Back to Journals » International Journal of General Medicine » Volume 14
The Systemic Inflammation-Based Prognostic Score Predicts Postoperative Complications in Patients Undergoing Pancreaticoduodenectomy
Authors Qu G , Wang D, Xu W , Wu K , Guo W
Received 6 January 2021
Accepted for publication 22 February 2021
Published 9 March 2021 Volume 2021:14 Pages 787—795
DOI https://doi.org/10.2147/IJGM.S299167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Guangzhen Qu, Dong Wang, Weiyu Xu, Kai Wu, Wei Guo
Department of General Surgery, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, People’s Republic of China
Correspondence: Wei Guo Email [email protected]
Background: Although many studies have confirmed the correlation between inflammation-based or nutritional markers and postoperative complications in patients undergoing colorectal cancer surgery, their correlation after undergoing pancreaticoduodenectomy (PD) remains unclear.
Methods: We retrospectively reviewed the clinical data of patients who underwent PD in Beijing Friendship hospital between 2018 and 2020. Univariate analysis, multivariate analysis, and receiver operating characteristic curve (ROC) were performed. We assessed the preoperative modified Glasgow Prognostic Score (mGPS), C-reactive protein/albumin ratio (CAR), C-reactive protein (CRP), postoperative Glasgow Prognostic Score (poGPS), CRP on postoperative day 3 (POD3) and CAR on POD3. The optimal cut-off values were determined by performing logistic regression analysis.
Results: Of the 172 patients who underwent PD, 74 (43.0%) developed complications, of whom 27 (15.7%) had clinically relevant postoperative pancreatic fistulas (CR-POPF) and 36 (20.9%) had positive drainage fluid cultures. Elevated levels of preoperative mGPS (P< 0.001), poGPS (P< 0.001), CRP (P< 0.001) and CAR on POD3 were associated with postoperative complications. CRP on POD3 (OR=1.028, 95% CI=1.017– 1.039, P< 0.001) was an independent risk factor associated with postoperative complications in both univariate and multivariate analyses. CAR on POD 3 showed the largest area under the curve (AUC=0.883, P< 0.001). Compared with CAR< 4.86, CAR ≥ 4.86 on POD3 was associated with a higher probability of complications (85.5% vs 14.6%, P< 0.001), especially CR-POPF (33.3% vs 4.9%, P< 0.001), intra-abdominal infection (36.2% vs 10.7%, P< 0.001) with a positive drainage fluid culture.
Conclusion: CAR, an inflammatory response-based marker, can effectively predict early postoperative complications in patients undergone PD.
Keywords: C-reactive protein, albumin, Glasgow Prognostic Score, pancreaticoduodenectomy, complications prediction
Introduction
Pancreatic cancer is a highly lethal malignancy that ranks fourth for cancer-related deaths in the United States and second for digestive system cancer-related deaths.1 Surgical resection is the only potentially curative treatment for pancreatic tumors. Pancreaticoduodenectomy (PD), which was performed and refined by Whipple in 1935 based on the experience of his predecessors, was used to resect pancreatic neoplasms.2 Large clinical centres use improved surgical techniques and critical care to treat patients with postoperative complications, which decreased the mortality rates after PD to around 2–3%.3 Despite this reduction in mortality, postoperative complication rates of PD persist at 30–40%,4,5 even in some large centres. Common complications include bleeding, delayed gastric emptying, and fistula formation from any of the three enteric anastomoses: gastrojejunostomy, hepaticojejunostomy, and pancreaticojejunostomy.6–9 Postoperative pancreatic fistula (POPF), the most common complication, may prolong the stay and cause life-threatening secondary complications such as delayed abdominal bleeding.10 Timely management of these complications may reduce patient suffering and psychological burden. Inflammatory response-based prognostic scoring systems have gained increasing attention for predicting postoperative complications, and those systems include C-reactive protein/albumin ratio (CAR), modified Glasgow prognostic score (mGPS) and postoperative Glasgow prognostic score (poGPS). In a meta-analysis of 1832 patients undergoing colorectal surgery,11 it concluded that C-reactive protein (CRP) on postoperative day (POD) 4 had an improved negative predictive value for infectious complications. A study12 suggested that the decline in albumin level in the early postoperative period reflects the magnitude of surgical trauma and was associated with adverse postoperative outcomes. Another study reported13 that CAR on POD3 was an independent predictor of clinically relevant POPF. CRP and albumin alone may only reflect the extent of the inflammatory response and the nutritional condition. Based on this, we assess the postoperative inflammatory response and nutritional status of the patient in combination with CRP and albumin. We evaluated the significance of CAR, mGPS and poGPS for predicting early postoperative complications in patients who underwent PD.
Methods
Patient Cohort and Data Collection
Data from 172 patients who underwent PD between June 2018 and October 2020 at Beijing Friendship hospital were obtained and retrospectively reviewed. All the surgeries were performed by three experienced hepatobiliary pancreatic surgeons. The following preoperative clinical data of the patients were recorded: age, gender, body mass index (BMI), proportion of patients with hypertension and diabetes mellitus, the American Society of Anaesthesiologists (ASA) status, preoperative serum CRP (mg/L) and serum albumin (g/L), preoperative CAR and patients with preoperative mGPS≥1. The upper CRP limit was 8 mg/L and the lower albumin limit was 35 g/L. Intraoperative data included operative methods, operative time (mins), blood loss (mL), intraoperative blood transfusion (mL). Postoperative outcomes, such as CRP (mg/L), albumin (g/L), CAR on POD3, poGPS score ≥1, hospitalization days, reoperation, mortality and tumour types, were assessed. Among the malignancies, in addition to pancreatic cancer, periampullary carcinoma malignancy was commonly observed, with 36 cases of ampullary carcinoma, 14 cases of duodenal carcinoma and 45 cases of distal bile duct carcinoma (Table 1). The preoperative and postoperative inflammatory response indexes were based on mGPS and poGPS (Table 2). POPF was defined according to the latest International Study Group of Pancreatic Surgery (ISGPS).14 Clinical relevant POPF referred to grade B/C fistula. The postoperative complications were classified according to the Clavien-Dindo classification system.15 Intra-abdominal infection with the positive bacterial culture of drainage fluid is defined as an infection of pathogenic bacteria cultured in peritoneal drainage fluid. Hospitalization days refer to the time from operation to discharge. Reoperation is defined as surgery performed because of life-threatening postoperative complications. Postoperative mortality included death before discharge and within 30 days after surgery. All the information of patients was confidential, all patients signed informed consent, and this retrospective study was conducted in accordance with the Declaration of Helsinki.
 |
Table 1 Univariate Analysis of Risk Factors Associated with Postoperative Complications |
 |
Table 2 The Composition and Contents of Modified Glasgow Prognostic Score (mGPS) and Postoperative Glasgow Prognostic Score (poGPS) |
Surgical Technique and Perioperative Management
The surgical techniques included open pancreaticoduodenectomy (OPD), laparoscopic pancreatoduodenectomy (LPD) and laparoscopic conversion to open surgery. After the patient was given general anaesthesia, the upper abdominal incision was taken, the pancreatic head was dissected at a distance of 1–2 cm from tumour. A stent tube with a diameter matching with the pancreatic duct was inserted into the pancreatic duct to around 8–9 cm distance. The pancreatic stump was then closed together with the jejunum, and 5–0 Vicryl thread was used to make pancreaticojejunal mucosa anastomosis, the other end of the pancreatic fluid drainage tube was put into the distal jejunum, and then six needles were sutured. After suturing, the posterior wall was intermittently suture using 3–0 prolene, and the suture was tightened and knotted. Another 3–0 prolene thread was used to make continuous suture through the anterior wall of the pancreas with the plasma muscle layer of the jejunum, and the knot was pulled and tied. Biliary and gastrointestinal anastomosis was performed after the completion of the pancreaticojejunostomy. Finally, two silicone drainage tubes were placed near pancreaticoenteric and bilioenteric anastomosis.
Statistical Analysis
Statistical analysis was performed using SPSS version 23.0 (IBM Corp., Armonk, NY: IBM Corp.) and MedCalc version 19.6 (MedCalc Software bvba, Ostend, Belgium) software. Continuous variables are expressed as mean ± standard deviation, while categorical variables are presented as proportion. The statistical significance of quantitative variables between groups was determined using independent sample student’s t test, whereas that of qualitative variables was determined using chi-square test or Fisher’s exact test. To determine the independent risk factors of postoperative complications, variables showing a certain degree of correlation (P <0.05) with postoperative outcomes in univariate analysis were selected for multivariate logistic regression analysis. Receiver operating characteristics (ROC) curve analysis was performed for preoperative mGPS, preoperative CAR and CRP on POD3 and poGPS and CAR on POD3. Youden’s index was calculated to select the optimal cut-off value of the CAR and patients were categorized into two groups for comparison. The area under the curve (AUC) values along with corresponding 95% confidence intervals (CI) were used to calculate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy at the optimal cut-off value of these indicators for predicting postoperative complications.
Results
Patient Characteristics
Out of 172 patients who underwent PD, 74 (43.0%) developed postoperative complications, whereas 98 (57.0%) did not. As shown in Table 2, the mean age of the patients was 60.1 ± 9.5 years. A total of 105 (61.0%) patients were males and 67 (39.0%) were females. Out of the 74 patients who developed postoperative complications, two died because of postoperative complications.
Univariate and Multivariate Analysis of Possible Risk Factors for Postoperative Complications
We categorised patients into two groups: complication and without complication. The average age of patients with postoperative complications was higher than that of patients without complications (62.2±7.1 vs 58.5±10.8, P=0.007). The sex ratio between the two groups was significantly different (74% male vs 51% male, P=0.002). Univariate analysis showed that preoperative CRP level (19.02±33.98 vs 8.75±15.07, p=0.033), preoperative albumin level (35.59±4.40 vs 37.26±4.08, p=0.011) preoperative CAR (0.55±0.94 vs 0.21±0.4, p=0.008), the number of people with mGPS≥1 (40, 54.05% vs 28, 28.57%, p<0.001) and blood loss were statistically different between the two groups (Table 1). The postoperative serum biochemical indexes such as CRP and albumin levels, CAR and poGPS were significantly associated with postoperative complications (p <0.01) (Table 1). The mGPS, which incorporates serum albumin and CRP levels, reflects systemic inflammation and nutritional status. Similarly, CAR and poGPS are dependent on CRP and albumin levels; therefore, we did not include mGPS, CAR and poGPS in multivariate analysis. In multivariate analysis, independent factors related to postoperative complications such as gender (male/female, OR = 0.012; 95% CI = 0.051–0.694, P = 0.012), age (OR = 1.083; 95% CI = 1.009–1.162, P = 0.027) and POD3 CRP level (OR = 1.028, 95% CI = 1.017–1.039, P <0.001) were included (Table 3). Although there was a significant correlation between preoperative CRP level and postoperative complications in univariate analysis, it was not statistically significant in multivariate analysis (P=0.072). For CRP on POD3 was an independent risk factor for predicting complications, We speculated that CAR and poGPS calculated by CRP and albumin would be more sensitive than preoperative CRP and mGPS in predicting postoperative complications.
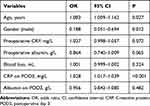 |
Table 3 Multivariate Analysis of Factors Associated with Postoperative Complications |
Significance of CRP, CAR, mGPS and poGPS in Predicting Postoperative Complications
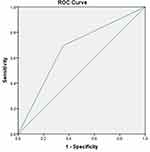
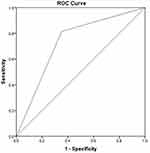
We analysed the receiver operating characteristic curve of all five indicators (preoperative mGPS and CAR, CRP, CAR, and poGPS on POD3) representing inflammatory response (Figure 1) (Table 4). The AUC of the five inflammatory response indexes was more than 0.6, with the smallest value for preoperative CRP (0.606, P = 0.033) and 0.883 (P <0.001) for CAR on POD3. For the preoperative CAR, when the optimal cut-off value was 10.98, the AUC was 0.606 (P = 0.033), the sensitivity was 40%, the specificity was 83.56%, the PPV was 66.70%, the NPV was 62.90%, and the accuracy was 54.90%. For preoperative mGPS, the AUC was 0.667 (P < 0.001) and accuracy was 64.70%. The preoperative mGPS score was divided into three grades (0, 1 and 2). When the optimal cut-off value was 0, its corresponding sensitivity, specificity, PPV and NPV were 66.67%, 63.01%, 59.70% and 69.70%. For poGPS, AUC was 0.780 (P <0.001) and the accuracy was 78.40%. It was divided into 3 levels by its score (0, 1 and 2 points), with the optimal cut-off value at 0 point (sensitivity, 77.03%; specificity, 79.57%; PPV, 75.00%; NPV, 81.30%), 1 point (sensitivity, 5.41%; specificity, 97.85%; PPV, 56.50%; NPV, 56.50%) and 2 point (sensitivity, 0; specificity, 100%; PPV, 55.70%). For CRP on POD3, AUC was 0.870 (P <0.001), and at an optimal cut-off value of 142 mg/L, sensitivity, specificity, PPV, NPV and accuracy were 82.43%, 79.57%, 85.10%, 76.20% and 77.80%, respectively. For CAR on POD3, AUC was 0.883 (P <0.001), and at an optimal cut-off value of 4.86, sensitivity, specificity, PPV, NPV and accuracy were 79.73%, 86.02%, 84.20%, 81.90% and 82%, respectively. The CAR value on POD3 was the highest positive predictive value and was shown to be more effective in distinguishing the patients in complication and without complication groups. As CAR value on POD3 was calculated by taking the ratio of CRP and albumin values that reflect the dynamic change in the degree of the inflammatory response and nutritional status in the early postoperative period, we will further analyse its significance in predicting postoperative complications.
 |
Table 4 Accuracy of Risk Factors in Predicting Postoperative Complications |
CAR for Predicting Postoperative Complications and Infection
A total of 103 patients with low CAR (CAR <4.86) and 69 patients with high CAR (CAR ≥4.86) were recorded. Out of 103 patients, 15 patients (14.6%) developed postoperative complications (Table 5). Out of 69 patients, 59 patients (85.5%) developed postoperative complications. Significant differences were observed between complication and without complication groups, and the complications were graded as Grade I, II, IIIa, IIIb and IVa. (P ≤0.01). However, for Grade IVb and V complications, no difference was observed between the two groups, probably because of the small number of patients (two, each) who developed Grade IVb and V complications. Out of the two patients who developed grade V complications, one died because of abdominal haemorrhage and the other died because of decreased immune function caused by infection and poor nutritional status. In patients who developed complications of grade III or greater, there were distinct differences between the two groups (7, 6.8% vs 23, 33.3% P<0.001). In patients who developed grade III or higher grade complications, distinct differences were observed between the two groups (7, 6.8% vs 23, 33.3% P <0.001). To predict clinically relevant POPF, statistical significance was observed in pancreatic duct diameters between the two groups (median 5 vs 3, P = 0.023), whereas no significant statistical difference was observed in pancreatic texture between the two groups (8.74% vs 17.39%, P = 0.095). This can be because of the fact that pancreatic texture assessment depends on the subjective judgment of the surgeon (Table 5). Prediction of postoperative drain culture-positive intra-abdominal infection showed that intra-abdominal infection was significantly different between the two groups (10.7% vs 36.2%, P <0.001). From the peritoneal drainage fluid culture, we found that common pathogenic species in postoperative abdominal infection were Enterococcus faecium, Pseudomonas aeruginosa and Escherichia coli (Table 6). The AUC was 0.671 (95% CI=0.572–0.77, P<0.001) (Figure 2). The CAR value on POD3 was closely related to clinically relevant POPF occurrence (AUC = 0.732, 95% CI = 0.633–0.830, P <0.001) (Figure 3).
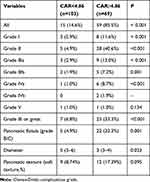 |
Table 5 Incidence of Postoperative Complications Between Two Groups According to Postoperative C-Reactive Protein/Albumin Ratio (CAR) |
 |
Table 6 CAR of Two Groups in Predicting Intra-Abdominal Infection |
Discussion
To our knowledge, no previous study has implicated postoperative CAR in the prognoses of patients who underwent pancreaticoduodenectomy. For patients with pancreatic head cancer, PD is the only treatment that can cure the cancer. In recent years, the mortality rate after PD has decreased to about 5%; however, the incidence of postoperative complications is 50–60%.16–18 Surgical tissue cutting injury, leakage of digestive fluid during operation and hypothermia caused by prolonged anaesthesia can initiate an inflammatory response in the body. CRP is a positive acute-phase inflammatory reactant synthesized by the liver and a marker of systemic inflammation and a widely used serum marker.19 Albumin is a negative acute phase reactant, its content in the blood decreases with increasing degrees of inflammation. Hypoalbuminemia correlates with the severity of inflammation, prognosis of the disease and mortality.20,21 Therefore, CRP and albumin levels are the two markers that represent a balance between inflammation and nutritional status. Muttillo22 reported that CRP and albumin levels were not associated with 1, 3, and 5-year survival after PD. They represent only recent inflammation and nutritional status after PD. An increase in CRP level and a decrease in albumin level before surgery may indicate a higher risk of developing postoperative complications. Therefore, experienced surgeons may administer hypoalbuminemia or control inflammation in patients before PD.
Sakamoto13 reported that serum CRP level on POD 3 in patients with clinical relevant POPF was significantly higher than levels in patients with non-clinical relevant POPF. A significant association was observed between the regulation of serum CRP levels and the polymorphisms in interleukin-6 promoter.23 Hubner12 reported that early postoperative albumin drop reflects the magnitude of surgical trauma and is correlated with adverse clinical outcomes. To accurately predict the postoperative complications, we determined the ratio of CRP and albumin values as an ideal indicator. Postoperative CAR and poGPS were based on CRP and albumin levels and were highly potential for determining postoperative complications. Among them, CAR on POD3 beyond preoperative or postoperative CRP alone as well as preoperative mGPS offers the best possibility to distinguish patients with complications from those without complications.
In patients who underwent PD, clinicians mainly focus on POPF as it is the most common complication after PD.24 However, POPF merely represents a fatal complication after PD. Therefore, the focus should be moved from POPF to other major complications such as infection and bleeding. Xiaolong Ge25 reported that CAR ≥ 2.2 on POD3 could predict the postoperative complications of colorectal cancer. Our study also showed that CAR on POD3 is closely associated with postoperative complications after PD. When the CAR value is ≥4.86 on POD3, the patient has a higher risk of developing POPF and infection. Therefore, antibiotics can be used to treat abdominal infection and POPF injury can be cured by adjusting the position and placement time of the drainage tube. The results of drainage fluid bacterial culture could help select targeted antibiotics, as well as avoid the increased resistance associated with long-time usage of antibiotics.
Purvi26 suggested that postoperative complications and mortality were increased in females and in patients older than 74 years, which is consistent with our study. Both univariate and multivariate analyses indicated that age and gender were associated with postoperative complications. The limitations of our study include the small sample size, high proportion of male patients. Apart from these, a retrospective study has its own limitations. We did not perform subgroup analyses for OPD, LPD and conversion of laparoscopy to open PD.
Conclusion
Our study shows that CAR level has an accurate predictive value for postoperative complications in the early days of PD. Early and effective prediction of postoperative complications and related interventions could help reduce the hospitalization of patients, reduce the economic burden, and enable the smooth transition of the perioperative period in patients who underwent PD.
Ethics Statement and Consent to Participate
All the patients have signed written informed consent forms for their data to be used in the study and the data were kept confidential. The ethics of this study was authorized by the Beijing Friendship Hospital Affiliated to Capital Medical University and followed the guidelines outlined in the Declaration of Helsinki.
Acknowledgment
We would like to thank Fei Xue, Mingyue Zhu and Kai Wu of Beijing Friendship Hospital for their data collection and contributions to this article.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi:10.3322/caac.21332
2. Griffin JF, Poruk KE, Wolfgang CL. Pancreatic cancer surgery: past, present, and future. Chin J Cancer Res. 2015;27(4):332–348. doi:10.3978/j.issn.1000-9604.2015.06.07
3. Ziegler KM, Nakeeb A, Pitt HA, et al. Pancreatic surgery: evolution at a high-volume center. Surgery. 2010;148(4):702–710. doi:10.1016/j.surg.2010.07.029
4. Tzeng C-WD, Katz MHG, Fleming JB, et al. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg. 2014;18(1):146–156. doi:10.1007/s11605-013-2371-6
5. Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18(8):2126–2135. doi:10.1245/s10434-011-1594-6
6. Beane JD, House MG, Miller A, et al. Optimal management of delayed gastric emptying after pancreatectomy: an analysis of 1089 patients. Surgery. 2014;156(4):939–946. doi:10.1016/j.surg.2014.06.024
7. Zyromski NJ, Vieira C, Stecker M, et al. Improved outcomes in postoperative and pancreatitis-related visceral pseudoaneurysms. J Gastrointest Surg. 2007;11(1):50–55. doi:10.1007/s11605-006-0038-2
8. McMillan MT, Vollmer CM, Asbun HJ, et al. The characterization and prediction of ISGPF grade C fistulas following pancreatoduodenectomy. J Gastrointest Surg. 2016;20(2):262–276. doi:10.1007/s11605-015-2884-2
9. Ceppa EP, McCurdy RM, Becerra DC, et al. Does pancreatic stump closure method influence distal pancreatectomy outcomes? J Gastrointest Surg. 2015;19(8):1449–1456. doi:10.1007/s11605-015-2825-0
10. Wolfgang CL, Pawlik TM. Pancreaticoduodenectomy: time to change our approach? Lancet Oncol. 2013;14(7):573–575. doi:10.1016/S1470-2045(13)70159-1
11. Warschkow R, Beutner U, Steffen T, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256(2):245–250. doi:10.1097/SLA.0b013e31825b60f0
12. Hübner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schäfer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016;2016:8743187. doi:10.1155/2016/8743187
13. Sakamoto T, Yagyu Y, Uchinaka EI, et al. Predictive significance of C-reactive protein-to-albumin ratio for postoperative pancreatic fistula after pancreaticoduodenectomy. Anticancer Res. 2019;39(11):6283–6290. doi:10.21873/anticanres.13838
14. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584–591. doi:10.1016/j.surg.2016.11.014
15. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
16. Duffas J-P, Suc B, Msika S, et al. A controlled randomized multicenter trial of pancreatogastrostomy or pancreatojejunostomy after pancreatoduodenectomy. Am J Surg. 2005;189(6):720–729. doi:10.1016/j.amjsurg.2005.03.015
17. Seiler CA, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92(5):547–556. doi:10.1002/bjs.4881
18. Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136(4):391–398. doi:10.1001/archsurg.136.4.391
19. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi:10.1172/JCI18921
20. Winter JM, Cameron JL, Yeo CJ, et al. Biochemical markers predict morbidity and mortality after pancreaticoduodenectomy. J Am Coll Surg. 2007;204(5):1029–1036. doi:10.1016/j.jamcollsurg.2007.01.026
21. Yin M, Si L, Qin W, et al. Predictive value of serum albumin level for the prognosis of severe sepsis without exogenous human albumin administration: a prospective cohort study. J Intensive Care Med. 2018;33(12):687–694. doi:10.1177/0885066616685300
22. Muttillo EM, Ciardi A, Saullo P, et al. A prognostic score for predicting survival in patients with pancreatic head adenocarcinoma and distal cholangiocarcinoma. In Vivo. 2021;35(1):507–515. doi:10.21873/invivo.12285
23. Okada Y, Takahashi A, Ohmiya H, et al. Genome-wide association study for C-reactive protein levels identified pleiotropic associations in the IL6 locus. Hum Mol Genet. 2011;20(6):1224–1231. doi:10.1093/hmg/ddq551
24. Reid-Lombardo KM, Farnell MB, Crippa S, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007;11(11):1451–1459. doi:10.1007/s11605-007-0270-4
25. Ge X, Cao Y, Wang H, et al. Diagnostic accuracy of the postoperative ratio of C-reactive protein to albumin for complications after colorectal surgery. World J Surg Oncol. 2017;15(1):15. doi:10.1186/s12957-016-1092-1
26. Parikh P, Shiloach M, Cohen ME, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB (Oxford). 2010;12(7):488–497. doi:10.1111/j.1477-2574.2010.00216.x
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.