasound microflow assessment to distinguish malignant from benign solid breast masses: association between ultrasound parameters and histologic microvessel densities. Korean J Radiol. 2019;20(5):759–772. doi:10.3348/kjr.2018.0515
23. Chen S-C, Cheung Y-C, Su C-H, Chen M-F, Hwang T-L, Hsueh S. Analysis of sonographic features for the differentiation of benign and malignant breast tumors of different sizes. Ultrasound Obstet Gynecol. 2004;23(2):188–193. doi:10.1002/uog.v23:2
24. Korpraphong P, Tritanon O, Tangcharoensathien W, Angsusinha T, Chuthapisith S. Ultrasonographic characteristics of mammographically occult small breast cancer. J Breast Cancer. 2012;15(3):344–349. doi:10.4048/jbc.2012.15.3.344
25. Moy LSP, Moore R, Satija S, et al. Specificity of mammography and US in the evaluation of a palpable abnor- mality: retrospective review. Radiology. 2002;225:176–181. doi:10.1148/radiol.2251010999
26. Welch HG, Prorok PC, O’malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438–1447. doi:10.1056/NEJMoa1600249
27. Xiao X, Jiang Q, Wu H, Guan X, Qin W, Luo B. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound-a preliminary study in China. Eur Radiol. 2017;27(6):2443–2450. doi:10.1007/s00330-016-4628-4
28. Weidner N, S J, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:
29. Schmitz AC, Peters NHGM, Veldhuis WB, et al. Contrast-enhanced 3.0-T breast MRI for characterization of breast lesions: increased specificity by using vascular maps. Eur Radiol. 2007;18(2):355–364. doi:10.1007/s00330-007-0766-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.