Back to Journals » Patient Preference and Adherence » Volume 14
The SPUR Model: A Framework for Considering Patient Behavior
Authors Dolgin K
Received 8 November 2019
Accepted for publication 18 December 2019
Published 16 January 2020 Volume 2020:14 Pages 97—105
DOI https://doi.org/10.2147/PPA.S237778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Kevin Dolgin
Observia, Paris, France
Correspondence: Kevin Dolgin
Observia, 16 Rue Brancion, Paris 75015, France
Tel +33 1 81 80 24 50
Email [email protected]
Background: Medication nonadherence is a global problem that requires urgent attention. Roughly half of all drugs that are prescribed for chronic treatments are not taken by the patients in question. Initiatives designed to support patients and help them modify their behavior are enhanced by personalization, and a number of profiling tools exist to help customize such interventions. Most of these tools were originally designed as paper-based questionnaires, but the growth of digital adherence technologies (DATs) illuminate the need for the development of digital profiling systems that can interact with fully automated patient interfaces.
Objective: The objective of this study was to examine existing frameworks from medicine, psychology, sociology, consumer behavior, and economics to elaborate a comprehensive, quantitative profiling approach that can be used to drive the customization of patient support initiatives.
Results: Building primarily on Icek Ajzen’s Theory of Planned Behavior (TPB), the Health Belief Model (HBM) was used to inform the beliefs about behavior posited in the TPB, while incorporating established factors regarding self-efficacy in the “control” elements of the TPB and selected social and psychological factors in the other constituents of the model. The resulting SPUR (Social, Psychological, Usage, Rational) framework represents a holistic, profiling tool with detailed, quantitative outputs that describe a patient’s behavioral risks and the drivers of that risk.
Conclusion: An interactive, digital questionnaire built around SPUR represents a potentially useful tool for those desirous of building interactive digital support programs for patients with chronic diseases.
Keywords: adherence, compliance, health beliefs, chronic diseases, review
Introduction
According to the World Health Organization (WHO),1 poor adherence to treatment of chronic diseases is a worldwide problem of “striking magnitude” and the burden of poor adherence is growing worldwide as the prevalence of chronic disease increases. The WHO goes on to point out that the consequences of poor adherence to long-term chronic therapies are both poor health outcomes and increased health-care costs. In 2012, global avoidable cost due to non-adherence was estimated at $269 billion.2 The impact of non-adherence led the WHO to agree with Hayne’s contention that “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments”.3
The WHO estimates that roughly 50% of medications prescribed for chronic diseases are actually taken.1 Even in life-threatening cases adherence rates can be much lower than expected, with adherence rates measured as low as 77.3% in post-transplant immunosuppressant drugs4 and 71% for oral oncology drugs.5 A 2012 meta-analysis of adherence in drugs that prevent cardiovascular disease found an adherence rate of 57% in more than 370,000 patients.6 Over the past several years, stakeholders in the healthcare world have intensified their efforts to both understand this issue and to put into place patient support programs that will help address non-adherence. Addressing non-adherence through targeting intervention is one of the few topics on which everyone is in agreement: patients certainly benefit from increased support and payers would very much like to reduce overall costs by enhancing adherence to those drug treatments which they have decided are beneficial; health-care professionals would like to ensure that the treatments they prescribe are being followed and the pharmaceutical industry benefits by increased sales of their products. Physicians have traditionally been poor at determining patient adherence. As early as 1978, Roth et al7 found that physicians overestimated their patients’ adherence by 400%, and that the patients too overestimated their own adherence. In 2010, Copher et al8 found that physicians overestimated the number of adherent patients by over 60% and in the following year Trindade et al9 found similar overestimation of the adherence rates of IBD patients. In 2016, Clyne et al10 found a weak correlation between physician estimates of their patients’ adherence and objective measures, as well as a systematic bias among prescribers to assume that their patients are more adherent than the norm.
Given their difficulty in perceiving the problem, relying on health-care professionals alone to address patient non-adherence can lead to suboptimal outcomes.11 Furthermore, decisions about whether or not to take medication are typically made outside of a health-care institution, when the patient is not in direct contact with health-care professionals.
Many tools have been provided to health-care professionals to help prescribers more accurately assess adherence. The most widely used of these is the 4-question Morisky Medication Adherence Scale (MMAS 4).12 This tool has been and continues to be of great use to health-care professionals, but it and other tools like it typically require extra time and effort from often busy professionals. Both physicians and patients often cite a lack of time during visits, and surveys such as 2007’s Global Asthma Physician and Patient Survey13 underscore the need for more time spent on education and coaching.14 At the same time, a recent survey carried out in the United States indicates that only 11% of patients and 14% of physicians feel that doctors have the time they need to provide excellent care.15 Faced with the need to balance ease of use and thoroughness of analysis, behavioral profiling tools such as the MMAS-4 (and the later, 8-question MMAS-8) must sacrifice the latter to ensure the former. As such, existing tools have been criticized as being too restrictive to offer a basis for highly tailored behavioral interventions.16
The availability of digital solutions (Digital Adherence Technologies: DAT) provides effective new means of identifying patients at risk of non-adherence and promoting behavioral change while minimizing demands on physician time.17–19 However, this technology by its nature lacks the personalization that can be provided by a trained human during an interpersonal exchange. This gives rise to a need for more flexible and personalized digital support that takes into account each individual’s behavioral drivers and triggers without the need for human analysis. Tailored DATs have great potential to support patients effectively without undue demands on physician time and with much lower costs than traditional telephone-based programs, as demonstrated by a 2018 review of the literature on such technologies with tuberculosis patients20 as well as a 2019 study with hypertensive patients in the UK.21 These promising approaches warrant further development, including the design of DAT-friendly profiling tools. Such tools would be digital in nature, thorough in their quantification of the drivers of adherence, predictive of actual adherence behavior and easily incorporated into DATs.
The tools that are typically used to determine individual patient risk and behavioral needs were designed to be used by humans and do not incorporate the kind of continuous and detailed mathematical principles that can interact effectively with digital support programs. For example, most online retailers, such as Amazon.com, use Bayesian product recommendation engines such as that described in US patent 8.255.263 B2.22 Digital patient support programs could likewise benefit from a similar high degree of customization, yet they need the detailed, quantifiable personal profiling that drives them. As pointed out by Prochaska, Redding, and Evers, “… most [health behavior frameworks] have not even developed constructs that are subject to such mathematical principles.”23 We believe that the SPUR (Social, Psychological, Usage, Rational) framework, built on existing behavioral frameworks, can fill this gap by allowing detailed quantitative measures of established adherence behavioral drivers, determined through an interactive digital questionnaire. Such a questionnaire, designed from the start to be administered in a digital setting, can provide engaging intermediate feedback to patients while providing the kind of driver-specific measures that can serve as the foundation for personalized digital interaction. In order to achieve this in a valid way, such a tool must be built on solid theoretical frameworks.
Theoretical Frameworks
A number of frameworks are often cited when referring to patient adherence decision-making. Among the first of these was the Health Belief Model (HBM),24 first postulated over fifty years ago. This model has been verified in more recent studies,25 but it does not address the non-cognitive components of patient behavior, such as medication costs. Icek Ajzen’s Theory of Planned Behavior (TPB), and its predecessor the Theory of Reasoned Action do address these factors and have also often been applied to healthcare decision-making,26 as has the more recent derivative of the TPB, the Integrated Behavior Model.27 Prochaska and Clemente’s Transtheoretical Model (TTM) has also been a staple model, as has social cognition theory.28,29 On a more psychological level, Gérard Reach has postulated elements of relationship to authority (reactance), and he and others have borrowed from behavioral economics concepts regarding the ability to project into the future as determinants of adherence behavior.30
Outside of medical or even strictly behavioral academic domains, the field of consumer behavior has generated interesting frameworks for addressing adherence problems. Notably, issues of identity, described in the concept of the extended self, as researched by Russel Belk,31 provides insights into how individuals view consumption as an extension of self-identity. This has an impact both on the acceptance of their disease (as described by Graffigna and Barello 201832) and on their acceptance of the prescribed treatment.
Patient Support Programs (PSPs) built using these and other models have proven useful as public health initiatives at a population level.33 These models have been used to build patient-specific support, and they are also often used to profile patients and provide guidance to health-care professionals who can then offer tailored advice, education, and coaching. Some models have been specifically designed to profile patients, either in terms of their risk of non-adherence or the reasons behind this risk. One of the more widely used frameworks to investigate and affect patient behavior is the Patient Activation Measure (PAM).34 The goal of PAM is to measure the degree to which patients have been “activated” to engage with their own health; and PAM scores have been strongly correlated to outcomes.35,36 The Patient Health Engagement model32 purports to go beyond what the authors see as a “passivizing approach” to patients’ care to examine the “meaning and lived experiences” of patients, as well as their emotional and psychological make-up.
While all of these models have demonstrated their value both at the level of individual prescribers (or providers) as well as in the design of programs at population level, none were designed to provide a multi-dimensional behavioral assessment model that can be used to profile individuals and interface with a digital agent, such as a DAT driven by artificial intelligence. What is needed is a comprehensive model of patient behavior that can both accurately predict adherence and identify modifiable drivers of health behavior that deliver useful input to digital coaching and support systems.
In sum, the imperative is to build a model and an associated profiling tool that successfully meet the following criteria: 1) accurately predicts patient adherence to medication; 2) identifies actionable drivers of adherence behavior; 3) can be used in the absence of human interpretation so as to inform the customization of purely digital support programs; and 4) is based on accepted behavioral models drawn from different domains. Such a model can then be used both to further investigate the non-adherence phenomenon at the population level as well as to provide tailored support to individual patients. By combining established behavioral models with digital questionnaire technology, a “grand unified” model can be created to serve as the basis for the digital questionnaire.
The Proposed Model: SPUR
The core of the SPUR framework is Ajzen’s Theory of Planned Behavior (TPB). This theory, as seen in Figure 1, is a well-established approach to consider complex decision-making. It has been successfully and widely applied to general healthcare behavior,37 and a smaller number of studies have shown its utility in predicting medication adherence,38,39 although its use has been criticized in this context for being simplistic, too rational in nature, not taking into account psychological factors such as identity, and for not having demonstrated impact when applied to health behavior.40 McEachan et al’s41 2010 meta-analysis of the use of the TPB determined that the model was predictive of health behavior and psychological constructs such as behavioral intent. Using Rothman and Salovey’s classification of health behaviors into those that prevent, detect, and cure health problems,42 McEachan et al determined that the type of behavior was important in assessing the applicability of the TPB and particularly, in the relative importance of its constituents. This was reinforced in McEachan et al’s subsequent 2016 meta-analysis.43 In both of these studies, however, the authors underlined that no satisfactory study had been made of the applicability of the TPB in cases of curative behaviors such as adherence to medication for chronic disease.
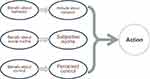 |
Figure 1 The theory of planned behavior. |
The lack of studies examining the TPB in curative behavior as well as the identified weaknesses of the approach led us to believe that augmenting the TPB with health-specific frameworks focusing on curative behavior can both enhance its applicability in this domain while further elaborating on it to provide quantifiable outputs that can then be used to drive algorithms in digital support programs for chronic disease management.
The TPB describes complex behavior as a function of attitudes about the behavior (driven by beliefs about the behavior and tempered by other psychological factors), subjective norms, and perceived control. Each of these is weighted to reflect its importance for each individual.
Drawing on the TPB, the SPUR model is focused specifically on chronic patients’ adherence behavior and includes elements of ancillary frameworks as mentioned above.
Specifically, SPUR includes concepts from the HBM to inform the cognitive elements of attitude formation within the TPB by detailing the perceived understanding of the patient and their beliefs about the prescribed behavior. Commonly cited practical reasons for non-adherence, such as financial difficulties, complexity of the treatment, etc.1 can inform the control elements of the TPB, typically referred to as self-efficacy in health behavior, and aforementioned behavioral factors such as reactance and discounting of future benefits can address how beliefs about behavior combine with the other elements to generate attitudes. The resulting model is specific to behaviors regarding chronic diseases and takes into account a number of established frameworks that have been demonstrated to influence adherence behavior, generating the following enhanced framework (Figure 2).
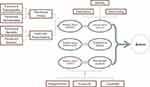 |
Figure 2 The concepts that constitute SPUR. |
The model is called “SPUR”, since the different elements fall into four major domains: Social, Psychological, Usage and Rational that are discussed below. The manner in which these four domains relate to the TPB can be seen in Figure 3.
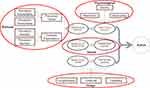 |
Figure 3 The SPUR framework in detail. |
Social Factors
Social elements in the case of medical adherence have also been studied at length. Most of the work in this area has focussed on perceived social support as a factor in patient adherence and has shown greater support improves adherence. This has been considered in general44 as well as in specific cases such as diabetes by Gu, et al45 as well as Shallcross et al46 in epilepsy and Kim, et al47 in HIV.
Social factors have been considered both with respect to the impact of those close to the patient and the role of society as a whole (e.g. the influence of socio-cultural expectations). This latter definition corresponds more closely to the TPB’s construct of social norms and can be clearly seen when considering the changing cultural acceptability of smoking in many Western societies and its impact on the prevalence of smoking. Likewise, the impact of social norms on general health-related behavior has been well considered (see, for example, Baer, Stacy, and Larimer48), although the direct impact of norms has been poorly studied in relation to actual medication adherence.
Psychological Factors
Many psychological constructs have been examined with respect to health behavior in cases of chronic disease. Mental illness itself, such as depression, has long been linked to non-adherence49–51 and treatment with anti-depression medication has been shown to increase adherence with depressed HIV patients.52 In SPUR, we have avoided examining the impact of mental disorders such as depression, bi-polar or Post-traumatic stress disorder, as they merit specific treatment beyond the type of behavioral support that is the focus of our research. We have therefore focussed on three compelling and well-documented non-clinical psychological factors: self-concept, reactance and the discounting of future values.
The Concept of the Self
In the case of medical adherence, there is a latent tendency to deny the existence or extent of the illness despite a cognitive understanding of the facts.53,54 While denial has been linked to well-being and general health54,55 the role of denial of personal traits and circumstances in behavior modification has not been adequately examined. There is some indication that denial is directly correlated to non-adherence, particularly in the case of mental illness. Greenhouse et al29 demonstrated that coping behaviors associated with denial were inversely correlated with adherence in bipolar disorder56 and Aldebot and Weisman de Mamani57 observed that denial led to lower adherence rates in schizophrenia. Outside of mental illnesses, denial has been identified as a significant contributor to non-adherence in pathologies ranging from cardiovascular disease58 to HIV.59
We thus hypothesize that questions of denial in the case of health are driven by conflicts between the pre-existing self-concept and the new identification with the disease state, e.g. as a “diabetic”, “cancer patient”, “asthmatic”, etc. In order to examine the concept of the self with respect to the consumption of medication, we turn to consumer behavior and notably, Russel Belk. Belk considered the effect of possessions on the “extended self” and the impact of consumption decisions.31,60,61 We believe that similar concepts can help elucidate a model to understand the impact of medication consumption on identity and therefore its impact on adherence. Specifically, does the person accept their illness and their status as “patient”? We hypothesize that denial of this attribute – equated to a refusal to incorporate it into their sense of identity – is detrimental to adherence.
In order to better examine this potential disconnect, it is useful to begin by considering the patient’s perceived identity and their concept of self, both from the point of view of the individual (i.e. Belk) and his or her relations to others and the community, i.e. Kashima.62 This becomes particularly relevant for individuals who express a degree of denial with respect to their disease. Kortte and Wegener53 have considered specifically denial of illness among patients across a number of pathologies and have investigated the psychological literature on the subject, ranging from Freud to modern thinkers. However, they have not considered the impact of denial on either sense of self directly, nor on consumer behavior, such as adherence.
Reactance
Reactance is the psychological tendency to resist authority. A number of studies have examined the impact of reactance on patient adherence. Fogarty and Young63 did not find an expected correlation between the use of an authoritative tone by physicians and patient behavior, however, it did lead the authors to conclude that the underlying degree of psychological reactance of the patient was a factor in their adherence behavior. Gérard Reach’s analysis of diabetic patients’ adherence35 bears this out, demonstrating a strong correlation between “obedient” behavior (wearing a seatbelt in the back seat of a car) and adherence, leading him to postulate that there are two elements to adherence behavior, a “passive” (i.e. driven by deference to authority) and a “motivational” element. In 2011, De La Cuevas et al49 determined that reactance was a stronger driver of adherence behavior than self-efficacy in patients suffering from depression.
Discounting and Prospect Theory
Researchers in the domain of behavioral economics have demonstrated the degree to which our asymmetrical valuing of losses over gains affects behavior.64 A number of researchers have investigated these factors with respect to adherence behavior.
Zhao et al37 demonstrated a significant difference in the impact of differential messaging on intended health behavior across people with different levels of focus on future outcomes,65 in which patients with high scores in Strathman’s Consideration of Future Consequences scale were significantly more sensitive to messages about future consequences than were subjects with lower scores, who were more likely to adhere when presented with messages concerning short-term benefits.
Lebeau et al,66 building on the work of Gérard Reach30 discovered a direct correlation between the discounting of future gains in type 2 diabetes patients and their measure of glycated hemoglobin (HbA1c).
Usage
The TPB’s inclusion of perceived control as an important behavioral driver leads to a consideration of what exactly this represents in adherence to treatment for chronic disease. This idea of self-efficacy has been closely studied and indeed, many patient support initiatives focus on it exclusively. Issues of control that affect adherence include non-access to medication due to physical constraints; financial difficulty;67 difficulty with self-administration, which can be the result of a multitude of causes including anxiety regarding self-injection;68 and forgetfulness.
Rational Factors
The HBM is the most widely and the longest used “expectancy-value” approach to health behavior. The fundamental behavioral choice is presented as a comparison of the outcome expectations of health behavior as compared to the perceived threat of not adopting that behavior. The former is further subdivided into the perceived benefits and the perceived threats of the behavior and further subdivided into the perceived gravity of the disease coupled with the perceived susceptibility of the subject to these nefarious consequences. While the HBM lacks the psychological, social and practical elements that have since been studied for their impact on health behavior, it does provide a good breakdown of the purely rational thought processes that can inform beliefs about health behavior that can then be incorporated into the TPB.
Conclusions and Next Steps
By building on the Theory of Planned Behavior, incorporating behavioral frameworks that are relevant to behavior in the case of medication adherence for patients with chronic diseases, the SPUR framework represents a coherent approach to build a quantifiable questionnaire that can be used to profile patients for adherence risk while identifying the drivers behind that risk. The inherently hierarchical structure of the framework, in which four major sets of drivers: social, psychological, utilitarian and rational are further broken down in accordance with relevant theories should allow both for assessment of an individual’s behavioral risk as well as analysis of the salient sources of that risk.
The next step will be to build a questionnaire based on the SPUR framework and test its predictive validity. We are in the process of doing exactly that and will be carrying out such a study across a range of pathologies in the near future. It is our hope that SPUR will prove to be a valuable tool for health-care professionals to determine the behavioral risks of each patient and construct support services for them that correspond to their personal behavioral drivers.
Abbreviations
DAT, Digital Adherence Technologies; HBM, Health Belief Model; PAM, Patient Activation Measure; PSP, Patient Support Program, SPUR, Social, Psychological, Usage, Rational; TPB, Theory of Planned Behavior; TTM, Transtheoretical Model; WHO, World Health Organization.
Acknowledgments
The author would like to thank John D. Piette, Reem Kayyali, Marie-Eve Laporte and Benoit Arnould for their input and critical review of the manuscript; Lea Kombargi for her support in preparing the manuscript; Béatrice Tugaut for her support in submitting the manuscript.
Author Contributions
The author contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agrees to be accountable for all aspects of the work.
Ethics Approval and Informed Consent
This study did not require any ethics approval.
Funding
This study was funded by Observia.
Disclosure
Kevin Dolgin is an employee of Observia. The author reports no other conflicts of interest in this work.
References
1. Sabaté E, World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003.
2. Aitken M, Gorokhovich L. Advancing the responsible use of medicines: applying levers for change. SSRN J. 2012. doi:10.2139/ssrn.2222541
3. Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2):CD000011. doi:10.1002/14651858.CD000011.pub3.
4. Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. doi:10.1097/01.tp.0000258599.65257.a6
5. Atkinson T, Rodríguez V, Gordon M, et al. The association between patient-reported and objective oral anticancer medication adherence measures: a systematic review. ONF. 2016;43(5):576–582. doi:10.1188/16.ONF.576-582
6. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882–887.e1. doi:10.1016/j.amjmed.2011.12.013
7. Roth HP, Caron HS. Accuracy of doctors’ estimates and patients’ statements on adherence to a drug regimen. Clin Pharmacol Ther. 1978;23(3):361–370. doi:10.1002/cpt1978233361
8. Copher R, Buzinec P, Zarotsky V, Kazis L, Iqbal SU, Macarios D. Physician perception of patient adherence compared to patient adherence of osteoporosis medications from pharmacy claims. Curr Med Res Opin. 2010;26(4):777–785. doi:10.1185/03007990903579171
9. Trindade AJ, Ehrlich A, Kornbluth A, Ullman TA. Are your patients taking their medicine? Validation of a new adherence scale in patients with inflammatory bowel disease and comparison with physician perception of adherence. Inflamm Bowel Dis. 2011;17(2):599–604. doi:10.1002/ibd.21310
10. Clyne W, McLachlan S, Mshelia C, et al. “My patients are better than yours”: optimistic bias about patients’ medication adherence by European health care professionals. Patient Prefer Adherence. 2016;10:1937–1944. doi:10.2147/PPA.S108827
11. Schulz PJ, Nakamoto K “Bad” literacy, the Internet, and the limits of patient empowerment. In:
12. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi:10.1097/00005650-198601000-00007
13. Canonica GW, Baena-Cagnani CE, Blaiss MS, et al. Unmet needs in asthma: Global Asthma Physician and Patient (GAPP) survey: global adult findings. Allergy. 2007;62(6):668–674. doi:10.1111/j.1398-9995.2007.01352.x
14. Greene J, Hibbard JH, Alvarez C, Overton V. Supporting patient behavior change: approaches used by primary care clinicians whose patients have an increase in activation levels. Ann Fam Med. 2016;14(2):148–154. doi:10.1370/afm.1904
15. Regina Corso Consulting. 2017 Patient Survey Report for the Physicians Foundation. Presented at the:
16. Tan X, Patel I, Chang J. Review of the four item Morisky Medication Adherence Scale (MMAS-4) and eight item Morisky Medication Adherence Scale (MMAS-8). Inov Pharm. 2014;5:3. doi:10.24926/iip.v5i3.347
17. Dolgin K. The Impact of text messaging in adherence programs. Eur J Person Centered Healthcare. 2015;3:1. doi:10.5750/ejpch.v3i1
18. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth Chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res. 2015;17(2):e52. doi:10.2196/jmir.3951
19. Wu Y, Yao X, Vespasiani G, et al. Mobile App-based interventions to support diabetes self-management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR Mhealth Uhealth. 2017;5:3. doi:10.2196/mhealth.6522
20. Subbaraman R, de Mondesert L, Musiimenta A, et al. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health. 2018;3(5):e001018. doi:10.1136/bmjgh-2018-001018
21. Kassavou A, Houghton V, Edwards S, Brimicombe J, Sutton S. Development and piloting of a highly tailored digital intervention to support adherence to antihypertensive medications as an adjunct to primary care consultations. BMJ Open. 2019;9(1):e024121. doi:10.1136/bmjopen-2018-024121
22. Smallwood RD (54) BAYESIAN PRODUCT RECOMMENDATION ENGINE.:12.
23. Prochaska JO, Redding C, Evers K. The transtheoretical model and stage of change. In: Health Behavior and Health Education. Karen Glanz, Barbara K. Rimer, Frances Marcus Lewis, (Eds). San Francisco, CA: Jossey-Bass Inc; 2002:60-84
24. Green EC, Murphy E. Health belief model. In: Cockerham WC, Dingwall R, Quah S, editors. The Wiley Blackwell Encyclopedia of Health, Illness, Behavior, and Society. Chichester, UK:John Wiley & Sons, Ltd; 2014:766–769. doi:10.1002/9781118410868.wbehibs410.
25. Carpenter CJ. A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Commun. 2010;25(8):661–669. doi:10.1080/10410236.2010.521906
26. Ajzen I. From intentions to actions: a theory of planned behavior. In: Action Control. SSSP Springer Series in Social Psychology. Julius Kuhl, Jürgen Beckmann (Eds). Berlin, Germany: Springer Berlin Heidelberg; 1985:11–39.
27. Montano D, Kazprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Health Behavior: Theory, Research and Practice. Karen Glanz, Barbara K. Rimer, K. Viswanath (Eds),
28. Riekert KA, Ockene JK, Pbert L, eds. Handbook of health behavior change.
29. Glanz K, Rimer BK, Viswanath K, eds. Health behavior: theory, research, and practice.
30. Reach G. A novel conceptual framework for understanding the mechanism of adherence to long term therapies. Patient Prefer Adherence. 2008;2:7.
31. Belk W. Possessions and the extended self. J Consum Res. 1988;15:139–168. doi:10.1086/jcr.1988.15.issue-2
32. Graffigna G, Barello S. Spotlight on the Patient Health Engagement model (PHE model): a psychosocial theory to understand people’s meaningful engagement in their own health care. Patient Prefer Adherence. 2018;12:1261–1271. doi:10.2147/PPA.S145646
33. Shillington A, Ganjuli A, Clewell J. The impact of patient support programs on adherence, clinical, humanistic, and economic patient outcomes: a targeted systematic review. PPA. 2016;711. doi:10.2147/PPA.S101175
34. Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4p1):1005–1026. doi:10.1111/j.1475-6773.2004.00269.x
35. Greene J, Hibbard JH, Sacks R, Overton V, Parrotta CD. When patient activation levels change, health outcomes and costs change, too. Health Aff. 2015;34(3):431–437. doi:10.1377/hlthaff.2014.0452
36. Kinney RL, Lemon SC, Person SD, Pagoto SL, Saczynski JS. The association between patient activation and medication adherence, hospitalization, and emergency room utilization in patients with chronic illnesses: a systematic review. Patient Educ Couns. 2015;98(5):545–552. doi:10.1016/j.pec.2015.02.005
37. Conner M, Sparks P. Theory of planned behaviour and health behaviour. In: Predicting Health Behaviour: Research and Practice with Social Cognition Models.
38. Bane C, Hughe CM, McElnay JC. Determinants of medication adherence in hypertensive patients: an application of self-efficacy and the Theory of Planned Behaviour. Int J Pharm Pract. 2006;14(3):197–204. doi:10.1211/ijpp.14.3.0006
39. Saal W, Kagee A. The applicability of the theory of planned behaviour in predicting adherence to ART among a South African sample. J Health Psychol. 2012;17(3):362–370. doi:10.1177/1359105311416875
40. Sniehotta FF, Presseau J, Araújo-Soares V. Time to retire the theory of planned behaviour. Health Psychol Rev. 2014;8(1):1–7. doi:10.1080/17437199.2013.869710
41. McEachan RRC, Conner M, Taylor NJ, Lawton RJ. Prospective prediction of health-related behaviours with the theory of planned behaviour: a meta-analysis.:49. Health Psychol Rev. 2011;5(2):97–144. doi:10.1080/17437199.2010.521684
42. Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: the role of message framing.:17. Psychol Bull. 1997;121(1):3. doi:10.1037/0033-2909.121.1.3
43. McEachan R. Meta-analysis of the Reasoned Action Approach (RAA) to understanding health behaviors. Ann Behav Med. 2016;50:21.
44. Amico KR, Mugavero M, Krousel-Wood MA, Bosworth HB, Merlin JS. Advantages to using social-behavioral models of medication adherence in research and practice. J Gen Intern Med. 2018;33(2):207–215. doi:10.1007/s11606-017-4197-5
45. Gu L, Wu S, Zhao S, et al. Association of social support and medication adherence in chinese patients with type 2 diabetes mellitus. Int J Environ Res Public Health. 2017;14(12):1522. doi:10.3390/ijerph14121522
46. Shallcross AJ, Becker DA, Singh A, et al. Psychosocial factors associated with medication adherence in ethnically and socioeconomically diverse patients with epilepsy. Epilepsy Behav. 2015;46:242–245. doi:10.1016/j.yebeh.2015.01.034
47. Kim S-H, McDonald S, Kim S, Foster C, Fidler S. Importance of self-motivation and social support in medication adherence in HIV-infected adolescents in the United Kingdom and Ireland: a multicentre HYPNet study. AIDS Patient Care STDS. 2015;29(6):354–364. doi:10.1089/apc.2014.0335
48. Baer JS, Stacy A, Larimer M. Biases in the perception of drinking norms among college students. J Stud Alcohol. 1991;52(6):580–586. doi:10.15288/jsa.1991.52.580
49. De Las Cuevas C, Peñate W, Sanz EJ. The relationship of psychological reactance, health locus of control and sense of self-efficacy with adherence to treatment in psychiatric outpatients with depression. BMC Psychiatry. 2014;14(1). doi:10.1186/s12888-014-0324-6
50. Goldstein CM, Gathright EC, Garcia S. Relationship between depression and medication adherence in cardiovascular disease: the perfect challenge for the integrated care team. PPA. 2017;11:547–559. doi:10.2147/PPA.S127277
51. Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Dia Care. 2008;31(12):2398–2403. doi:10.2337/dc08-1341
52. Yun LWH, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. JAIDS. 2005;38(4):432–438. doi:10.1097/01.qai.0000147524.19122.fd
53. Kortte KB, Wegener ST. Denial of illness in medical rehabilitation populations: theory, research, and definition. Rehabil Psychol. 2004;49(3):187–199. doi:10.1037/0090-5550.49.3.187
54. Prince R, Frasure-Smith N, Rolicz-Woloszyk E. Life stress, denial and outcome in ischemic heart disease patients. J Psychosom Res. 1982;26(1):23–31. doi:10.1016/0022-3999(82)90059-9
55. Levine J, Warrenburg S, Kerns R, et al. The role of denial in recovery from coronary heart disease. J Biobehav Med. Published 1987. http://journals.lww.com/psychosomaticmedicine/Fulltext/1987/03000/The_role_of_denial_in_recovery_from_coronary_heart.1.aspx.
56. Greenhouse WJ, Meyer B, Johnson SL. Coping and medication adherence in bipolar disorder. J Affect Disord. 2000;59(3):237–241. doi:10.1016/S0165-0327(99)00152-4
57. Aldebot S, Weisman de Mamani AG. Denial and acceptance coping styles and medication adherence in schizophrenia. J Nerv Ment Dis. 2009;197(8):580–584. doi:10.1097/NMD.0b013e3181b05fbe
58. Ganasegeran K, Rashid A. The prevalence of medication nonadherence in post-myocardial infarction survivors and its perceived barriers and psychological correlates: a cross-sectional study in a cardiac health facility in Malaysia. PPA. 2017;11:1975–1985. doi:10.2147/PPA.S151053
59. Nam SL, Fielding K, Avalos A, Dickinson D, Gaolathe T, Geissler PW. The relationship of acceptance or denial of HIV-status to antiretroviral adherence among adult HIV patients in urban Botswana. Soc Sci Med. 2008;67(2):301–310. doi:10.1016/j.socscimed.2008.03.042
60. Belk RW. Extended self and extending paradigmatic perspective. J Consum Res. 1989;16(1):129–132. doi:10.1086/jcr.1989.16.issue-1
61. Ahuvia AC. Beyond the extended self: loved objects and consumers’ identity narratives. J Consum Res. 2005;32(1):171–184. doi:10.1086/429607
62. Kashima Y, Yamaguchi S, Kim U, Choi SC, Gelfand MJ, Yuki M. Culture, gender, and self: a perspective from individualism-collectivism research. J Pers Soc Psychol. 1995;69(5):925–937. doi:10.1037/0022-3514.69.5.925
63. Fogarty JS, Youngs GA. Psychological reactance as a factor in patient noncompliance with medication taking: a field experiment1. J Appl Soc Psychol. 2000;30(11):2365–2391. doi:10.1111/j.1559-1816.2000.tb02441.x
64. Tversky A, Kahneman D. Loss aversion in riskless choice: a reference-dependent model. Q J Econ. 1991;106(4):1039–1061. doi:10.2307/2937956
65. Zhao X, Villagran MM, Kreps GL, McHorney C. Gain versus loss framing in adherence-promoting communication targeting patients with chronic diseases: the moderating effect of individual time perspective. Health Commun. 2012;27(1):75–85. doi:10.1080/10410236.2011.569002
66. Lebeau G, Consoli SM, Le Bouc R, et al. Delay discounting of gains and losses, glycemic control and therapeutic adherence in type 2 diabetes. Behav Processes. 2016;132:42–48. doi:10.1016/j.beproc.2016.09.006
67. Zullig LL, Peppercorn JM, Schrag D, et al. Financial distress, use of cost-coping strategies, and adherence to prescription medication among patients with cancer. J Oncol Pract. 2013;9(6S):60s–63s. doi:10.1200/JOP.2013.000971
68. Turner AP, Williams RM, Sloan AP, Haselkorn JK. Injection anxiety remains a long-term barrier to medication adherence in multiple sclerosis. Rehabil Psychol. 2009;54(1):116–121. doi:10.1037/a0014460
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
