Back to Journals » Infection and Drug Resistance » Volume 15
The Treatment Based on Ruxolitinib and Amphotericin B is Effective for Relapsed Leishmaniasis-Related Hemophagocytic Lymphohistiocytosis: A Case Report and Literature Review
Received 16 August 2022
Accepted for publication 19 October 2022
Published 11 November 2022 Volume 2022:15 Pages 6625—6629
DOI https://doi.org/10.2147/IDR.S384628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tingting Cui, Jingshi Wang, Zhao Wang
Department of Hematology, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, People’s Republic of China
Correspondence: Zhao Wang, Department of Hematology, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, People’s Republic of China, Tel +86-10-63138303, Email [email protected]
Background: Hemophagocytic lymphohistiocytosis (HLH) is known as a life-threatening syndrome, and Leishmania is the most common protozoan that triggers infection-related HLH. It is thus important to find the root cause and treat it effectively.
Case Report: This paper reports a 44-year-old man who developed antisynthetase antibody syndrome previously. The patient progressed rapidly to the extent of meeting the HLH-2004 diagnostic criteria, despite the unknown etiology. Although the patient was promptly treated in line with the HLH-1994 protocol to achieve remission, he still relapsed after glucocorticoid reduction. Afterwards, it was found out that HLH was secondary to Leishmania infection. The symptoms of HLH were alleviated quickly by the treatment with Ruxolitinib and Amphotericin B.
Conclusion: Etiological screening plays a crucial role in leishmaniasis-related HLH. An experienced pathologist and real-time PCR are essential for treating Leishmania. The treatment of Ruxolitinib and Amphotericin B proved effective in alleviating the relapse of visceral leishmaniasis-related HLH.
Keywords: hemophagocytic lymphohistiocytosis, visceral leishmaniasis, Amphotericin B, Ruxolitinib
Introduction
Hemophagocytic lymphohistiocytosis (HLH), a high cytokine inflammatory syndrome, is classified as primary HLH and secondary HLH (sHLH). sHLH is often induced by infectious, immunological, oncological diseases, drugs and gene replacement therapy. Leishmania is known as the most common protozoan triggering infection-related HLH.1–3 The persistently abnormal activation of cytotoxic T cells and overexpression of cytokines (including interferon-γ (IFN-γ) and interleukins 6 and 10 (IL-6 and IL-10)) are the major pathogenesis of HLH.4 For those patients with Leishmania infection, the immune cells infected with Leishmania would attract T cells and natural killer (NK) cells in the primary infectious sites and other organs.5 During this pathological process, these patients would suffer a series of proinflammatory cytokines, and the serum level of cytokines is closely related to the amount of Leishmaniasis in the bloodstream.5 Ruxolitinib inhibits inflammatory cytokines, reduces T-cell proliferation, and reverses organ damage by interfering with Janus kinase (JAK)-STAT signaling.6
The mortality rate among sHLH patients varied from 26.5 to 74.8%.7 Eighty-six percent of patients could benefit from the HLH-1994 protocol.8 It is reported in recent studies that the treatment based on Ruxolitinib for adult patients with sHLH and refractory/relapsed HLH (R/R HLH) is safe and has achieved favorable outcome.3,9–12 Since Leishmania infection would cause the overexpression of proinflammatory cytokines, it is assumed that the treatment based on Ruxolitinib may be also effective for Leishmaniasis-related HLH.
This paper reports a case of refractory Leishmaniasis-related HLH patient, whose symptoms were alleviated by the treatment based on Ruxolitinib and Amphotericin B.
Case Report
A 44-year-old male resided in Shanxi Province. This patient had a 5-year history of antisynthetase antibody syndrome and was treated with cyclosporine A. After hospital admission in January 2020, he developed a high fever of 38.6°C without cold and chills. The patient displayed breathlessness after walking 100 meters flat and struggled to squat independently. His symptoms and laboratory values showed no improvement despite broad-spectrum antimicrobials. In March 2020, the patient still suffered persistent fever. According to laboratory examination, WBC was 1.3×10^9/L, HB was 96 g/L, PLT was 113×10^9/L, FBG was 1.36 g/L, serum ferritin exceeded 1500 µg/L, NK-cell activity was 14.96%, and sCD25 was 12,947 pg/mL. ALT, AST, CK, and CK-MB were normal. The diagnosis of HLH was considered according to the symptoms displayed by the patient and the results of examination (Figure 1). HLH-directed therapy was initiated with methylprednisolone (10 mg/kg per day) and etoposide (100 mg/m2).13 After 2 weeks of chemotherapy, the patient’s body temperature returned to normal. As indicated by the results of laboratory examination, WBC was 10.8×10^9/L, HB was 95 g/L, PLT was 55×10^9/L, and FBG was 1.74 g/L.
 |
Figure 1 Patient Laboratory and Clinical Response based on HLH-2004 Clinical Criteria and Hscore. X indicates that a criterion was met. |
In June 2020, the patient developed a high fever of 39.5°C when the dose of prednisone was gradually reduced. The patient was unable to stand up independently. Before long, he exhibited pancytopenia, high serum ferritin, low NK-cell activity, high sCD25, splenomegaly, and hemophagocytosis in bone marrow (Figure 1). When analyzing the cause of HLH, we found out that he came from the endemic area. Bone marrow aspirate showed Leishman-Donovan bodies (Figure 2), and Leishmania infantum Noicolle DNA was detected in bone marrow. EBVDNA, CMVDNA, HIV serology and blood cultures were all negative. Except for primary HLH, malignancy HLH, rheumatological HLH and other types of infection all induced HLH. Eventually, the diagnosis of Leishmaniasis-related HLH was made. According to the recommendation made for the adults with HLH, the patient was treated with Amphotericin B (0.3 mg/(kg·d); dose adjusted for renal insufficiency). In the meantime, with informed consent obtained from the patient, he was also treated with Ruxolitinib in combination to relieve the displayed symptoms. 10 mg of it twice per day was prescribed on the basis of Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis.9
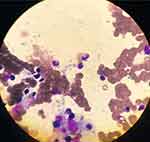 |
Figure 2 Bone marrow aspirate showed Leishman-Donovan bodies and hemophagocytosis. Wright’s staining, ×100 oil immersion lens. |
Within 24 hours of starting Ruxolitinib treatment, the patient became afebrile. In August 2020, the Leishman-Donovan bodies disappeared from the bone marrow aspirate, and the bone marrow Leishmania PCR turned negative. During a follow-up made in October 2020, the patient was treated with Ruxolitinib, and the results of laboratory examinations were normal (Figure 3).
Discussion
In China, visceral leishmaniasis (VL) was especially prevalent in Shanxi, Gansu, and Xinjiang. VL is an opportunistic disease in immunocompromised patients.14 Coming from the epidemic area of Shanxi Province, this patient had been receiving immunosuppressive therapy for quite long. Therefore, care shall be exercised when the etiology of HLH is identified for rare infections such as VL. An experienced pathologist and real-time PCR for Leishmania would be very helpful in this regard.
During the pathophysiological process of sHLH, it is difficult to eliminate infected cells due to the functional defects of NK and T cells, and IFN-γ is released in large amounts. After IFN-γ binds the IFN-γ receptor on macrophages, STAT1 is phosphorylated by JAK1/2 in the cytoplasm. When macrophages are activated, large amounts of cytokines are released, and red blood cells (RBCs) are engulfed.15
When Leishmania invades the host, it occupies the tissue macrophages in the spleen, liver, and bone marrow, thus causing a wide range of clinical symptoms. VL can develop into systemic symptoms, including splenomegaly, hepatomegaly, weight loss, persistent fever, and anemia. Cytokines are involved in the pathogenic process. IFN-γ is increased in symptomatic VL patients but reduced after treatment.16 IL-10 facilitates parasite reproduction and interferes with infection control.17 A higher IL-6 level in VL patients than 200 pg/mL is closely associated with mortality.18
Ruxolitinib is classified as a variety of JAK 1/2 inhibitor. Many of the key cytokines in HLH, including IFN-γ IL-6 and IL-10, could bind to the receptors that release signal via JAKs. The cytokine binding on the cell surface leads to the recruitment and activation (phosphorylation) of JAK proteins. This leads to the recruitment and activation (phosphorylation) of STAT proteins, STAT dimerization and nuclear translocation, which affects gene transcription. JAK inhibitors block the pathway at the JAK level and prevent STAT activation.19 A pre-clinical study demonstrated the in vivo efficacy of Ruxolitinib for sHLH, by suppressing the IFN-γ, IL-6 and IL-10 via inhibiting JAK 1/2.4 Currently, there have been promising results obtained to prove Ruxolitinib a means of treatment for HLH, including primary HLH, secondary HLH and R/R HLH.3,8 Anti-CD52 antibody alemtuzumab, anti-thymocyte globulin (ATG), IFN-gamma neutralizing antibody emapalumab, IL-18 binding protein, anti-IL-6R antibody tocilizumab were also reported for HLH.19
Amphotericin B eliminates Leishmania by binding to ergocalciferol on the cell membrane and forming pores to cause ion leakage. Ruxolitinib is effective in controlling body temperature and relieving inflammatory factor storm. Ruxolitinib is used in combination with Amphotericin B to treat a mouse model of VL. The mice treated with Ruxolitinib alone exhibited suppressed parasite growth in the liver. The parasite growth in the liver and spleen was inhibited after the use of ruxolitinib for treatment in combination with Amphotericin B.20 In a previous case, single-agent amphotericin B relieved VL-HLH and normalized body temperature after 3 days of treatment.21 In another report, 5 of 17 patients of VL-HLH died despite prompt antiparasitic and immunosuppressive treatment.22 The combination therapy is based on amphotericin B for Leishmania and Ruxolitinib to alleviate the cytokine storm. In our case, it was observed that the patient was quick to become afebrile after receiving the treatment with Ruxolitinib. Besides, the level of cytokines was reduced rapidly reduced during treatment. However, more clinical studies on VL-HLH are still required to determine when to start combination therapy.
Conclusion
Leishmania is the most common protozoan triggering infection-related HLH. The etiology of recurrent HLH should be identified with care. Experienced doctors should exercise care when browsing the bone marrow slices and PCR of Leishmania in bone marrow, which is conducive to gathering pathogenic evidence. The combined use of Ruxolitinib and Amphotericin B is effective not only in reducing severe cytokines storm, but also in treating VL.
Ethical Approval and Informed Consent
Since the data were anonymous and no threat to patients’ rights, the Ethics Committee of Beijing Friendship Hospital exempts the need for ethical approval. The patient provided written informed consent and agreed to the publication of the case.
Acknowledgments
We thank all authors for their contributions to this work.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Guo F, Kang L, Xu M. A case of pediatric visceral leishmaniasis-related hemophagocytic lymphohistiocytosis diagnosed by mNGS. Int J Infect Dis. 2020;97:27–29. doi:10.1016/j.ijid.2020.05.056
2. Galletta F, Cucinotta U, Marseglia L, et al. Hemophagocytic lymphohistiocytosis following gene replacement therapy in a child with type 1 spinal muscular atrophy. J Clin Pharm Ther. 2022;47(9):1478–1481. doi:10.1111/jcpt.13733
3. Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6(12):e630–e637. doi:10.1016/S2352-3026(19)30156-5
4. Das R, Guan P, Sprague L, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood. 2016;127(13):1666–1675. doi:10.1182/blood-2015-12-684399
5. de Araujo FF, Costa-Silva MF, Pereira AAS, et al. Chemokines in Leishmaniasis: map of cell movements highlights the landscape of infection and pathogenesis. Cytokine. 2020;147:155339. doi:10.1016/j.cyto.2020.155339
6. La Rosee P. Alleviating the storm: ruxolitinib in HLH. Blood. 2016;127(13):1626–1627. doi:10.1182/blood-2016-02-697151
7. Yildiz H, Van Den Neste E, Defour JP, Danse E, Yombi JC. Adult haemophagocytic lymphohistiocytosis: a Review. QJM. 2020. doi:10.1093/qjmed/hcaa011
8. Trottestam H, Horne A, Arico M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–4584. doi:10.1182/blood-2011-06-356261
9. Wang J, Wang Y, Wu L, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020;105(5):e210–e212. doi:10.3324/haematol.2019.222471
10. Boonstra PS, Ahmed A, Merrill SA, Wilcox RA. Ruxolitinib in adult patients with secondary hemophagocytic lymphohistiocytosis. Am J Hematol. 2021;96(4):E103–E105. doi:10.1002/ajh.26091
11. Wang J, Zhang R, Wu X, et al. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: a single-arm, multicentre, Phase 2 trial. Br J Haematol. 2021;193(4):761–768. doi:10.1111/bjh.17331
12. Yildiz H, Bailly S, Van Den Neste E, Yombi JC. Clinical management of relapsed/refractory hemophagocytic lymphohistiocytosis in adult patients: a review of current strategies and emerging therapies. Ther Clin Risk Manag. 2021;17:293–304. doi:10.2147/TCRM.S195538
13. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering committee of the histiocyte society. J Allergy Clin Immunol Pract. 2018;6(5):1508–1517. doi:10.1016/j.jaip.2018.05.031
14. Zheng C, Wang L, Li Y, Zhou XN. Visceral leishmaniasis in northwest China from 2004 to 2018: a spatio-temporal analysis. Infect Dis Poverty. 2020;9(1):165. doi:10.1186/s40249-020-00782-4
15. Zoller EE, Lykens JE, Terrell CE, et al. Hemophagocytosis causes a consumptive anemia of inflammation. J Exp Med. 2011;208(6):1203–1214. doi:10.1084/jem.20102538
16. Hailu A, van der Poll T, Berhe N, Kager PA. Elevated plasma levels of interferon (IFN)-gamma, IFN-gamma inducing cytokines, and IFN-gamma inducible CXC chemokines in visceral leishmaniasis. Am J Trop Med Hyg. 2004;71(5):561–567. doi:10.4269/ajtmh.2004.71.561
17. Gautam S, Kumar R, Maurya R, et al. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis. 2011;204(7):1134–1137. doi:10.1093/infdis/jir461
18. Dos Santos PL, de Oliveira FA, Santos ML, et al. The severity of visceral leishmaniasis correlates with elevated levels of serum IL-6, IL-27 and sCD14. PLoS Negl Trop Dis. 2016;10(1):e0004375. doi:10.1371/journal.pntd.0004375
19. Keenan C, Nichols KE, Albeituni S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol. 2021;12:614704. doi:10.3389/fimmu.2021.614704
20. Kumar R, Bunn PT, Singh SS, et al. Type I interferons suppress anti-parasitic immunity and can be targeted to improve treatment of visceral leishmaniasis. Cell Rep. 2020;30(8):2512–2525 e2519. doi:10.1016/j.celrep.2020.01.099
21. Diamantidis MD, Palioura A, Ioannou M, Tsangalas E, Karakousis K Sr. Hemophagocytic lymphohistiocytosis as a manifestation of underlying visceral leishmaniasis. Cureus. 2020;12(12):e11911. doi:10.7759/cureus.11911
22. Shi Q, Huang M, Li X, et al. Clinical and laboratory characteristics of hemophagocytic lymphohistiocytosis induced by Leishmania infantum infection. PLoS Negl Trop Dis. 2021;15(11):e0009944. doi:10.1371/journal.pntd.0009944
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

