Back to Journals » Clinical Interventions in Aging » Volume 13
The significance of the vitamin D metabolism in the development of periprosthetic infections after THA and TKA: a prospective matched-pair analysis of 240 patients
Authors Zajonz D, Prager F, Edel M, Möbius R, Daikos A, Fakler JKM , Josten C, Kratzsch J, Roth A
Received 16 April 2018
Accepted for publication 25 May 2018
Published 17 August 2018 Volume 2018:13 Pages 1429—1435
DOI https://doi.org/10.2147/CIA.S171307
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Dirk Zajonz,1,2,* Florian Prager,1,* Melanie Edel,1,2 Robert Möbius,1,2 Alexandros Daikos,1 Johannes KM Fakler,1 Christoph Josten,1,2 Jürgen Kratzsch,3 Andreas Roth1,2
1Department of Orthopaedics, Trauma and Plastic Surgery, University Hospital Leipzig, Leipzig, Germany; 2ZESBO – Zentrum zur Erforschung der Stuetz- und Bewegungsorgane, Leipzig, Germany; 3Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University of Leipzig, Leipzig, Germany
*These authors contributed equally to this work
Background: The importance of the vitamin D homeostasis in infections is already known. However, its significance in periprosthetic infections (PPIs) after total hip arthroplasty and total knee arthroplasty is largely unexplored. The aim of the study is to precisely analyze the vitamin D balance in patients with PPIs after total hip arthroplasty and total knee arthroplasty. Here, cases with primary endoprosthesis implants and aseptic loosening are used as comparison groups.
Materials and methods: In this prospective matched-pair analysis, patients with PPI at the hip and knee joints were included in the study group (SG). The control groups (CGs) consisted of patients with primary implants (CG I) and who underwent replacement surgery due to aseptic loosening (CG II). In addition to 25 OH vitamin D3 and calcium, bone mineral and protein parameters were determined. An osteoporosis-specific questionnaire was collected.
Results: There are no significant differences in the 25 OH-vitamin D levels between the SG (17.9±8.9) and both CGs (CG I: 16.8±6.90; CG II: 19.7±7.90). However, compared with the SG, significantly higher levels of calcium (Ca) and bone-specific alkaline phosphatase were found in both CGs in comparison with the SG. Significantly lower values concerning the protein balance in PPI were conspicuous. Acute PPI showed a significant reduction in 25 OH vitamin D3 compared with chronic infections (8.3±5.98 vs 21.6±8.40, P=0.002). Calcium and protein balance were also significantly reduced in acute PPIs.
Conclusion: Acute PPIs of the hip and knee joints show a significantly reduced calcium and 25 OH vitamin D3 levels as well as lowered proteins (albumin and total protein) compared with chronic infections as well as primary endoprostheses and aseptic replacement operations. Substitution of vitamin D3 and calcium with simultaneous adaptation of the protein balance is recommended in all PPIs, especially in the acute PPI.
Keywords: periprosthetic infection, protein deficiency, THA, TKA, vitamin D deficiency
Introduction
The importance of the vitamin D balance in infections is already known and scientifically proven. Vitamin D activates T lymphocytes and stimulates them to divide by means of an upregulation of antimicrobial peptides LL-37 and beta-defensins 2.1 Furthermore, vitamin D receptor polymorphism have essential immunomodulatory effects relating to infections.2 Especially in pathogens such as the influenza A virus, Mycobacterium tuberculosis and the HIV type 1, a strong interaction with vitamin D is described.3 The importance of vitamin D insufficiency in patients with severe sepsis is also known. Hence, in this study, patients with massive vitamin D deficiency showed a significantly higher mortality rate.4 Tiwari et al proved with his investigation that a strong vitamin D deficiency is associated with increased cytokine concentrations in diabetics, which had a negative impact especially on those with a foot infection.5 However, despite these works, the role of vitamin D in periprosthetic infections (PPIs) is largely unexplored. In this context, Maier et al compared the vitamin D level in patients with PPI at the hip and knee joints with patients with aseptic loosening in these joints. Here, this working group found a significantly lower vitamin D level in patients with PPI compared with patients with aseptic loosening.6 In contrast to this work, Signori et al describe a significantly higher vitamin D level in patients with PPI compared with patients with aseptic loosening.7 It is assumed that different sunlight exposures of patients in Italy and Germany as well as various selections of patient groups without strict exclusion or inclusion criteria are the reasons for these differences.6,7 However, a clear declaration is not evident from the two publications. It is also striking that none of these studies consider the importance of the nutritional status, particularly the protein balance. It is known that 99% of the body’s vitamin D exists in the protein-bound form. The vitamin D-binding protein (VDBP), which serves as the most important carrier protein for vitamin D3 and its intermediates, binds about 85%–90%, while 10%–15% are bound to albumin and other lipoproteins. Less than 1% is present as unbound component of the blood (Figure 1). In addition, the importance of the protein balance in PPIs is already known. An investigation showed that a preoperative albumin of <35 g/L is associated with a sevenfold increased risk of an infection after total endoprosthesis implantation.8
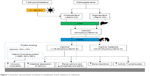  | Figure 1 Schematic representation of vitamin D metabolism and its influence on infections. |
The aim of this prospective matched-pair analysis is to analyze the vitamin D balance in case of PPIs at the hip and knee joints. Primary endoprosthesis implantations and cases with aseptic loosenings are used as comparison groups.
Materials and methods
In order to plan the study design and estimate an adequate sample size, a power analysis (G*Power, version 3.1.9.2.) was carried out in advance. Assuming a mean effect size (d=0.5) and a statistical test power of 80% (1–β=0.8), a sample size of 64 patients in every group (64= study group [SG] = control group [CG]) was required for the evidence of significant differences (α=0.05).
Furthermore, the vote of the university ethics committee was also positive (025-16-01022016). The study was conceived as a prospective monocentric single-center study (university maximum care provider in Europe). A matched-pair analysis was used. All study participants were of Caucasian descent.
The SG included patients with infections in their hip and knee joint implants, which were determined independently of this study. In addition, patients had to have written consent to participate in the study and must be at least 18 years of age. The definition of a PPI was based on the criteria of the International Consensus Meeting on Periprosthetic Joint Infection (ICMPJI) in 2013.9 Here, the classification is based on major and minor criteria. Major criteria are the presence of a fistula or the detection of identical bacteria in multiple samples. Minor criteria include inflammatory parameters and puncture results. Several of these minor criteria must be available.
The CGs were patients with primary implants (CG I) and revisions due to aseptic loosening (CG II) that have been adjusted according to age, gender and localization (matched-pair analysis). Aseptic loosening is defined as radiologically determined implant loosening with appropriate clinic and by puncture excluded PPI. An exclusion from the CG was carried out in cases of not suspected joint infection during surgery.
A total of 80 patients were included in the SG between June 1, 2016, and September 29, 2017. The corresponding inclusion in the CGs extended until November 3, 2017, with identical start. The CGs were adjusted in accordance with the matched pair method according to gender, age and localization (total hip arthroplasty [THA] or total knee arthroplasty [TKA]) of the SG. The specifics of the individual patient groups are shown in Table 1.
The following parameters were determined by peripheral venous blood sampling in all patients: 25 OH vitamin D3, parathyroid hormones, bone-specific alkaline phosphatase (AP), calcium (Ca), albumin, total proteins in serum, C-reactive protein (CRP), hemoglobin, creatinine and alanine aminotransferase. The blood values were determined at the time of inpatient admission prior to surgical treatment. The vitamin D status was defined as follows: deficiency: <20 ng/mL, insufficiency: 20–29 ng/mL and normal: 30–100 ng/mL.10
In addition, all patients completed a bone metabolism questionnaire with questions about the existence of a known osteoporosis including possible treatments, medication (cortisone derivatives, antiepileptic drugs or other psychotropic drugs, vitamin D, bisphosphonates, etc.), osteoporotic fractures, sports activities, time spending outdoors with exposure to sunlight and eating habits.
The statistical data were evaluated in Microsoft Excel 2013 (Redmond, WA, USA) and SPSS 24.0 (IBM, Armonk, NY, USA). Initially, the data were checked for normal distribution using the Shapiro–Wilk test. The nonparametric Mann–Whitney U test was used to compare the metric scaled variables. For the analysis of the nominal and ordinal scaled variables, the chi-squared test or Fisher’s test was used. The significance level was set to P<0.05.
Ethics approval
The ethics committee of the University Hospital Leipzig in Germany granted ethical approval (ref. no. 025-16-01022016). The committee is listed in the Institutional Review Board (IRB) of the Office for Human Research Protections (OHRP) IORG0001320, IRB00001750.
Consent to publish
Before the beginning of the study, all patients were informed and gave their written consent to treatment contract, the study and to the publication of their anonymized data.
Results
The evaluation of the laboratory tests is illustrated in Table 2. Significant differences in the CRP levels between the SG and both CGs are shown here. Significantly higher levels of calcium and bone-specific AP were also found in the CGs. However, this could not be confirmed with regard to 25 OH vitamin D3. A detailed analysis, which excludes patients who had already received antiosteoporotic therapy at the time of examination, showed no significant changes in these results. It was also noticeable that patients with PPI showed significantly lower levels of protein balance (albumin in serum, number of patients with lower levels of albumin and total protein in serum). The analysis of the questionnaires revealed significantly more patients having osteoporosis-induced fractures, manifesting a loss of size and taking osteoporosis medication corresponding to the higher number of patients with known osteoporosis. There were also significantly lower values in the SG than in the CG in terms of time spending outdoors and moving time (activities such as shopping or housework).
A detailed analysis of the SG after exclusion of patients who have already received antiosteoporotic treatment (21/80 excluded) is shown in Table 3. This is divided into acute PPI 25.4% (15/59) and chronic infections 74.6% (44/59). There were significant differences in bone metabolism (Ca and 25 OH vitamin D3) and protein balance (albumin and protein in serum) between acute and chronic PPIs (Table 3). Moreover, significant differences between acute PPI and CG I as well as CG II could be determined.
Discussion
Many aspects of the complex vitamin D metabolism are well known and researched. Today, we know that its homeostasis depends not only on adequate nutritional intake but also on intact kidney and liver function as well as sufficient exposure to sunlight (Figure 1).11,12 Its significance in infections, especially in septic processes, is also extensively described.2–5,13 In vitro studies have revealed that vitamin D3 has inhibitory effects on strains of Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumoniae, Escherichia coli and other bacteria. In the presence of vitamin D3, they were killed or a distinct inhibition of growth was induced.14,15 However, its importance in infections of the musculoskeletal system is insufficiently evaluated, although a widespread prevalence of vitamin D deficiency is known in orthopedic patients.12,16 So, there is a lack of analyses, especially according to PPIs. A paper pointing to the importance of vitamin D in PPIs was published by Traven et al in 2017. In a retrospective analysis of 126 revisions after THA, its working group ascertained that a low vitamin D level was associated with an increased risk of complications, especially periprosthetic joint infections, as the reason for a revision operation.17 Another interesting work was published by Maier et al in 2014. They found an association between an extremely low vitamin D level and periprosthetic joint infections.6 The authors concluded that vitamin D supplementation could be a safe and easy way to reduce the risk of PPIs.6 In a study from 2015 Signori et al described another thesis.7 Interestingly, all patients with an infection showed a higher vitamin D concentration (17.7±5.3 ng/mL) than the noninfected patients (15.1±5.6 ng/mL). In particular, significantly higher values were observed in patients with prosthetic joint infections (18.5±6.5 ng/mL) compared with aseptic loosening (13.6±9.4 ng/mL). The authors postulate that this is due to a rather unbalanced patient collective or the higher average age of Maier’s probands.7 The various light exposures of the two study collectives (Maier in the area around Mainz in Germany, Signori in the area around Milan in Italy) also seem conceivable.10 In our analysis, no significant differences between the vitamin D values in the CGs and the SG with PPI were found. Nevertheless, it should be noted that the majority of all patient groups showed a low vitamin D level (Table 2). In our study, it is also noticeable that patients of the SG showed a significantly worse protein status (Table 2). However, the association of hypoalbuminemia and a general protein deficiency with PPI has already been proven.18,19 Among other things, this relevant lack of albumin and protein means that fewer vitamin D can be bound to proteins. A closer examination of acute and chronic infections indicated that acute PPIs show a significant deficiency of vitamin D and calcium. This phenomenon confirms the data of Maier et al.6 This compound is also described in the studies on sepsis.4 Therefore, it must be assumed that no distinction between acute and chronic infections has been made in the two described studies, which in turn could explain the different results. In a retrospective analysis, the distinction between acute and chronic infections seems more important than the distinction between PPI in general and primary implantation or replacement surgery. Unfortunately, this interesting factor could not be analyzed more precisely due to the small size of the SG. The analysis of a larger number of patients with PPI seems necessary from this point of view.
Ultimately, our results show that vitamin D deficiency is one cause of PPI. This hypothesis could be further substantiated by the determination of the VDBP level in serum.20,21 However, these investigations are expensive, which is why they cannot be comprehensively used. Another possibility would be a genetic examination of the gene expression of the VDBP, which provides only limited information about the absolute VDBP.22 It is known that some gene variations influence the expression and treatment of infectious diseases. Its significance regarding HIV, tuberculosis and rheumatic fever has been well studied.23
Finally, further investigations seem to be necessary in order to understand the pathogenesis of vitamin D homeostasis in connection with PPI and to be able to deduce valid recommendations for the therapy. Nevertheless, we support the thesis that in patients with PPI, especially in acute PPI, vitamin D3 should be substituted together with Ca. However, this should be done including the protein balance, which should also be compensated by food substitution. Therefore, further randomized and placebo-controlled studies are also required. Because it is largely unclear, whether a substitution of vitamin D with or without additional compensation of the protein balance also contributes to an improvement in the outcome of patients with PPI. Although Hegde et al were able to show in vivo by means of a PPI mouse model that vitamin D3 deficiency leads to increased bacterial load as well as neutrophil infiltration and that this effect can be reversed by preoperative supplementation, valid clinical data are missing.24
Conclusion
In general, all patients showed a low vitamin D level in the threshold range, independently of the group. Acute PPIs in the hip and knee joints show a significantly lower calcium and vitamin D3 levels as well as a reduced protein level (albumin and total protein) compared with primary endoprostheses and aseptic revisions as well as chronic infections. From our point of view, it is, therefore, advisable to replace vitamin D3 and calcium while simultaneously adjusting the protein balance in all PPIs, especially in the acute PPI.
Limitations
Unfortunately, due to limited financial resources, it has not yet been possible to carry out investigations relating to the VDBP and the genetic variances. In addition, there is a lack of data on the outcome after substitution of the vitamin D balance, although the collective is too small for substantial statements. Another limitation is the difference in time between inital surgery and observed PPI. This varies considerably between 9 days and 42 months. This can significantly influence the results.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors wish to acknowledge the support of the non-profit German Arthritis Society (Deutsche Arthrose-Hilfe e.V.) and its president Helmut H Huberti, MD by grant P339. We acknowledge the support of the German Research Foundation (DFG) and the University Hospital Leipzig within the program of Open Access Publishing. This study was funded by the non-profit German Arthritis Society (Deutsche Arthrose-Hilfe e.V.) and the German Research Foundation (DFG) and the University Hospital Leipzig within the program of Open Access Publishing.
Author contributions
DZ analyzed and interpreted all patient data and was a major contributor in writing the manuscript. FP and AD carried out the data collection and contributed significantly to the preparation of the manuscript. ME and RM were responsible for the translation and have jointly performed the statistical analyzes. JK carried out the laboratory chemical tests. DZ, JKMF, CJ and AR were mainly responsible for the patient treatment and contributed as assistants to the preparation of the work. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50(3):194–200. | ||
Torres C, Sánchez de la Torre M, García-Moruja C, et al. Immunophenotype of vitamin D receptor polymorphism associated to risk of HIV-1 infection and rate of disease progression. Curr HIV Res. 2010;8(6):487–492. | ||
Abhimanyu A, Coussens AK. The role of UV radiation and vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16(3):314–338. | ||
Trongtrakul K, Feemuchang C. Prevalence and association of vitamin D deficiency and mortality in patients with severe sepsis. Int J Gen Med. 2017;10:415–421. | ||
Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr. 2014;112(12):1938–1943. | ||
Maier GS, Horas K, Seeger JB, Roth KE, Kurth AA, Maus U. Is there an association between periprosthetic joint infection and low vitamin D levels? Int Orthop. 2014;38(7):1499–1504. | ||
Signori V, Romanò CL, de Vecchi E, Mattina R, Drago L. May osteoarticular infections be influenced by vitamin D status? An observational study on selected patients. BMC Musculoskelet Disord. 2015;16:183. | ||
Greene KA, Wilde AH, Stulberg BN. Preoperative nutritional status of total joint patients. Relationship to postoperative wound complications. J Arthroplasty. 1991;6(4):321–325. | ||
Fayaz HC, Jupiter JB. The Zeitgeist of Challenging the Evidence. A Perspective on the International Consensus Meeting on Periprosthetic Joint Infection. Arch Bone Jt Surg. 2017;5(1):32–38. | ||
Wacker M, Holick MF. Sunlight and vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5(1):51–108. | ||
Maier GS, Horas K, Seeger JB, Roth KE, Kurth AA, Maus U. Vitamin D insufficiency in the elderly orthopaedic patient: an epidemic phenomenon. Int Orthop. 2015;39(4):787–792. | ||
Jiménez-Garrido C, Gómez-Palomo JM, Rodriguez-Delourme I, Durán-Garrido FJ, Nuño-Álvarez E, Montañez-Heredia E. The kidney, liver, index surgery and C reactive protein score is a predictor of treatment response in acute prosthetic joint infection. Int Orthop. 2018;42(1):33–38. | ||
van Etten E, Decallonne B, Bouillon R, Mathieu C. NOD bone marrow-derived dendritic cells are modulated by analogs of 1,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2004;89–90(1–5):457–459. | ||
Feindt E, Ströder J, Ströder J. Zur antimikrobiellen Wirkung von Vitamin D3 [The antimicrobial effect of vitamin D3]. Klin Wochenschr. 1977;55(10):507–508. | ||
Youssef DA, Miller CW, El-Abbassi AM, et al. Antimicrobial implications of vitamin D. Dermatoendocrinol. 2011;3(4):220–229. | ||
Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300–2304. | ||
Traven SA, Chiaramonti AM, Barfield WR, et al. Fewer complications following revision hip and knee arthroplasty in patients with normal vitamin D levels. J Arthroplasty. 2017;32(9S):S193–S196. | ||
Yi PH, Frank RM, Vann E, Sonn KA, Moric M, Della Valle CJ. Is potential malnutrition associated with septic failure and acute infection after revision total joint arthroplasty? Clin Orthop Relat Res. 2015;473(1):175–182. | ||
Bohl DD, Shen MR, Kayupov E, Cvetanovich GL, Della Valle CJ. Is hypoalbuminemia associated with septic failure and acute infection after revision total joint arthroplasty? A study of 4517 patients from the National Surgical Quality Improvement Program. J Arthroplasty. 2016;31(5):963–967. | ||
Mathias E, Tangpricha V, Sarnaik A, Farooqi A, Sethuraman U. Association of vitamin D with cathelicidin and vitamin D binding protein in pediatric sepsis. J Clin Transl Endocrinol. 2017;10:36–38. | ||
Chaykovska L, Heunisch F, von Einem G, et al. Urinary vitamin D binding protein and KIM-1 are potent new biomarkers of major adverse renal events in patients undergoing coronary angiography. PLoS One. 2016;11(1):e0145723. | ||
Daffara V, Verdoia M, Rolla R, et al. Impact of polymorphism rs7041 and rs4588 of vitamin D binding protein on the extent of coronary artery disease. Nutr Metab Cardiovasc Dis. 2017;27(9):775–783. | ||
Malik S, Fu L, Juras DJ, et al. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci. 2013;50(1):1–22. | ||
Hegde V, Dworsky EM, Stavrakis AI, et al. Single-dose, preoperative vitamin-D supplementation decreases infection in a mouse model of periprosthetic joint infection. J Bone Joint Surg Am. 2017;99(20):1737–1744. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



