Back to Journals » Clinical Ophthalmology » Volume 16
The Safety Profile of FDA-Approved Epithelium-Off Corneal Cross-Linking in a US Community-Based Healthcare System
Authors Ang MJ, Darbinian JA, Hoskins EN, Holsclaw DS, Sudesh S, Chandra NS
Received 20 January 2022
Accepted for publication 15 March 2022
Published 11 April 2022 Volume 2022:16 Pages 1117—1125
DOI https://doi.org/10.2147/OPTH.S359224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michael J Ang,1 Jeanne A Darbinian,2 Eliza N Hoskins,3 Douglas S Holsclaw,3 Sudha Sudesh,3 Naveen S Chandra3
1Department of Ophthalmology, California Pacific Medical Center, San Francisco, CA, USA; 2Division of Research, Kaiser Permanente Northern California, Oakland, CA, USA; 3Department of Ophthalmology, The Permanente Medical Group, Oakland, CA, USA
Correspondence: Naveen S Chandra, The Permanente Medical Group, 320 Lennon Lane, Walnut Creek, CA, 94598, 94598, Tel +1 925-906-2550, Fax +1 925-906-2332, Email [email protected]
Purpose: To determine the occurrence of early post-operative complications following FDA-approved epithelium-off corneal cross-linking in the United States.
Materials and Methods: This multicenter, retrospective cohort study identified patients who underwent epithelium-off (epi-off) corneal cross-linking (CXL) for keratoconus and post-refractive keratectasia within the Kaiser Permanente Northern California healthcare system between 2016 and 2018. Post-operative complications including delayed epithelial healing, infection, and loss of visual acuity were recorded.
Results: The study included 878 eyes of 654 patients. The mean age was 27± 9.4 years (range 7– 71). Five hundred ninety-nine patients (91.6%) had keratoconus while 55 had post-refractive corneal ectasia (8.4%). Forty-seven eyes had prolonged follow-up because of the occurrence of complications in the early post-operative period. The respective rates of delayed epithelial healing, and keratitis were 3.9% (95% CI 2.7– 5.3%), and 1.5% (95% CI 0.8– 2.5%). Four approaches for management of delayed epithelial healing were compared; epithelium healing duration was the longest in the repeat bandage contact lens (BCL) group (23.8 days) and the shortest in the antibiotic ointment group (14.3 days), with statistically significant differences (p < 0.05) in the healing time between these 2 groups.
Conclusion: The concern for early clinical complications after epi-off CXL often leads to delayed CXL intervention and further keratoconus progression with increased economic burdens. A large retrospective review of 878 eyes found that FDA-approved epi-off CXL protocol appears to be safe with low occurrence rates of early post-operative complications. The recommended management for delayed epithelial healing is using antibiotic ophthalmic ointment.
Keywords: keratoconus, epithelium-off, corneal cross-linking, CXL, post-operative complications, epithelial healing
Introduction
Keratoconus is a severe, sight-threatening disorder that can significantly impair vision and is the most common primary corneal ectasia.1–3 It is often asymmetric and bilateral and it is a progressive disease that can result in distortion and thinning of the cornea, which can lead to irregular astigmatism and corneal scarring that is not correctable with spectacles beyond the mildest stages of the disease.1–3 The onset of keratoconus typically occurs in adolescence or young adulthood and, if left untreated, may result in a reduced quality of life and represents an economic burden for individuals and health systems.4–6
In 2016, a drug-device combination for epithelium-off corneal cross-linking (epi-off CXL) received Food and Drug Administration (FDA) approval as the first and only therapy demonstrated to slow or arrest the progression of keratoconus.7,8 Economic analyses in Canada, the Netherlands, Norway, and the United Kingdom support the hypothesis that providing CXL during the early stages of progressive keratoconus proves to be cost-effective, limiting the need for more invasive and costly procedures like corneal transplantation at the later stages of the disease.5,6,9,10 In 2020, a study conducted in the USA further states that the economic value of cross-linking is maximized when applied earlier in the disease process and/or younger age and extends to improved work productivity and quality of life.11
As with any surgical procedure, there is a risk of infection. After more than 15 years of widespread use of epi-off CXL around the world, various sporadic case reports of infectious keratitis associated with epithelial debridement have been published in the literature.12–14
In 2009, Koller et al conducted a prospective study on 117 eyes of 99 patients and found that the complication rate was 2.9% (95% CI: 0.6–8.5%) and the failure rate was 7.6%. Sterile infiltrates were found in 7.6% of eyes and central stromal scars in 2.8%.12
In 2019, Nicula et al published 10-year results of standard Epi-Off CXL in 113 eyes of 90 keratoconus patients in Romania. They noticed a statistically significant improvement of all keratometry measurements. They had 6 cases of sterile infiltrates which responded to steroid treatment and a case of delayed epithelial healing with good evolution. They did not encounter any case with infective keratitis.15
Another study in 2019 by Iqbal et al found that out of 49 eyes (of 28 keratoconus patients), only 2 eyes (4.2%) of a pediatric patient with vernal keratoconjunctivitis progressed by the end of the fifth year. Five eyes (10.2%) had delayed epithelial healing. All of them were treated by removal of contact lens, cessation of topical steroid, and adding a preservative-free eye lubricant, four eyes improved and only one had a persistent epithelial defect.16
Currently, there is a paucity of large cohort data from the United States to help identify the actual rate of infectious keratitis and delayed epithelial healing following epithelium-off corneal cross-linking. This study aims to determine the occurrence of early post-operative complications following corneal cross-linking in the United States.
Materials and Methods
Setting
This is a retrospective observational multicenter study conducted within the membership of Kaiser Permanente Northern California (KPNC), an integrated healthcare system with more than 4.5 million members. The membership is racially and ethnically diverse and, except at extreme of incomes, demographically similar to the underlying population.17 The study adhered to the tenets of the Declaration of Helsinki and its amendments and was approved by the KPNC Institutional Review Board with a waiver of consent due to its retrospective nature. All patients’ data were anonymized.
Inclusion and Exclusion Criteria
Medical records of all eyes that underwent epithelium-off corneal cross-linking for the FDA-approved indications, ie keratoconus, and post-refractive keratectasia, from November 1, 2016, to December 31, 2018, in the KPNC system were identified from procedure codes specific to KPNC or by appointment types specific for the procedure and linked to ophthalmology visits with a provider known to have performed epi-off CXL. Exclusion criteria included loss to follow-up within 31 days of treatment and any eye that had epi-off CXL performed for an indication that was not approved by the FDA (eg, pellucid marginal corneal degeneration and corneal ulcer).
Cross-Linking
Epithelium-off corneal cross-linking was performed with the current FDA-approved UVA device and riboflavin ophthalmic solutions.7 A topical ophthalmic anesthetic was administered in the treatment eye before removal of the central 9 mm of the epithelium. Subsequently, two riboflavin solutions (Photrexa Viscous, 0.146% riboflavin ophthalmic solution plus 20% dextran, and Photrexa, 0.146% riboflavin ophthalmic solution without dextran, Glaukos, San Clemente, CA, USA) were alternately applied topically to the corneal surface at one-minute intervals for 30 minutes.18 Once the riboflavin absorption was confirmed by slit-lamp examination, ultrasound pachymetry was performed to ensure a minimum of 400µm at the thinnest corneal location before initiating UVA irradiation. If the thinnest corneal location was less than 400μm, the hypotonic Photrexa (Glaukos) was applied at a rate of 1 drop every 10 seconds while pachymetric measurements were performed in 2-minute intervals. This was repeated until a corneal thickness of at least 400 μm was obtained. Once this pachymetry criterion was met, the cornea was exposed to ultraviolet A (365-nm) light for 30 minutes at an irradiance of 3.0 mW/cm2 (KXL System, Glaukos). During ultraviolet exposure, the alternating applications of the two topical riboflavin solutions were continued every minute. Following the procedure, antibiotic and corticosteroid eye drops were applied, a soft contact lens bandage (BCL) was placed, and the eye was reexamined at the slit lamp. Some patients were also prescribed topical steroids.
Data Acquisition
Follow-up, as a proxy for an opportunity to use clinical services, especially ophthalmology-related, was from the date of the indexed epi-off CXL procedure until 15 months afterward or October 31, 2019, whichever occurred first. Data obtained from electronic sources included demographics (age, sex, race/ethnicity), eye(s) involved, and underlying diagnosis.
For the cases with post-operative visual acuity data, the date of the most recent post-procedure visual acuity measurement was used as the end of follow-up provided it was within this specified range. Where postoperative visual acuity measurements were not available, the date of last active health plan enrollment during the defined study period was used to mark the end of follow-up.
Potential post-procedure complications were initially identified by flagging all cases who, within 31 days of the epi-off CXL procedure, had four or more ophthalmology visits and/or clinical encounters linked diagnosis codes for all types of keratitis and/or corneal ulcers. The medical records of these cases were reviewed by the investigator to ascertain evidence of delayed epithelial healing, infections, or both. In addition, sample cases were selected from the remaining cases, which had three or fewer ophthalmology visits within 31 days postoperatively. These cases were reviewed to ascertain if postoperative complications occurred among the subjects not initially flagged based on the algorithm. This sample included all eyes with one post-operative visit plus 10% of the cases with 2–3 visits.
Time to epithelial healing was categorized as healed by the second postoperative visit (which ranged from 5 to 8 days postoperatively). When prolonged healing required further intervention, the number of days and treatments employed to fully close the epithelial defect was recorded. When infection was present, type of keratitis (bacterial, fungal, herpetic, etc.), along with pathogens isolated in culture, treatment administered, and days until resolution were documented. Visual acuity change was determined by reviewing an individual chart of all cases that incurred a documented post-procedure complication. For pre- and post-operative visual acuity measurements, Snellen acuity was converted to the logarithm of the minimum angle of resolution (logMAR).
Statistical Analysis
Descriptive statistics included counts and percentages for categorical variables (such as race/ethnicity) and means ± standard deviations (SDs) for continuous data (such as age at the procedure). Binomial proportions and exact 95% confidence intervals (CI) were computed to report the proportion of the cohort that incurred postoperative complications. All analyses were performed using SAS version 9.4 software (SAS Institute, Inc., Cary, North Carolina).
Results
Figure 1 shows the 885 eyes treated in 658 subjects for FDA-approved conditions. Among the original 885 eyes, 112 charts were flagged for frequent visits (≥4 within 31 days) or coding of corneal ulcer or infectious keratitis. After a thorough review, 47 eyes in 44 subjects were found to have delayed healing (n = 34 eyes in 31 unique subjects) or keratitis (n = 13 subjects and eyes). No additional cases of delayed epithelial healing or keratitis were identified among the 91 sample cases selected from the remaining 773 that had one to three postoperative visits within the 31-day window. The sample group consisted of all 16 eyes with only one postoperative ophthalmology visit and an approximately 10% stratified random sample of cases with two (n = 45) and three (n = 30) visits, respectively.
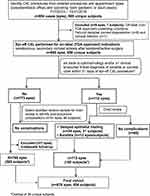 |
Figure 1 Identification of epithelium-off CXL cases. |
Seven of the original 885 cases (eyes) had insufficient follow-up data after the index epi-off CXL procedure and were excluded from the study, leaving 878 eyes from 654 unique subjects. The mean age was 27.0±9.4 years (range 7–71 years). As shown in Table 1, approximately two-thirds (n = 432) of the patients identified were male. A total of 224 patients had epi-off CXL performed on both eyes, with a mean interval between them of 145 days (range 0–518 days). Seventy-two percent of the subjects in the study were non-white. Nearly 44% had one year of follow-up, 27.7% had six months, 18.7% had three months, and 10.1% had one month of follow-up.
 |
Table 1 Characteristics of the Cohort: The 654 Unique Subjects Who Underwent Epithelium-Off Corneal Crosslinking (Epi-Off CXL), 2016–2018 |
Thirty-four of the 878 eyes (3.9%; exact 95% CI 2.7 to 5.4%) had delayed healing confirmed by review of the medical records. The mean number of days to complete epithelialization for these 34 eyes was 18.6±7.5 days (range 9–35); median (IQR) 16 days (13, 22). Treatment of delayed epithelial healing included antibiotic ointment four times per day without BCL (7 cases), continued routine care with postoperative steroid and antibiotic drops without BCL (7 cases) or with repeat BCL (10 cases), and application of a pressure patch with antibiotic ointment (10 cases). Mean epithelium healing duration was the longest in the repeat BCL group (23.8 days) and the shortest in the antibiotic ointment group (14.3 days), with statistically significant differences (p < 0.05) in the healing time between these 2 groups (Figure 2).
Thirteen of the 878 eyes (1.5%; 95% CI 0.8–2.5%) of 13 subjects exhibited a sign of keratitis, which warranted the treating ophthalmologist to code the visit as corneal ulcer or keratitis. These cases presented between postoperative days 1–30 and resolved between postoperative days 7–83. Of the affected eyes, four were right and nine were left; seven (53.8%) were in male patients. The CXL treatment dates evenly span the whole study period. No culture-proven cases of infectious keratitis were identified: 6 (46%) eyes had cultures performed, all of which were negative. No cultures were obtained for the remaining 7 because the infiltrates were small and peripheral or clinical presentation suggested viral etiology. Clinical presentation and therapeutic response implied the etiologies to include herpetic (4 eyes), bacterial (3 eyes), fungal (1 eye), inflammatory (2 eyes), and undetermined (3 eyes).
Of the four suspected cases of herpetic keratitis, three presented in 2017, and only one presented in 2018. None of the patients who developed herpetic keratitis had a history of facial HSV or were taking peri-operative oral antiviral prophylaxis. These cases presented with a variety of findings, ranging from disciform keratitis or dendrite on post-operative day 1 to late epithelial defect.
The remaining nine eyes with suspected keratitis presented with a subepithelial or anterior stromal opacity with an overlying epithelial defect. Three eyes responded to antibacterial topical therapy (including fortified vancomycin eye drops, gatifloxacin eye drops, and/or tobramycin ophthalmic ointment) alone, so are deduced to be of bacterial etiology. One eye with multiple small infiltrates, without an epithelial defect, failed to respond until topical and oral anti-fungal therapies were added. In one eye, infiltrates did not respond to antibiotic therapy but rapidly responded to 1% prednisolone acetate eye drops alone. Two eyes had infiltrates that responded to a topical antibiotic-steroid combination. One eye presented with a small late infiltrate that resolved on its own without any added topical treatment apart from the postoperative drops. One eye presented with infiltrate and immune rings which responded to a combination of a topical antibiotic, a topical steroid, and oral prednisone taper from 60mg QD over 10 days.
Safety Profile
Differences between pre- and post-operative visual acuity measurements for the 47 cases that incurred complications are summarized in Tables 2 and 3. Thirty-eight of the 47 eyes (80.9%) maintained or improved vision at the last examination up to one year, with a mean 3 lines of improved vision (range 0–5 lines). Nine eyes (19.1%) lost a line or more of vision, four of which resulted from infectious keratitis and the remainder from delayed epithelial healing. Of the 9 eyes, six eyes lost 1 line of best corrected vision (BCVA), one eye lost 2 lines of BCVA (mostly in the 20/30 to 20/60 range), one eye lost 1 line of UCVA, and one eye lost 5 lines of UCVA (both in the 20/100 range).
 |
Table 2 The 47 Cases That Incurred a Post-Procedure Complication: Δlogmar (Post-Procedure Va Minus Pre-Procedure Va), by Type of Correctiona |
 |
Table 3 The 47 Eyes That Incurred a Post-Procedure Complication: Measures of Dispersion for Change in logMAR Between Pre- and Post-Operative Measurements, by Category |
Discussion
This study aimed to determine the rate of early post-operative complications following FDA-approved epi-off CXL in a large US community-based healthcare system. The data shows a low occurrence of early post-operative complications, including delayed epithelial healing (3.9%), and infectious keratitis (1.5%) in a large cohort of 878 eyes. While CXL has been widely used internationally for many years, it has only recently received FDA approval for use in the United States (2016).
In the international literature, there are only a few large studies to identify the rates of delayed epithelial healing and infectious keratitis following epi-off CXL and those rates vary between 4% and 26% of the eyes, depending on the study.
In a recently published summary of US multicenter studies, Greenstein et al reported that between 23% (for keratoconus cases) and 26% (for ectatic cases) of eyes presented an epithelial defect after one week19 but in their safety-dedicated study of 117 eyes, Koller et al found that in patients following treatment with epi-off CXL, epithelial healing was complete at a mean of 3.25 days±1.4 days (range 1–8 days).12 Iqbal et al recently reported a delayed epithelial healing rate in 11 eyes (14%) in their retrospective study of 79 eyes who underwent epi-off CXL combined with photorefractive keratectomy.20 Price et al reported that the epithelium was not fully healed at the 1-week post-operative examination in 31 of 717 (4%) of treated eyes.17 For these eyes, treatment involved removing the bandage contact lens, and healing was usually complete by the subsequent examination.17 Hersh et al in their multicenter trial reported that 23 of 101 (23%) patients had an epithelial defect after 1 week.7
The proportion in this study’s cohort with delayed epithelial healing was found to be 3.9% and defined delayed epithelial healing by failure to fully re-epithelialize by the second postoperative visit 5–8 days postoperatively. The rates in this study are compatible with the rates in the international literature. Rubbing of the eyes is very common after CXL and could be a cause for delayed epithelial healing, so using an eye shield is recommended to prevent it.
Treatment of the persistent epithelial defect differs by providers, as guided by clinical presentations. However, these cases were treated by any of the 4 following clinical approaches: BCL removal with adding antibiotic ointment, prolonging postoperative antibiotic and steroid eye drops either with BCL removal, or with repeat BCL, or pressure patching with antibiotic ointment. Interestingly, patients treated with BCL replacement took the longest to fully re-epithelialize. This potentially suggests that the standard BCL rubs against the apex of the cone, thus slowing the re-epithelization of the central cornea.
Infectious and sterile keratitis are concerning complications following epi-off CXL.21 Sporadic case reports have documented microbial keratitis occurring following epi-off CXL with etiologies including herpetic, bacterial, fungal, and acanthamoeba13,14,22–24 Infectious keratitis rates following epi-off CXL have been reported to range from <0.01% to 1.6%.25,26 Shetty et al reported a case series of 4 eyes that developed moxifloxacin-resistant Staphylococcus aureus infectious keratitis.26 In their study, they retrospectively analyzed 2350 patients (1715 conventional CXL, 310 transepithelial CXL, and 325 accelerated CXL) who underwent CXL for keratoconus at a tertiary care center. All 4 patients who developed keratitis had conventional CXL and were on chronic preoperative oral/topical steroids for chronic disorders, such as vernal conjunctivitis, asthma, or eczema. No such chronic steroid use was identified preoperatively in this series. Godefrooij et al found an infection rate of 1.6% after various types of CXL.25 In this study, a low occurrence of postoperative keratitis was observed (13 out of 885 eyes, 1.5%). Six of the 13 cases were cultured; however, no culture-proven cases of infectious keratitis were identified. Seven cases were not cultured due to peripheral infiltrates or clinical herpetic presentation. Presumed etiologies of keratitis from clinical appearance and therapeutic response in this study were similar to those previously reported in the literature, which have mostly been of herpetic and bacterial etiologies. This study had one case of presumed fungal keratitis following CXL, which demonstrated multifocal infiltrates that failed to respond to anti-bacterial treatment. Ultimately, the infection responded to appropriate topical anti-fungal treatment. Two cases only responded to steroids, implying a possibly inflammatory etiology, and three cases responded to combination therapy of topical antibiotic and steroid eye drops. All cases responded to treatment.
Regarding safety criteria, visual outcome was chosen as an indication of safety, given main value of this procedure is to stabilize the cornea and prevent further loss of vision. Of the 47 eyes with complications, nine (19%) lost uncorrected or best corrected visual acuity by the final postoperative visit due to cornea scarring. This outcome of four such eyes resulted from infectious keratitis and five from delayed epithelial healing.
Limitations of this study include the retrospective design and short-term follow-up of approximately one year or less. Other limitations were that this study did not measure epithelial defect size and cultures were obtained in only half of the post-operative keratitis patients. The study was conducted in one integrated institution at 15 sites by as many surgeons on a population of patients that reflects the underlying community. Given the disparity in healthcare delivery in the USA, the results cannot be generalized to the United States, but it gives an idea about the safety profile of CXL and hopefully will help other centers improve their performance. Finally, it would have been more accurate to review every encounter of all patients in detail. However, due to time and funding limitations, manual chart review was primarily performed on cases that were identified by the authors’ algorithm for suspected postoperative complications (frequent visits and/or diagnostic evidence of infection). Thus, other information relevant to post-procedure complications were unable to be captured uniformly.
Strengths of this study include the high proportion of the cohort (approximately 71%) that had at least six months of follow-up during the study period, the large study size, and the number of treating surgeons, reflecting clinical practice with the only FDA approved CXL system at the time of the study. Future research should include more data on long-term follow-up and the efficacy of CXL.
The concerns for early clinical complications after epi-off CXL often lead to delayed treatment and further keratoconus progression with an increased economic burden. These complications were found to have low incidence rates in this large cohort study. Epi-off CXL, using the current FDA-approved clinical protocol in a US community-based healthcare setting, appears to be safe with a low risk of immediate postoperative complications. Further studies are necessary to evaluate longer and more detailed outcomes of the procedure.
Disclosure
Funding from Kaiser Permanente Community Benefit Grant. The authors declare no other conflicts of interest for this work.
References
1. Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi:10.1016/0039-6257(84)90094-8
2. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi:10.1016/S0039-6257(97)00119-7
3. Ihalainen A. Clinical and epidemiological features of keratoconus genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol Suppl. 1986;178:1–64.
4. Godefrooij DA, Gans R, Imhof SM, et al. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94:675–678. doi:10.1111/aos.13095
5. Godefrooij DA, Mangen MJJ, Chan E, et al. Cost-effectiveness analysis of corneal collagen crosslinking for progressive keratoconus. Ophthalmology. 2017;124:1485–1495. doi:10.1016/j.ophtha.2017.04.011
6. Leung VC, Pechlivanoglou P, Chew HF, et al. Corneal collagen cross-linking in the management of keratoconus in Canada: a cost-effectiveness analysis. Ophthalmology. 2017;124:1108–1119. doi:10.1016/j.ophtha.2017.03.019
7. Hersh PS, Stulting RD, Muller D, et al. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124:1259–1270. doi:10.1016/j.ophtha.2017.03.052
8. Strmeňová E, Vlková E, Michalcová L, et al. [The effectiveness of corneal cross-linking in stopping the progression of keratoconus]. Ceska Slovenska Oftalmol. 2014;70:218–222. Slovak.
9. Salmon HA, Chalk D, Stein K, et al. Cost effectiveness of collagen crosslinking for progressive keratoconus in the UK NHS. Eye. 2015;29:1504–1511. doi:10.1038/eye.2015.151
10. Sandvik GF, Thorsrud A, Råen M, et al. Does corneal collagen cross-linking reduce the need for keratoplasties in patients with keratoconus? Cornea. 2015;34:991–995. doi:10.1097/ICO.0000000000000460
11. Lindstrom RL, Berdahl JP, Donnenfeld ED, et al. Corneal cross-linking versus conventional management for keratoconus: a lifetime economic model. J Med Econ. 2021;24:410–420. doi:10.1080/13696998.2020.1851556
12. Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–1362. doi:10.1016/j.jcrs.2009.03.035
13. Pérez-Santonja JJ, Artola A, Javaloy J, et al. Microbial keratitis after corneal collagen crosslinking. J Cataract Refract Surg. 2009;35:1138–1140. doi:10.1016/j.jcrs.2009.01.036
14. Sharma N, Maharana P, Singh G, et al. Pseudomonas keratitis after collagen crosslinking for keratoconus: case report and review of literature. J Cataract Refract Surg. 2010;36:517–520. doi:10.1016/j.jcrs.2009.08.041
15. Nicula C, Pop R, Rednik A, et al. 10-year results of standard cross-linking in patients with progressive keratoconus in Romania. J Ophthalmol. 2019;2019:1–5. doi:10.1155/2019/8285649
16. Iqbal M, Elmassry A, Badawi AE, et al. Visual and refractive long-term outcomes following standard cross-linking in progressive keratoconus management. Clin Ophthalmol. 2019;13:2477–2488. doi:10.2147/OPTH.S232954
17. Price MO, Fairchild K, Feng MT, et al. Prospective randomized trial of corneal cross-linking riboflavin dosing frequencies for treatment of keratoconus and corneal ectasia. Ophthalmology. 2018;125:505–511. doi:10.1016/j.ophtha.2017.10.034
18. Saleh S, Koo EB, Lambert SR, et al. Outcomes after corneal crosslinking for keratoconus in children and young adults. Cornea. 2021. doi:10.1097/ICO.0000000000002730
19. Greenstein SA, Hersh PS. Corneal crosslinking for progressive keratoconus and corneal ectasia: summary of US multicenter and subgroup clinical trials. Transl Vis Sci Technol. 2021;10:13. doi:10.1167/tvst.10.5.13
20. Iqbal M, Elmassry A, Tawfik A, et al. Evaluation of the effectiveness of cross-linking combined with photorefractive keratectomy for treatment of keratoconus. Cornea. 2018;37:1143–1150. doi:10.1097/ICO.0000000000001663
21. Çerman E, Özcan DÖ, Toker E. Sterile corneal infiltrates after corneal collagen cross-linking: evaluation of risk factors. Acta Ophthalmol. 2017;95:199–204. doi:10.1111/aos.13218
22. Rama P, Di Matteo F, Matuska S, et al. Acanthamoeba keratitis with perforation after corneal crosslinking and bandage contact lens use. J Cataract Refract Surg. 2009;35:788–791. doi:10.1016/j.jcrs.2008.09.035
23. Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg. 2009;35:588–589. doi:10.1016/j.jcrs.2008.09.029
24. Kymionis GD, Portaliou DM, Bouzoukis DI, et al. Herpetic keratitis with iritis after corneal crosslinking with riboflavin and ultraviolet A for keratoconus. J Cataract Refract Surg. 2007;33:1982–1984. doi:10.1016/j.jcrs.2007.06.036
25. Godefrooij DA, Roohé SL, Soeters N, et al. The independent effect of various cross-linking treatment modalities on treatment effectiveness in keratoconus. Cornea. 2020;39:63–70. doi:10.1097/ICO.0000000000002168
26. Shetty R, Kaweri L, Nuijts RM, et al. Profile of microbial keratitis after corneal collagen cross-linking. Biomed Res Int. 2014;2014:1–7. doi:10.1155/2014/340509
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

