Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
The Science of Absorbable Poly(L-Lactide-Co-ϵ-Caprolactone) Threads for Soft Tissue Repositioning of the Face: An Evidence-Based Evaluation of Their Physical Properties and Clinical Application
Authors Wong V
Received 1 October 2020
Accepted for publication 8 December 2020
Published 13 January 2021 Volume 2021:14 Pages 45—54
DOI https://doi.org/10.2147/CCID.S274160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Vincent Wong
Vindoc Aesthetics, London W1G 9PF, UK
Correspondence: Vincent Wong
Vindoc Aesthetics, 10 Harley Street, London W1G 9PF, UK
Tel +44 20 7299 0380
Email [email protected]
Abstract: The use of bioabsorbable threads has become a common minimally invasive technique for the nonsurgical lifting of sagged facial tissues. It entails the passage of barbed threads that form a support structure under the skin of the face and neck to mechanically reposition sagging tissue. Poly(L-lactide-co-ϵ-caprolactone) has long been used as absorbable sutures and as such has a well-demonstrated efficacy and safety profile. This biomaterial also has a well-defined biocompatibility and degradation profile. All studies reviewed in this paper show that thread lifting with absorbable barbed threads is an effective and well-tolerated procedure for correction of ptosis in facial and neck soft tissue and is associated with minor and reversible adverse effects. Most patients and surgeons consider the procedure satisfactory, with good to excellent results. This publication reviews the literature and clinical data supporting the degradation, absorbability, biocompatibility, safety, and effectiveness of these threads when used for tissue repositioning and facial rejuvenation procedures.
Keywords: poly(L-lactide-co-ϵ-caprolactone), non-surgical face lift, absorbability, microscopic evaluation, histology
Introduction
Bioabsorbable threads have recently become a popular option for rejuvenation and lifting of ptotic facial tissue.1 Customarily, the process entails the subcutaneous insertion of the threads along a planned course under the skin via needles or cannulas. This avoids the need for general anesthesia and for large incisions, which are requirements for traditional surgical face lifting, and does not have the same downtime associated with this procedure.2–4 Because of these advantages and the relative safety of bioabsorbable thread lifting, many patients have recently sought this treatment.
Modern advancements in thread technology have hence been introduced, such as the presence of barbs, cogs, or cones along certain portions of the threads. These ‘barbs’ grasp, lift and suspend and support sagging tissue. Despite its many versions, threads follow the same fundamental principle, that is mechanically repositioning or lifting sagging facial tissue in order to achieve a younger, rejuvenated, and attractive appearance.5
Depending on the direction of the barbs, threads can be categorized either as unidirectional or bidirectional. Unidirectional threads present barbs that are all angled in one direction and hence requiring anchoring to a fixed structure, often a facial ligament.6 Bidirectional threads have barbs in opposing direction and a central smooth area. They do not require anchoring points. When inserted, bidirectional threads are unable to move either way and hence, require a precise insertion technique that is often reserved for advanced users.5
These threads constitute a large group of synthetic biomaterials that are commonly used for medical devices such as drug delivery systems, implants, and surgical threads.7 Most products in the market today are made from polydioxanone (PDO), poly-L-lactic acid (PLLA), polyglycolic acid (PGA), and polycaprolactone (PCL).6,8,9 Research interest in random copolymers of L-lactide and ε-caprolactone, specifically poly(L-lactide-co-ε-caprolactone) (PLCL), has increased steadily as their potential in a wide range of biomedical applications has been realized. PLCL is a polyester manufactured from ring-opening polymerization of the cyclic ester monomers of lactide, glycolide, and ε-caprolactone.10 Unlike the rest, PLCL is used in more long-term applications, such as in controlled-release drug delivery systems and absorbable nerve guides for axon regeneration, often requiring a period of resorption that is extended, ie, more than 1 year.7
Given these properties, certain manufacturers have developed their proprietary threads that are clinically designed for a wide variety of applications intended for different facial types.
PLCL Threads: Common Features and Applications
Materials obtained from PLCL undergo slower degradation than other copolymers derived from its component aliphatic compounds.7 At least two surgical threads used for soft tissue repositioning have specifically been manufactured with PLCL. The anti-ptosis suture Excellence Visage thread was shown to have gradual degradation in subcutaneous implant models of about 40% in 18 months,11 which is relatively longer than earlier generation absorbable threads (PDO and PLLA are fully hydrolyzed within 18 months).12 Another product, Definisse™ threads [or Happy Lift™ (Revitalizing) or Dermafil™] have been shown in clinical studies to induce a cutaneous reaction of subcutaneous fibrosis, which remained stable after resorption of the thread beyond 12 months.13 The literature reviewed in this are peer-reviewed publications on these PLCL threads.
These threads were designed to have a barbed tri-dimensional geometry to enable them to maintain a mechanical lifting action for repositioning of sagging facial tissue, and a histological revitalizing action from the stimulation of fibroblasts and the synthesis of collagen, hyaluronic acid and elastin around the thread.14
Once placed, PLCL is cleaved into its components, polylactic acid and polycaprolactone, which are ultimately hydrolyzed into lactic acid and 6-hydroxycaproic acid.15 These degradation products are eventually resorbed through specific metabolic pathways16,17 with low tissue reactivity.12 This degradative process takes about 9 to 12 months, which is ample time for tissue regeneration that could lead to a sustained lifting effect.14
In this paper, the physical properties of PLCL, including its rheologic, stereomicroscopic characteristics and its composition, as well as clinical studies that evaluate its safety and efficacy are discussed. A description of commonly employed techniques in thread placement for several indications is also available here as reference for beginning practitioners.
Materials and Methods
A search of the Medline database and Google scholar using the keywords “bioabsorbable,” “clinical trials,” “evidence-based,” “meta-analysis,” “physical properties,” “poly(L-lactide-co-ε-caprolactone),” “review,” “suspension threads,” “systematic review”, or “thread lifting” was conducted. Only recent studies within the last 10 years dated on or earlier than 31 December 2019 were included. Finally, only journal articles that have been published or translated in English were included in the review. From a total of 634 hits, only aesthetic articles were selected specifically mentioning PLCL and face lifting. Three clinical studies, two consensus publications, and other relevant publications, for a total of 30 papers have been reviewed.
A written informed consent to publish anonymized before and after photos has been obtained from the patients.
Results and Discussion
Indications
Common indications for PLCL threads include contouring, lifting, and reinforcement of the middle and lower thirds of the face and neck. These are also used to eliminate jowls and ptosis of the mental area.14,18 Other manufacturers highlight the use of PLCL threads to lift other areas such as the arms, abdomen, and inner thighs. In addition, specific variations have been incorporated in the design, size, and needle type used to enable the use of the threads on specific regions of the face (Table 1).14 For simplicity, the discussion in this paper will be limited to application of the thread to the face.
 |
Table 1 PLCL Device Configurations14 |
Most manufacturers use free-floating threads that are used to partially lift the soft tissue of the malar area with mild to moderate ptosis.18,19 Through specific techniques, such as the Soft Tissue Reshaping (STR) Technique, thread placement can achieve a slightly pronounced lateral and upward lift of the cheeks. In contrast, threads made with two needles on each end, or the so-called double needle threads, are used to address the movement of tissue into two vectors: (1) transversal and (2) sagittal, for repositioning and reshaping of the forehead, cheeks, jawline, as well as the neck. Various techniques have been developed employing specific entry points (IN), exit points (OUT), reshaping lines (RL) and in some cases an intermediate point (M). Safety lines (SL) are demarcated to avoid adverse events associated with damage to major vessels of the face.14 Other indications, such as that for anchorage threads can achieve fixed anchor lifting along the malar area or along the jawline to counter gravity.14 Lastly, a number of threads have been used for the nose for nasal reshaping or as a minimally invasive option for nose lifting.14,18–20
Microscopic, Rheological, and Histologic Characteristics
PLCL threads have been evaluated for their general morphology, tensile strength, rheological creep, recovery, and mechanical strength.21 Stereomicroscopic analysis showed that specimens of PLCL threads had notches that created periodic, bi-directional barbs along their lengths (Figure 1A–C).21 Histological analysis of the tissue surrounding these threads from two different patients showed that after 2 months, these threads connect to the high and lateral dermis retinacula cutis system, to the SMAS, and to the downward muscular bands. No apparent signs of inflammation were found in these specimens.13 Studies conducted on other PLCL brands have shown that after 14 days, a layer of connective tissue surrounds the thread. Interestingly, there is a reported gradual increase in thickness of the layers by day 30 and a subsequent decrease seen by day 60 likely due to reorganization of the fibroblasts and maturing of connective tissue.22
 |
Figure 1 Microscopic Images of PLCL threads: Stereomicroscopy was performed at magnifications of 6.7x (A), 20x (B) and 40x (C); PLCL threads (12 cm and 23.2) cm were both seen to have notches that create periodic, bi-directional barbs along their lengths.14 |
In a limited histologic study conducted by the author to evaluate thread morphology of a single thread after 40 days application, there appeared to be minimal immunologic response in this sample with only few mast cells adhering to the thread lining. Beyond 15 months, there were also no visible signs of untoward tissue reactions (Figure 2).23
 |
Figure 2 Mast cells seen around a PLCL thread 40 days after insertion. |
After absorption of the thread, a more permanent and homogenous fibrous capsule replaces the thread while it becomes resorbed. In a cross section of skin taken 2 months after insertion, a fragment of thread is seen as a dark-colored ovoid figure surrounded by the cells and connective tissue creating a fibrotic capsule. There were no overt signs of inflammation seen in the examined tissue. After absorption, a more permanent collection of scarred connective tissue replaces the capsule while the absorbed thread is no longer discernible.13 This capsule apparently enhances tissue firmness. An increased thickness of the dermis was suggestive of the formation of collagen, elastin, and other connective tissue (Figure 3).23
 |
Figure 3 Histological assessment of poly(L-lactide-co-ε-caprolactone) showing that it is fully absorbed in a fibrous tissue collected after 18 months of treatment (H & E, original magnification 920). |
The stability of the threads, which were not fully resorbed until at least 12 months after the procedure, is believed to provide a substantial scaffold where development of connective tissue and angiogenesis could occur. These properties likely contributed to clinical results of gradual increase in lift and revitalization for up to 6 to 12 months after the procedure.23
The properties above demonstrate that the threads have excellent tissue integration. The inflammatory and collagenesis process is seen local to where the threads have been placed, and there are no extensive reactions nor are there extended puckering seen on the skin when it heals. This is an important point as it means that thread placement has no effect on the movement of the facial muscles of expression in the treated area. For mild and moderate ptosis, the density and length of barbs can effectively lift tissue safely. Patients are unable to feel or see the threads under the skin, and the recovery time is usually short (eg, within 5 days). Patients can return to work or daily activities without any signs of having a procedure done. In the recovery phase, there are less limitations for the patient as the threads are strong and do not break easily.
A Review of Efficacy and Safety Studies of PLCL Threads
The safety and effectiveness of PLCL threads have been demonstrated in clinical studies by Rezaee Khiabanloo et al,24 Savoia et al,13 and Kalyan et al.25
In the study by Rezaee Khiabanloo and colleagues, 193 patients were enrolled and underwent thread lifting procedures to the eyebrows, midface, jawline, and neck using PLCL threads in conjunction with Silhouette Soft threads. These patients aged 25 to 89 years old underwent correction of the jawline (46.1%), midface (33.7%), eyebrows (12.4%), and neck (7.8%). Patient-perceived level of satisfaction increased from 94% in the first week after surgery to 99% in the sixth month after surgery, while surgeon satisfaction increased from 94% to 99%, and from 83% to 98% for surgeons 1 and 2, respectively.24 The most reported adverse event was ecchymosis (40.9%), followed by complications of dimples (28.5%), tumefaction (18.1%), and pain (5.2%).24 Overall, the results of this study showed that facial rejuvenation using combination of PLCL threads and Silhouette Soft threads is effective, safe, and is associated with minor complications.24
Savoia and colleagues evaluated patient-perceived surgical outcomes associated with PLCL threads in eyebrow lifting, forehead lifting, mid-face lifting (zygomatic malar), upper-mandibular region lifting, and high cervical region lifting. Female patients aged between 37 and 65 years old, with average aging signs and required a lifting of modest degree were enrolled in the study.13 Of the 37, 89% considered the results of their thread lift procedures satisfactory, of which 65% considered the results “excellent” and 24% “good.” Although an 11% regarded their results “unsatisfying,” none of the patients asked for the removal of the threads immediately after the operation. Further, the optimal cosmetic effect was maintained after 6 months of treatment.13 Histological analysis performed on two patients treated with the free-floating threads revealed no signs of acute inflammation, necrosis, or other significant pathological phenomenon in all the specimens analyzed. After 2 months, it was possible to detect some connective structure and fibroblastic cells surrounding the thread in the subcutaneous tissues, consistent with the properties of the barbed threads.13 The most frequent minor complication was the presence of small ecchymosis 62%. Mild erythema was noticed in 41%. These complications lasted for a maximum of 3 weeks with none of the patients requesting any treatment. Mild transitory aesthesia of a maximum of 2 months was encountered in two patients. Mild post-operation tumefaction was also observed in 41% of patients that were successfully treated with non-steroidal drugs. All the blood, urine, and electrocardiogram tests were normal during the testing period, and all participants could return to their everyday activities within 3 days of treatment.13 Overall, this study demonstrated the effectiveness of PLCL threads when used for facial tissue lifting. Microscopic analysis showed that the procedure also stimulated the synthesis of collagen providing greater structure and elasticity to the skin. Additionally, no acute inflammatory responses were elicited by the procedure.13
Kalyan and Wong evaluated the surgical outcomes associated with double needle and free-floating threads in relation to soft tissue lifting in the zygomatic and mandibular areas of the face. Free-floating bidirectional threads were used for the zygomatic area, while double needle convergent bidirectional threads were used for the jaw line and submental area. Twenty-three female patients aged between 32 and 57 years old, with sagging soft tissue that required mild to moderate lifting, were enrolled in the study. This study showed that significant improvement in tissue sagging was observed in all patients, with 21.7% of patients considering their results “good,” 43.5% considered their results “very improved,” and 34.8% said they noticed “exceptional improvement.” About 65.2% were satisfied with the treatment, 26% were highly satisfied, and 8.8% were neither satisfied nor dissatisfied. The most frequent complications observed in this study were bruising (87.0% of patients) and transient and mild skin puckering (47.8%). Mild erythema was also observed. No other complications were observed through the study period and no patients asked for the removal of the threads post-procedure. Additionally, no significant acute inflammatory response following treatment with the double needle and free-floating threads was detected.25
Guidance Relevant to Technique of PLCL Thread Placement
Only a few guidelines on bioabsorbable barbed monofilament threads for facial mobilization and lifting have been published thus far. In terms of patient selection with matching thread, Wong and colleagues have specified that PLCL threads are useful for patients necessitating rejuvenation and moderate lift.26 Fundaro and colleagues have recently outlined procedural and technical guidelines on the use of bidirectional PLCL threads for a variety of indications.27 Berardesca and colleagues have outlined essential techniques and outlined management of complications in a recently published book/manual.14 The recommendations below are largely derived from these articles.
Pre-Operative Preparation
Select patients with light to moderate signs of aging and normal to thick skin with trophic or hypertrophic subcutaneous tissue. The recommended patient profile may include the following characteristics:14
- Sagging skin or lower face due to aging
- Aesthetic improvement achievable with tissue repositioning
- Full comprehension of the procedure and post-operative issues, as well as the possible side-effects and complications
- Intention and motivation for aesthetic medicine treatment
- Compliance to the aftercare indications and availability for follow-up
- With good doctor-subject relationship to increase mutual trust
The procedure is contraindicated in the following conditions:14,27
- Recognizable personality disorders
- Unrealistic subject expectations
- Substance abuse
- Severe systemic/immunologic illnesses/bleeding disorders
- Local or systemic infections
- Acne/rosacea at or near entry/exit points
- Pregnant or breastfeeding mothers
- Prior reaction to fillers/threads at same treatment area
- Facial/scalp eczema or psoriasis
- Intolerance or allergies to thread material and lidocaine/epinephrine
- History of keloids/hypertrophic scarring
- Uncontrolled diabetes
Subject assessment should include evaluation of the skin, face shape, aging processes, anatomic landmarks, and other aesthetic concerns. Facial shapes can be classified as either round, rhombic, diamond, or “melted ice cream.” Fundaro and colleagues outlined the use of the Aging Type Classification (ATC) system (Table 2) to categorize their patients.14,27
 |
Table 2 Aging Type Classification (ATC) System27 |
Fundaro states that patients who are classified as type 1 and 2 are suitable as first choice for volume augmentation of hypotrophic fat compartments using filler injections. After volume restoration and in the presence of sagging, they can be treated with suspension threads. In type 3 patients, aging is characterized mainly by the ptosis of hypertrophic superficial fat compartments but this time with volume reduction of deep fat. The repositioning of superficial fat with threads could be the first therapeutic choice followed by volume restoration of deep compartments with fillers. Type 4 patients are characterized by an excessive facial volume and consequent soft tissue ptosis; therefore, the only choice is volume repositioning with suspension threads. The weight of fatty tissue and quality of retinacula cutis and its interaction with threads affect the overall repositioning effect. If there is high fat volume or if the quality of the SMAS has already been severely compromised by the aging process, soft tissue repositioning may have limited effects.27
To ensure asepsis, conduct insertion in an operating theatre or a minor procedure room. Before commencing, apply antiseptic solutions to disinfect the area of skin and outline the face using a dermographic pen. These will aid with accurate placement of the thread to ensure the desired aesthetic result.14
Operative Technique
Threads placed too superficially in the dermal plane become uncomfortably palpable and may even be visible. In this plane, the threads will also not achieve the correct degree of lifting of the tissues nor stimulate collagen production.28 On the other hand, threads placed too deeply present a greater risk of damage to deep anatomical structures such as the arteries, veins, and nerves of the face.29 Therefore, it is essential to identify the correct tissue layers of the face for placement. Correct placement in the subcutaneous and supra-SMAS tissue ensures adequate lifting. Regardless, gross scarring is not typically a dilemma for patients undergoing thread placement as the needle entry points are relatively unnoticeable when adequately healed.
After this, use a local anesthetic keeping in mind the extent of the procedure and the experience of the surgeon.5 There have been insertion techniques identified that are suitable for certain facial types (Figure 4). These techniques have been discussed extensively by Berardesca and Fundaro in their respective publications.14,27
 |
Figure 4 Landmarks of the different insertion techniques based on the principal direction of the repositioning vectors.14 The Lateral Vector (LV) techniques are typically used in Caucasian facial types and include the: (A) Jawline Reshaping (JR), (B) Malar Reshaping (MR) and (C) Lateral Reshaping (LR) techniques. The Vertical Vector (VV) techniques are mostly used in Asian facial types and include the: (D) Oval Reshaping – H technique (OR-H) and the (D) Oval Reshaping – Vertical technique (OR-V). The Anti-Gravity Reshaping (AGR) techniques may be used for large areas of ptosis and include the (F) Malar (AGR-M) and (G) Jawline (AGR-J) technique. The Soft Tissue Reshaping (STR) technique (H) is used to create more volume in the malar area. Abbreviations: Legend: IN, entry point; M, midpoint; OUT, exit point; R, vector route; SL, safety line; TA, thread axis. |
Post-Operative Care
After careful placement of the thread, to reduce the risk of complications during recovery, the following post-operative procedures should be done:5
- Short-term prophylactic antibiotics are to be prescribed.
- Swelling and bruising can be prevented by applying ice packs to the treated area.
- Explain to the patient that post-operative pain can be managed with oral medications such as acetaminophen, but NSAIDs, such as ibuprofen, should be avoided to limit bruising.
- It should be recommended to the patients to keep their head elevated when sleeping on the first night after the procedure to reduce swelling.
- Movement of the face may be restricted by applying tape to the treated areas of the face for about a week.
- Patients should also be advised to sleep on their back for a week and to avoid straining muscles around their mouth or massages of any kind for 3 weeks.
- Patients should be advised to resume non-strenuous activities the day after the procedure, while normal activities can usually be resumed within 7 days.
- Mild bruising, neural pain or paraesthesia after insertion, oedema, slight inflammation, skin dimpling, and irregularities/asymmetry may occur. Advise the patient to return if with persistence of any of these symptoms.27
- The complications that require urgent care include haemorrhage, thread rupture or barb unhooking, thread visibility or palpability, extrusion, infection, sialocele formation, or parotid duct rupture.27
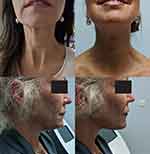
Images taken before and after show the effects of thread lifts in various indications after 9 and 12 months (Figure 5).
Conclusion
Bioabsorbable monofilament PLCL threads have been used by aesthetic medicine practitioners worldwide for years. It has a good biocompatibility profile, with excellent safety and tolerability. Studies conducted on PLCL threads, mainly on Definisse™ threads, have shown that these are effective and well tolerated for facial rejuvenation procedures. PLCL threads can be used to perform three-dimensional tissue repositioning and to provide a noticeable lifting effect for mild to moderate sagging tissue. None of the patients in the assessed studies developed serious complications. Most adverse responses resolve either spontaneously or with minimal supportive therapy. In these studies, most patients and surgeons consider the procedure satisfactory with good to excellent clinical results.
Results last over 18 months as shown in clinical studies due to the collagen-stimulating properties and because PLCL threads are stronger than earlier generation threads. More studies evaluating the histology of PLCL threads on different regions of the face and in different patient types, and in comparison with other thread types, should help to objectively elucidate the possible extent of variations in immunologic response (ie, by ex vivo analysis) in the future. Also, more applications such as body treatments (eg, breast lift or buttock lift) are worth exploring and threads may also be improved based on indications (eg, shorter thread for the nose). Recent articles and/or technical guide documents have been published and are recommended for reading by learning practitioners.
Acknowledgments
I would like to thank RELIFE S.r.l. for providing an unrestricted educational grant.
Disclosure
The author has consultancy contracts with RELIFE S.r.l., reports grants from RELIFE S.r.l., during the conduct of the study, personal fees from RELIFE S.r.l., outside the submitted work, and no other potential conflicts of interest for this work.
References
1. Azevedo H, Reis R. Understanding the enzymatic degradation of biodegradable polymers and strategies to control their degradation rate. In: Reis R, Roman J, editors. Biodegradable Systems in Tissue Engineering and Regenerative Medicine. New York: CRC Press; 2005:179–182.
2. Wu WTL. Barbed sutures in facial rejuvenation. Aesthetic Surg J. 2004;24:582–587. doi:10.1016/j.asj.2004.09.007
3. Wu WTL. Commentary on Facial rejuvenation with fine barbed threads: the simple MIZ-lift. Aesthetic Plast Surg. 2014;38:75–77. doi:10.1007/s00266-013-0262-6
4. Sulamanidze M, Sulamanidze G. APTOS suture lifting methods: 10 years of experience. Clin Plast Surg. 2009;36:281–306. doi:10.1016/j.cps.2008.12.003
5. Kalra R. Use of barbed threads in facial rejuvenation. Indian J Plast Surg. 2008;41:S93–S100. doi:10.1055/s-0039-1700480
6. Yongtrakul P, Sirithanabadeekul P, Siriphan P, et al. Thread lift: classification, technique, and how to approach to the patient. Int J Medical, Heal Biomed Bioeng Pharm Eng. 2016;10:547–555.
7. Jelonek K, Kasperczyk J, Li S, et al. Novel Poly(L-lactide-co-ε-caprolactone) matrices obtained with the use of Zr[Acac]4 as nontoxic initiator for long-term release of immunosuppressive drugs. Biomed Res Int. 2013;2013:607351. doi:10.1155/2013/607351
8. Tavares JDP, Oliveira CACP, Torres RP, et al. Rejuvenescimento facial com fios de sustentação. Braz J Otorhinolaryngol. 2017;83:712–719. doi:10.1016/j.bjorl.2017.03.015
9. Park JH, Lee BK, Park SH, et al. Preparation of biodegradable and elastic poly(ε-caprolactone-co-lactide) copolymers and evaluation as a localized and sustained drug delivery carrier. Int J Mol Sci. 2017;18:671. doi:10.3390/ijms18030671
10. Fernández J, Etxeberria A, Sarasua JR. Synthesis, structure and properties of poly(L-lactide-co-ε-caprolactone) statistical copolymers. J Mech Behav Biomed Mater. 2012;9:100–112. doi:10.1016/j.jmbbm.2012.01.003
11. Sulamanidze GM, Sulamanidze MA, Sulamanidze KM, et al. The subcutaneous tissue reaction on poly (l-lactide-co-caprolactone) based threads. Int J Clin Exp Dermatology. 2018;3:1–9.
12. Molea G, Schonauer F, Bifulco G, et al. Comparative study on biocompatibility and absorption times of three absorbable monofilament suture materials (Polydioxanone, Poliglecaprone 25, Glycomer 631). Br J Plast Surg. 2000;53:137–141. doi:10.1054/bjps.1999.3247
13. Savoia A, Accardo C, Vannini F, et al. Outcomes in thread lift for facial rejuvenation: a study performed with Happy LiftTM revitalizing. Dermatol Ther. 2014;4:103–114.
14. Berardesca E, Cirillo P, Fundaro SP, et al. Reshaping with Barbed Threads.
15. Ramot Y, Nyska A, Markovitz E, et al. Long-term local and systemic safety of poly(l-lactide-co-epsilon-caprolactone) after subcutaneous and intra-articular implantation in rats. Toxicol Pathol. 2015;43:1127–1140. doi:10.1177/0192623315600275
16. Goonoo N, Jeetah R, Bhaw-Luximon A, et al. Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft fur Pharm Verfahrenstechnik eV. 2015;97:371–391. doi:10.1016/j.ejpb.2015.05.024
17. Malikmammadov E, Tanir TE, Kiziltay A, et al. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed. 2018;29:863–893. doi:10.1080/09205063.2017.1394711
18. APTOS. Excellence for facial rejuvenation. Available from: https://www.prime-journal.com/aptos-excellence-for-facial-rejuvenation/.
19. Xline: the X concept. Available from: http://www.xcelens.ch/x-lineingleseweb.pdf.
20. Can N. Aptos thread lifting methods of the nasal tip lifting a minimally invasive approach for nose reshaping. Aesthetic Med. 2018;4:14–19.
21. Sundaram H. The new science of threads: an evidence-based paradigm for physiological tissue mobilisation and reshaping. Prime J. 2019;48–54.
22. 3rd generation aptos threads with HA for simultaneous lifting and rejuvenation. Available from: https://aptos.cz/en/post/3rd-gereration-aptos-threads.
23. Wong V. Efficacy of bidirectional barbed polycaprolactone threads in repositioning of soft tissue in the neck. J Clin Exp Dermatol Res. 2017;8:1.
24. Rezaee Khiabanloo S, Jebreili R, Aalipour E, et al. Outcomes in thread lift for face and neck: A study performed with Silhouette Soft and promo Happy Lift double needle, innovative and classic techniques. J Cosmet Dermatol. 2019;18:84–93.
25. Kalyan R. The efficacy of polycaprolactone threads in zygomatic and mandibular lifting: consecutive study from a single practitioner ’s experience. Int J Clin Exp Dermatology. 2017;1:3–5.
26. Wong V. Hanging by a thread: choosing the right thread for the right patient. J Dermatology Cosmetol. 2017;1:86–88.
27. Fundaro SP, Goh CL, Hau KC, et al. Expert consensus on soft tissue repositioning using absorbable barbed suspension double needle threads in Asian and Caucasian patients. In press 2021.
28. Mian I. PDO Threadlifting. Available from: https://aestheticsjournal.com/feature/pdo-threadlifting.
29. Jacono A SMAS facelift rhytidectomy. Available from: https://emedicine.medscape.com/article/841892-overview.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.