Back to Journals » International Journal of Nanomedicine » Volume 15
The Role of Tantalum Nanoparticles in Bone Regeneration Involves the BMP2/Smad4/Runx2 Signaling Pathway
Authors Zhang G, Liu W, Wang R, Zhang Y, Chen L, Chen A, Luo H, Zhong H, Shao L
Received 8 January 2020
Accepted for publication 22 March 2020
Published 14 April 2020 Volume 2020:15 Pages 2419—2435
DOI https://doi.org/10.2147/IJN.S245174
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
This paper has been retracted.
Guilan Zhang, 1, 2 Wenjing Liu, 3 Ruolan Wang, 1 Yanli Zhang, 3 Liangjiao Chen, 4 Aijie Chen, 3 Haiyun Luo, 5 Hui Zhong, 1 Longquan Shao 1, 2
1Department of Stomatology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Construction and Detection in Tissue Engineering, Guangzhou 510515, People’s Republic of China; 3Department of Prosthodontics, Stomatological Hospital, Southern Medical University, Guangzhou 510280, People’s Republic of China; 4Department of Orthodontics, Stomatological Hospital, Guangzhou Medical University, Guangzhou, 510150, People’s Republic of China; 5Department of Endodontics, Stomatological Hospital, Southern Medical University, Guangzhou 510280, People’s Republic of China
Correspondence: Longquan Shao
Department of Stomatology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, People’s Republic of China
Tel +86 (0)20 15989283921
Email [email protected]
Background: In recent years, nanomaterials have been increasingly developed and applied in the field of bone tissue engineering. However, there are few studies on the induction of bone regeneration by tantalum nanoparticles (Ta NPs) and no reports on the effects of Ta NPs on the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and the underlying mechanisms. The main purpose of this study was to investigate the effects of Ta NPs on bone regeneration and BMSC osteogenic differentiation and the underlying mechanisms.
Materials and Methods: The effects of Ta NPs on bone regeneration were evaluated in an animal experiment, and the effects of Ta NPs on osteogenic differentiation of BMSCs and the underlying mechanisms were evaluated in cell experiments. In the animal experiment, hematoxylin-eosin (HE) staining and hard-tissue section analysis showed that Ta NPs promoted bone regeneration, and immunohistochemistry revealed elevated expression of BMP2 and Smad4 in cells cultured with Ta NPs.
Results: The results of the cell experiments showed that Ta NPs promoted BMSC proliferation, alkaline phosphatase (ALP) activity, BMP2 secretion and extracellular matrix (ECM) mineralization, and the expression of related osteogenic genes and proteins (especially BMP2, Smad4 and Runx2) was upregulated under culture with Ta NPs. Smad4 expression, ALP activity, ECM mineralization, and osteogenesis-related gene and protein expression decreased after inhibiting Smad4.
Conclusion: These data suggest that Ta NPs have an osteogenic effect and induce bone regeneration by activating the BMP2/Smad4/Runx2 signaling pathway, which in turn causes BMSCs to undergo osteogenic differentiation. This study provides insight into the molecular mechanisms underlying the effects of Ta NPs in bone regeneration.
Keywords: tantalum nanoparticles, bone regeneration, osteogenic differentiation, Smad4, BMSCs
Introduction
In recent years, nanomaterials have been applied locally in bone tissue engineering to augment tissue regeneration, enhance osseointegration of implants, and prevent infections.1–3 Several nanomaterials, such as variety of metals and their oxides, layered double hydroxides, zeolites, and carbon in different forms, have been used for tissue engineering applications.1,4–6 Among them, tantalum (Ta) has been used for implants in both orthopedics and dentistry.7–9 Ta nanomaterials are increasingly being explored as alternatives to metals with good biocompatibility in the manufacture of implantable medical devices.8,10 However, there are few studies on the bone regeneration induced by Ta nanoparticles (Ta NPs).11 Therefore, in this study, we explored the effects of Ta NPs on bone regeneration in an animal experiment. In bone regeneration, bone mesenchymal stem cells (BMSCs) are considered promising seed cells for tissue engineering applications, especially due to their excellent potential for differentiation into osteoblasts, chondrocytes, adipocytes, neurons, and other cell types.12,13 The outcomes of BMSC proliferation and differentiation are highly influenced by the surrounding environment.14 Nanomaterials play significant roles in determining the fate of BMSCs.15–17 However, the influence of Ta NPs on the fate of BMSCs has not been reported. Therefore, in this study, we explored the influence of Ta NPs on BMSCs through cell experiments.
The bone morphogenetic protein (BMP) family plays a crucial role in osteoblast differentiation.18 As an important member of the BMP family, BMP2 participates in bone regeneration and BMSC differentiation.18 BMP2 is initially identified by its ability to promote osteogenesis and interacts with other could signaling pathways. Recent studies have shown that BMP2 plays important roles in bone mass homeostasis and osteocyte function and is activated in BMSCs.19 The osteogenic capability of BMP2 has been extensively studied, and recombinant proteins are currently being investigated in the fields of fracture healing and spinal fusion. BMP2 is a primary participant in postnatal skeletal homeostasis, and the osteogenic signal provided by BMP2 is required for the inherent reparative capacity of bones.19 In previous work, we discovered that BMP2 induced the expression of not only genes commonly associated with ossification and mineralization but also other genes.20 Interestingly, a recent study showed that BMP2 addition to culture medium rapidly induced the expansion of isolated mouse skeletal stem cells.19 BMP2 is tightly regulated by ligand availability, receptor activation, and intracellular signaling. Drosophila mothers against decapentaplegics (Smads) are crucial downstream mediators of BMP signal transduction.21
Recombinant human mothers against decapentaplegic homolog 4 (Smad4) can form complexes with other activated Smads (Smad1/5/8); the resulting heterodimers complex with diverse transcription factors (coactivators or corepressors) to regulate gene expression in the nucleus.22,23 Furthermore, the Smad4 protein pathway has been found to enhance osteoblast differentiation. Smad4 is the only common Smad involved in BMP2 signaling.24,25 Conditional deletion of Smad4 in osteoblasts leads to reduced bone mineral density, decreased bone volume, a decreased bone formation rate, and a reduced number of osteoblasts.26 Controlling Smad4 is a good way to regulate bone formation. In vitro Smad4 ablation partially suppresses BMP2-induced osteoblast differentiation.27 In vivo silencing of Smad4 in chondrocytes results in dwarfism with a severely disorganized growth plate and ectopic bone collars in the perichondrium.26 In the Smad4-deficient growth plate, the resting zone is expanded, whereas chondrocyte proliferation is reduced, and hypertrophic differentiation is accelerated.28 Deletion of Smad4 in mature osteoblasts causes reduced bone mass and decreased osteoblast proliferation and differentiation.29 Embryonic deletion of Smad4 in preosteoblasts causes stunted growth, spontaneous fractures and a variety of features observed in osteogenesis imperfecta, cleidocranial dysplasia, and Wnt-deficiency syndromes.30 Postnatal deletion of Smad4 in preosteoblasts increases the mitosis rate of cells on trabecular bone surfaces and in primary osteoblast cultures and delays differentiation and matrix mineralization by primary osteoblasts.25 In summary, Smad4 has multiple roles in osteogenic differentiation and bone regeneration.
In addition to playing a role in BMP2 signaling, Smad4 induces runt-related transcription factor 2 (Runx2) degradation in a ubiquitin proteasome-dependent manner, which directly affects osteoblast differentiation.31 The Smad4 complex transcribes Runx2 and interacts with Runx2 to initiate the expression of other osteoblast genes.32 Thus, Smad4 expression is mediated by Smad4/Runx2 signaling at the transcriptional level, and Smad4 regulates Runx2 activity and the expression of other osteoblast genes in a feedback loop.33,34 The cytoskeleton has been shown to control the activation of Smad4/Runx2 signaling in mesenchymal cells upon external stimulation, and cell morphology remodeling and cytoskeletal organization can affect stem cell lineage commitment.14 Furthermore, the cytoskeletal network allows cells to transfer external mechanical stimuli into the nucleus and activates external stimuli-induced mechanotransduction transducers on the membrane.
NPs have been shown to promote the expression of BMP2.35 Furthermore, NPs have been shown to influence Smad expression36 and can promote the expression of the Runx2 gene.37 In addition, recent studies have confirmed that NPs can promote the differentiation of osteoblasts through the BMP2/Smad/Runx2 signaling pathway.38
We previously hypothesized that Ta NPs play an important role in promoting BMSC osteogenic commitment and that this role is regulated by crosstalk between Smad4 and Runx2 signaling via BMP2. To test this hypothesis, we analyzed osteogenic differentiation, cytoskeletal organization, BMP2 secretion, and Smad4/Runx2 signaling activity in BMSCs cultured on Ta NPs. We then silenced Smad4 expression with Smad4 inhibitors and monitored BMSC osteogenic function and predicted downstream signaling events. We confirmed that Ta NPs act as positive regulators in the osteogenic differentiation of BMSCs via the BMP2-induced Smad4/Runx2 signaling network. This result suggests that Ta NPs could play important roles in bone regeneration. Our study provides insight into the molecular mechanisms associated with Ta NP-induced bone regeneration.
Experimental Materials and Methods
Characterization of Ta NPs
Ta NPs were purchased from Sigma-Aldrich (Catalog number: 593486, USA). The morphology of the material was observed by scanning electron microscopy (SEM; LEO1530VP, Germany) and transmission electron microscopy (TEM; JEM-2100F, Hitachi, Japan). The crystal phase was analyzed by X-ray diffraction (XRD; Bruker D8 ADVANCE, Germany). The hydration particle size and zeta potential (change in surface charge) were analyzed in a mixture of Ta NPs with deionized water by nanoparticle analyzer (SZ-100Z, HORIBA, Japan). Ta NPs (0.75 mg) were placed in a 1.5-mL EP tube, packed in a sterilization bag and sterilized by 60Co irradiation. The sterilized Ta NPs were suspended in 30 mL of complete culture medium, placed in a cell breaker and subjected to sterile ultrasound for 30 min, and prepared at concentrations of 0, 5, 10, 15, 20, and 25 µg/mL.
In vivo Animal Model
Thirty-six Sprague-Dawley (SD) rats (approximately 250 g) were equally assigned into 3 groups: (1) blank (control), (2) hydroxyapatite (HA, purchased from Sigma-Aldrich, USA; Catalog number: 55496), and (3) HA-Ta NPs (1: 1 mass ratio mixing). Mandible defects (5 mm in diameter, 1 mm in depth) were created on the left side of the mandible following described surgical procedures.39 The defects were then filled with prepared HA or HA-Ta NPs. The surgical procedure was performed as shown in (Figure S1). After 8 or 12 weeks, each rat was anesthetized and sacrificed, and the mandible was extracted. The animal experimental protocol was approved by the Biomedical Research Ethics Committee of Southern Medical University (Permit Number: L2018109). The name of the guidelines followed for the welfare of the laboratory animals is “Laboratory animal—Guideline for ethical review of animal welfare (Standard number: GB/T 35892–2018)”.
Histological and Histomorphometric Evaluations
All specimens were fixed for 4 weeks to prepare undecalcified and decalcified histological sections. The undecalcified slices for histological sections (80–100 μm) were prepared using a modified microtome (Leica, Germany), polished to remove grinding marks and stained with 1.2% trinitrophenol and 1% acid fuchsin (Van-Gieson). The decalcified sections (4 μm) were cut and placed on slides for further staining. For hematoxylin-eosin (HE) staining, the sections were dewaxed with xylene, washed with a series of ethanol dilutions, stained with hematoxylin, rinsed, stained with an eosin solution, dehydrated, cleared and sealed. For BMP2 (Proteintech, USA) and Smad4 (CST, USA) antigen staining, samples were first deparaffinized and rehydrated, and then antigen unmasking was performed. Then, the sections were treated to eliminate endogenous peroxidase activity and blocked with goat serum. The samples were then incubated with appropriate primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies. The sections were examined with a light microscope (Leica, Germany). Quantitative analyses of above images were performed using an image analysis system (Image-Pro Plus 6.0 software, USA).
BMSCs Culture
BMSCs were purchased from Cygen (USA) to cultivate BMSCs by the whole bone marrow culture method and stored at −180°C in liquid nitrogen. BMSCs were cultured in essential medium with 10% fetal bovine serum and 1% antibiotics for expansion. A standard culturing environment of 37°C in a humidified atmosphere with 5% CO2 was utilized. For the induction of osteogenic differentiation, BMSCs were cultured in osteogenic medium (OM, Cygen, USA) for 7, 14 and 21 days for experiments including those evaluating alkaline phosphatase (ALP) activity, mineralization, and protein and gene expression.
Cell Proliferation and Cycling
Cells were cultured in a cell incubator for 24 h, and the original medium was discarded. Different concentrations of a Ta NP suspension were added, with 100 µL added for 12, 24, or 48 h. At each time point, different concentrations of the Ta NP suspension were discarded, and the cells were rinsed with phosphate buffered saline solution (PBS). Then, 20 µL of Cell Counting Kit-8 (CCK-8; Kumamoto, Japan) solution and 100 µL of complete medium were added to each well and incubated in a cell incubator for 2 h. The absorbance was detected with a microplate reader at a wavelength of 450 nm. The original medium was discarded, and 2 mL of Ta NP suspension at different concentrations was added to the cultures for 24 h. The six groups were centrifuged, PBS was used for resuspension, and then the groups were centrifuged to prepare the cell cycle detection reagent (Beyotime, China). After an incubation at 37°C for 45 minutes, the cell cycle distribution was detected by flow cytometry (BD, USA). Cells in the S phase of the cell cycle were analyzed.
BMSC Ingestion of Ta NPs and the Cell Cytoskeleton
Cells were cultured in a cell incubator for 24 h, and the original medium was discarded. Then, 2 mL of Ta NP suspension at different concentrations were added to the cultures for 24 h. Trypsin was used to digest the cells, which were slowly poured into the proper amount of a fixative solution; the cells were then fixed overnight at 4°C, fixed again in osmic acid, dehydrated with gradient ethanol, replaced, dried and sectioned. The uptake of Ta NPs by BMSCs was observed under transmission electron microscope (TEM: JEM-2100F, Hitachi, Japan). The treatment of the cells was the same as the treatment described above. The cells were fixed overnight with 4% paraformaldehyde at 4°C, dyed with ghost pen cyclopeptide (Sigma, USA), washed with deionized water, dyed with 4,6-diamidino-2-phenylindole (Invitrogen, USA), and rinsed with deionized water. The cytoskeletal changes in BMSCs were observed under an inverted fluorescence microscope (Olympus, Japan).
Osteogenic Differentiation Assays
BMSCs were cocultured with six different concentrations of Ta NPs in suspension for 24 h, with or without the addition of a Smad4 inhibitor for 24 h, with the most suitable concentration administered alone for 24 h or the most suitable concentration and the inhibitor administered for 24 h. The BMSC supernatant was collected at 7 and 14 days after osteogenic induction, and the BMSCs were rinsed with PBS, fixed with 4% paraformaldehyde and stained with an ALP staining kit (Beyotime, China). ALP staining was observed under an inverted microscope. The BMSC supernatant was detected by using an ALP detection kit (Beyotime, China).
The treatment of cells was the same as that described above. Alizarin red (Sigma, USA) was used to quantify the calcification of samples after osteogenic induction (14 and 21 days). After staining for 10 minutes, the samples were rinsed with deionized water three times, and images were acquired under a light microscope (Leica, Germany). The stain was then eluted in 10% cetylpyridinium chloride in 10 mM sodium phosphate (pH = 7.0). The optical density (OD) value was obtained at 565 nm with an enzyme-labeling instrument.
The treatment of cells was the same as that described above. BMP2 production by osteoblasts was measured after an incubation of 7, 14 and 21 days using enzyme-linked immunosorbent assay (ELISA; Proteintech, USA). The culture medium of each group was extracted and incubated with an anti-BMP2 antibody overnight at 4°C and then with a HRP-conjugated secondary antibody. Tetramethylbenzidine was used as a chromogenic substrate for HRP. The absorbance values were then measured at 450 nm after stopping the reaction via the addition of hydrochloric acid. BMP2 production was calculated based on the absorbance calibration curve.
Cell Immunofluorescence
BMSCs were cocultured with six different concentrations of Ta NPs in suspension for 24 h, with or without the addition of a Smad4 inhibitor for 24 h, with the most suitable concentration administered alone for 24 h or the most suitable concentration and the inhibitor administered for 24 h. BMSC immunofluorescence specific for Smad4 was used to determine the expression levels in cells after osteogenic induction (14 days). The cells were treated with 4% POM fixation, 0.5% Triton X-100 treatment, 2% BSA sealing, PBS rinsing, anti-Smad4 (CST, USA) primary antibody, 4°C incubation overnight, fluoresceine isothiocyanate (FITC)-labeled secondary antibody (Proteintech, USA) addition, incubation, 4,6-diamino-2-phenyl indole (DAPI) addition, and incubation at room temperature. Cellular fluorescence was observed via FV10i confocal microscopy (Olympus, Japan) and analyzed using Image-Pro Plus 6.0 software.
Osteogenesis-Related Gene and Protein Expression
BMSCs were cocultured with six different concentrations of Ta NPs in suspension for 24 h, with or without the addition of a Smad4 inhibitor for 24 h, with the most suitable concentration administered alone for 24 h or the most suitable concentration and the inhibitor administered for 24 h. The primers for five osteogenesis-related genes, namely, ALP, BMP2, Smad4, osteopontin (OPN), and Runx2, are listed in Table S1. After 7, 14 and 21 days of osteogenic induction, total RNA was extracted from each group of BMSCs with Trizol after discarding the original medium. Reverse transcription of RNA into cDNA was performed using the prime script RT Kit (Takara, Japan). Finally, real-time PCR (ABI, USA) was performed using SYBR Premix ExTM Taq II (Takara, Japan) to amplify the cDNA samples. The results were normalized to the results for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the 2-ΔΔCt method was used to analyze mRNA expression levels.
The treatment of cells was the same as that described above. The osteogenesis-related proteins were BMP2 (Proteintech, USA), Runx2 (Proteintech, USA), and Smad4 (CST, USA), and GAPDH (Proteintech, USA) was used as an internal reference. After 7, 14 and 21 days of osteogenic induction, proteins were extracted with RIPA lysis buffer. The concentration was determined by BCA protein (Thermo Fisher, USA) analysis. Equivalent protein amounts were separated by gel electrophoresis and transferred to a PVDF membrane. The membranes were incubated overnight with primary antibodies and then with the corresponding secondary antibody. Detection was performed by using an ECL kit (WBLKS0500, Merck Millipore, USA), and the results were analyzed using the Tanon 5200 Automatic Chemiluminescence Image Analysis System (China) and Image-Pro Plus 6.0 software.
Statistical Analysis
Through SPSS19.0 (SPSS, USA) statistical software analysis of the relevant data obtained in this experiment. Comparisons between two groups were performed by Student’s t-test, and comparisons among 3 or more groups were analyzed by one-way ANOVA followed by the Student-Newman-Keuls post hoc test. p<0.05 was considered statistically significant.
Results
Characterization of Ta NPs
Ta NPs were observed to be spherical particles, which were generally uniform in size and shape (Figure 1A and B). The particle size of the Ta NPs was 20 nm, which conformed to the expectations for biological nanomaterials (Figure 1B). Figure 1C shows the phase of Ta NPs as determined by XRD. The particle size of the Ta NP suspension was 47 ± 5.2 nm (Figure 1D), and the zeta potential of the Ta NPs was 19.6 ± 5.2 mV.
Effects of Ta NPs on Bone Regeneration
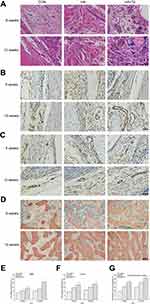
The expression of BMP2 and Smad4 during bone regeneration induced by Ta NPs is shown in Figure 2A–C, E and F. At both 8 and 12 weeks, the expression of BMP2 and Smad4 in the HA-composite Ta NPs group was significantly higher than that in the control and HA groups (p<0.05). These results demonstrated that the HA-composite Ta NPs produced high expression of BMP2 and Smad4 during bone regeneration.
The results of bone regeneration are shown in Figure 2D and G. After 8 or 12 weeks, limited new bone tissue (red) grew into defect areas, with abundant, loosely packed fibrous and osteoid tissue (blue and purple) occupying the central area. At 8 weeks, the bone volume fraction in the HA-composite Ta NPs group (21.2%) was significantly higher than that in the control group (10.0%, p<0.05). The HA group also exhibited moderate new bone ingrowth (16.1%); the new ingrowth was at a level higher than that of the control group (p<0.05) but lower than that of the HA-composite Ta NPs group (p<0.05). At 12 weeks, the bone volume fraction in the HA-composite Ta NPs group (26.2%) was significantly higher than that in the control group (15.0%, p<0.05). The HA group also exhibited moderate new bone ingrowth (20.9%); ingrowth in this group was greater than that of the control group (p<0.05) but less than that of the HA-composite Ta NPs group (p<0.05). These data demonstrated that the HA-composite Ta NPs had a positive influence on bone regeneration.
Cell Proliferation and Cycle Distribution
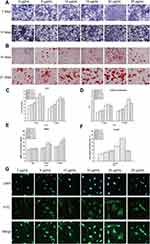
The results for the cell cycle distribution are shown in Figure 3A. Compared with the control group, the 10, 15, 20 and 25 µg/mL Ta NP suspension groups showed increased proportions of cells in the S phase of the cell cycle (p< 0.05, Figure 3B). The results for the 24- and 48-h time points indicated that the Ta NP suspension promoted the proliferation of BMSCs. The Ta NP suspensions with concentrations of 15, 20 and 25 µg/mL had stronger effects than the control treatment (p< 0.05, Figure 3C). The effects of the Ta NP suspensions were weaker at 12 h than at the longer time points. There was no significant difference between 24 h and 48 h. Hence, 24 h was chosen as the time point for further evaluation in this study.
Cell Uptake and the Cytoskeleton
The results in Figure 4A show that compared with control treatment, treatment with 5, 10, 15, 20, or 25 µg/mL Ta NPs promoted cell extension, and the 20 µg/mL Ta NP suspension induced some interaction with surrounding cells, as evidenced by extended pseudopods. Figure 4B shows that Ta NPs were apparent in the cytoplasm, but no Ta NPs were observed in the nucleus after 24 h of cell coculture with 10, 15, or 20 µg/mL Ta NP suspension. Among the treatments, the 20 µg/mL Ta NP suspension achieved the highest cell uptake. No Ta NPs were found in the cytoplasm or nucleus for the Ta NP suspensions of the other concentrations.
Osteogenic Differentiation and Cellular Immunofluorescence
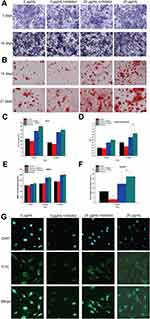
BMSC osteogenic differentiation was upregulated by Ta NPs, as revealed by ALP production, extracellular matrix (ECM) mineralization, BMP2 secretion and cellular immunofluorescence (Figure 5).
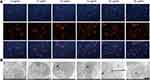
In Figure 5A and B, a more saturated staining color indicates increased production induced by the Ta NPs relative to production in the control treatment. The ALP production and ECM mineralization quantification results shown in Figure 5C and D demonstrated that the values of the 15, 20 and 25 µg/mL Ta NP groups were significantly higher than those of the control group (p<0.05). BMP2 production, as a marker of mid- and late-stage osteoblast differentiation, was evaluated for each group after incubation for 7, 14 and 21 days using enzyme linked immunosorbent assay (ELISA) (Figure 5E). The assay results were consistent with the ECM mineralization quantification results for the activity observed on days 14 and 21. In general, Ta NPs enhanced osteogenic differentiation, with 20 µg/mL Ta NPs yielding the greatest enhancement. In the cellular immunofluorescence tests (Figure 5G), the fluorescence intensity results indicated increased Smad4 expression in the Ta NP groups compared with that in the control group. The percentage of positive area quantification results shown in Figure 5F demonstrated that the 15 and 20 µg/mL Ta NP group results were significantly higher than those of the control group (p<0.05).
Expression of Osteogenic Genes and Proteins
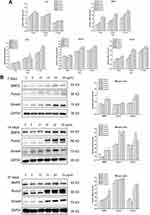
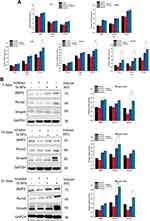
The osteogenesis-related gene expression quantification results are shown in Figure 6A. In general, Ta NPs enhanced the expression of ALP, BMP2, OPN, Runx2 and Smad4. For ALP, at 7 days, 15 and 20 µg/mL Ta NPs produced significantly higher expression than control treatment (p<0.05), and at 14 days, 20 and 25 µg/mL Ta NPs produced significantly higher expression than the control treatment (p<0.05). For BMP2, at 7 days, the 10, 15 and 20 µg/mL Ta NP treatments yielded significantly higher expression than control treatment (p<0.05); at 14 and 21 days, the 20 and 25 µg/mL Ta NP treatments yielded significantly higher expression than control treatment (p<0.05). For OPN, at 14 days, expression was significantly higher in the 20 µg/mL Ta NP group than in the control group (p<0.05); at 21 days, expression was significantly higher in the 20 and 25 µg/mL Ta NP groups than in the control group (p<0.05). For Runx2, at 14 and 21 days, the 15, 20 and 25 µg/mL Ta NP treatments yielded significantly higher expression than the control treatment (p<0.05). For Smad4, at 7 days, 20 and 25 µg/mL Ta NPs produced significantly higher expression than control treatment (p<0.05), and at 14 and 21 days, 15, 20 and 25 µg/mL Ta NPs produced significantly higher expression than control treatment (p<0.05).
The protein expression levels of BMP2, Runx2 and Smad4 are shown in Figure 6B. For BMP2 and Smad4, at 7 days, the 15, 20 and 25 µg/mL Ta NP treatments yielded significantly higher expression than the control treatment (p<0.05). For BMP2 and Runx2, at 14 days, 15, 20 and 25 µg/mL Ta NPs induced significantly higher expression than control treatment (p<0.05). For Smad4, at 14 days, the expression levels in the 20 µg/mL Ta NP group were significantly higher than those in the control group (p<0.05). For BMP2 and Runx2, at 21 days, 20 and 25 µg/mL Ta NPs induced significantly higher expression than control treatment (p<0.05). For Smad4, at 21 days, 15, 20 and 25 µg/mL Ta NPs induced significantly higher expression than control treatment (p<0.05). In general, the interaction of 20 µg/mL Ta NPs with BMSCs promoted high expression of osteogenesis-related genes and proteins.
Effects of Ta NPs on the Smad4/Runx2 Signaling Pathway
Osteogenic Differentiation and Cellular Immunofluorescence
BMSCs were cocultured in four groups with different concentrations and assessed for ALP production, ECM mineralization, BMP2 secretion and cellular immunofluorescence (Figure 7).
In Figure 7A and B, a more saturated staining color indicates increased production induced by the Ta NPs relative to production under control treatment. The ALP production quantification results shown in Figure 7C demonstrated that 20 µg/mL + inhibitor and 20 µg/mL Ta NPs induced significantly higher production than control treatment (p<0.05). The ECM mineralization quantification results shown in Figure 7D demonstrated that 20 µg/mL + inhibitor and 20 µg/mL Ta NPs induced significantly more mineralization than control treatment (p<0.05) and mineralization under 0 µg/mL Ta NPs was significantly higher than that under 0 µg/mL + inhibitor (p< 0.05). At 21 days, 20 µg/mL + inhibitor yielded significantly lower mineralization than 20 µg/mL Ta NPs (p<0.05). For BMP2 secretion (Figure 7E), at 14 and 21 days, 20 µg/mL + inhibitor and 20 µg/mL Ta NPs produced significantly higher secretion than control treatment (p<0.05). In cellular immunofluorescence tests (Figure 7G), the fluorescence intensity indicated depressed Smad4 expression in the 20 µg/mL + inhibitor group relative to Smad4 expression in the 20 µg/mL group. The results regarding percentage of positive area are shown in Figure 7F. The values of the 20 µg/mL group were significantly higher than those of the 20 µg/mL + inhibitor group (p<0.05). There were significant differences between the control group and the other three treatment groups (p<0.05). In general, ALP expression, ECM mineralization and Smad4 cellular immunofluorescence decreased after adding Smad4 inhibitors.
Expression of Osteogenic Genes and Proteins
The gene expression results for ALP, BMP2, OPN, Runx2 and Smad4 are shown in Figure 8A. For ALP, at 7 and 21 days, 20 µg/mL + inhibitor and 20 µg/mL induced significantly higher gene expression than 0 µg/mL Ta NPs (p<0.05). For BMP2, at 7, 14 and 21 days, 20 µg/mL + inhibitor and 20 µg/mL induced significantly higher expression than 0 µg/mL Ta NPs (p<0.05). For OPN, at 14 and 21 days, 20 µg/mL + inhibitor and 20 µg/mL yielded significantly higher gene expression than 0 µg/mL Ta NPs (p<0.05), and significantly lower expression was observed for 0 µg/mL + inhibitor than for 0 µg/mL Ta NPs (p<0.05) and for 20 µg/mL + inhibitor than for 20 µg/mL Ta NPs (p<0.05). Runx2 gene expression was consistent with the expression of OPN. For Smad4, at 7, 14 and 21 days, 20 µg/mL + inhibitor and 20 µg/mL induced significantly higher expression than 0 µg/mL Ta NPs (p<0.05). At 14 and 21 days, the expression for 0 µg/mL + inhibitor was significantly lower than that for 0 µg/mL Ta NPs (p<0.05). At 7, 14 and 21 days, 20 µg/mL + inhibitor yielded significantly lower expression than 20 µg/mL Ta NPs (p<0.05). According to the above results, 20 µg/mL Ta NPs was superior to 20 µg/mL + inhibitor in inducing the expression of osteogenesis-related genes. The expression levels of Smad4, Runx2 and OPN decreased after adding Smad4 inhibitors.
The protein expression results for BMP2, Runx2 and Smad4 are shown in Figure 8B. For BMP2, at 7, 14 and 21 days, 20 µg/mL + inhibitor and 20 µg/mL induced significantly higher expression than 0 µg/mL Ta NPs (p<0.05). For Runx2, at 7, 14 and 21 days, expression under 20 µg/mL + inhibitor and 20 µg/mL was significantly higher than that under 0 µg/mL Ta NPs (p<0.05). At 14 and 21 days, expression under 0 µg/mL + inhibitor was significantly lower than that under 0 µg/mL Ta NPs (p< 0.05), and that with 20 µg/mL + inhibitor was significantly lower than that with for 20 µg/mL Ta NPs (p<0.05). For Smad4, at 7, 14 and 21 days, 0 µg/mL + inhibitor yielded significantly lower expression than 0 µg/mL Ta NPs (p<0.05). At 7 and 21 days, 20 µg/mL + inhibitor yielded significantly lower expression than 20 µg/mL Ta NPs (p<0.05). At 14 days, 20 µg/mL Ta NPs induced significantly higher expression than 0 µg/mL Ta NPs (p<0.05). According to the above results, 20 µg/mL was superior to 20 µg/mL + inhibitor in promoting the expression of osteogenesis-related proteins. The expression levels of Smad4 and Runx2 decreased after adding Smad4 inhibitors.
Discussion
Previous studies have demonstrated the induction of bone formation by Ta NPs in mixed biological scaffolds.11 In this study, Ta NPs were confirmed through the analysis of hard-tissue sections to promote bone formation (Figure 2D). In addition, HE (Figure 2A) and immunohistochemistry confirmed that Ta NPs promote the expression of BMP2 and Smad4 (Figure 2B and C). These results provide insight into the molecular mechanisms by which Ta NPs promote bone formation and may facilitate the development of methods to exploit Ta NPs for this purpose. However, the roles of Ta NPs in the lineage commitment of BMSCs (the key cells in bone reconstruction) and the underlying mechanisms related to these roles, especially the precise molecular events that regulate the Smad4/Runx2 pathway, have not been well studied. Our present study showed that Ta NPs (20 µg/mL) promoted BMSC osteogenic differentiation and that BMSC osteogenic differentiation varied depending on Ta NP concentration and was closely related to increased BMP2 activity (Figure 5E). Furthermore, we identified critical roles of Ta NPs in promoting the osteogenic differentiation of BMSCs and regulating the crosstalk of the Smad4/Runx2 pathway though BMP2. Our findings shed light on the mechanisms underlying Ta NP cues for BMSC commitment and may facilitate the application of nanobiomaterials in bone tissue engineering and regenerative medicine. As bone formation depends on the commitment of BMSCs to the osteoblast lineage and BMSC proliferation and differentiation, BMSCs are the focus of considerable research for tissue engineering and regenerative medicine applications.12 In this study, BMSCs were used to study the signaling pathways related to Ta NPs.
The Ta NPs were uniform spherical NPs with a particle size of 50 nm, as determined by SEM and TEM (Figure 1A and B). The analysis of hydrated particle size revealed that particle size in Ta NP suspension was 47 ± 5.2 nm (Figure 1D), which indicated the occurrence of Ta NP aggregation; however, this aggregation did not affect the uptake of Ta NPs by cells because the particle size was within the nanoscale range.1 Furthermore, the particle size of 47 ± 5.2 nm was consistent with the TEM observations. The phase of Ta NP determined by XRD (Figure 1C) was consistent with that of standard Ta. Zeta potential (19.6 ± 5.2 mV) measurements revealed sequential changes in the surface charges in each step.
To test the toxicity of Ta NPs to BMSCs, we observed cell proliferation by CCK8 and cell cycle tests. The results showed that at a certain concentration (20 µg/mL) and time point (24 h), Ta NPs not only were nontoxic to cells but also promoted the proliferation of BMSCs (Figure 3). In addition, we found that Ta NPs could pass through the cell membrane into the cytoplasm and were scattered throughout the cytoplasm (Figure 4B). This dispersal may have been due to the hydrated particle size of Ta NPs of 47 ± 5.2 nm, allowing the Ta NPs to pass through the phospholipid bilayer of the cell membrane, consistent with previous reports.1,2,40
Our results showed that the cytoskeleton of cells changed following exposure to Ta NPs (Figure 4A). Nanomaterials have been reported able to affect the fate of cells,1,16 suggesting that the cytoskeletal changes observed in the present study were related to the entry of Ta NPs into cells. The cytoskeleton has been reported to influence osteogenic differentiation.41 As the mechanism explored in this study was BMP2/Smad4/Runx2 signal pathway, the cytoskeleton and its related pathways were not studied, but we aim to analyze the cytoskeleton in detail in our future research. The presence of Ta NPs at a low concentration (20 µg/mL) and over a short time (24 h) can account for the observed biological effects (on ALP activity, ECM mineralization, BMP2 secretion, and the expression of osteogenic proteins and genes; Figures 5 and 6). The results of cell and animal experiments suggest multiple influences of Ta NP cues. (Figure 2B and C).
Ta NPs enhanced BMSC differentiation, and this enhancement was associated with elevated BMP2 ligand and BMP2 receptor expression, enhanced BMP2 secretion and expressions, and Smad4/Runx2 signaling activation. BMP2 promoted the activation of Smad4/Runx2 signaling and the consequent enhancement of BMSC differentiation by Ta NPs. Our results demonstrate that the biological effects of Ta NP cues on cells are mediated by the BMP2/Smad4/Runx2 pathway. The Ta NPs cued the upregulation of BMP2 expression (Figure 5E). It may be that during the osteogenic differentiation of BMSCs, Ta NPs enter these cells and upregulate the secretion and expression of BMP2. BMP2 initiates Smad4 and Runx2 expression, giving rise to increased levels of Smad4 and Runx2 products. These increased levels lead to cytoplasmic Smad4 accumulation and nuclear translocation; as a result, Smad4/Runx2 signaling is activated, and target osteogenesis-related gene expression is initiated. Ta NPs influence BMSC morphology and significantly enhance BMSC differentiation, as evidenced by the increased mRNA expression of ALP, BMP2, OPN, Smad4, and Runx2 (Figure 6A); the increased protein expression of BMP2, Smad4, and Runx2 (Figure 6B); and the elevations in BMP2 secretion and ECM mineralization (Figure 5). ALP is an early marker of osteoblast expression in osteogenic differentiation;5,42 accordingly, ALP gene expression in our samples at day 21 did not differ from that at day 7 or 14. OPN is a late marker expressed only by mature osteoblasts in osteogenic differentiation;21 accordingly, no difference in OPN gene expression at 7 days was found among our samples.
Smad4/Runx2 signaling is critical in osteogenesis.31 Several research groups are investigating the role of Smad4/Runx2 signaling in mediating the cell response to biomaterials.1,34 Increasing evidence indicates that Smad4/Runx2 signaling is involved in the responses of cells to Ta NPs. As Smad4 nuclear translocation and accumulation constitute the markers of Smad4/Runx2 signaling activation,31 we investigated Smad4 and Runx2 protein levels. Elevated nuclear Smad4 protein levels were found after Ta NP treatment, confirming the activation of Smad4/Runx2 signaling by the Ta NPs. The induction of increased Smad4 production (Smad4 expression evaluated by immunocytochemistry, Figure 5F) by the Ta NPs was also found. Thus, the increases in Runx2 protein levels mediated by the Ta NPs can be attributed to the elevated amounts of Smad4. Hence, the Runx2 concentration is restricted to a baseline to maintain the inactivation of Smad4/Runx2 signaling.43 Theoretically, the increased Smad4 and Runx2 amounts can be ascribed to two possible reasons: increased Smad4 production and decreased Smad4 degradation.44,45 The mRNA expression of Smad4 was increased (Figure 6A) by Ta NPs, indicating that the elevated total Smad4 level induced by the Ta NPs contributed, at least partially, to the increase in Smad4 production product levels.
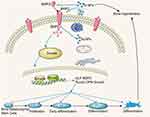
We hypothesized that the mechanism through which the 20 µg/mL Ta NP suspension promotes the osteogenic differentiation of BMSCs is the BMP2/Smad4/Runx2 signaling pathway (Figure 9). To test this hypothesis, the effects of a Smad4 inhibitor were investigated by ALP secretion evaluation, ECM mineralization assessment, BMP2 secretion detection, immunocytochemistry (Figure 7) and measurement of the expression of related osteogenic genes (ALP, BMP2, OPN, Runx2 and Smad4; Figure 8A) and proteins (BMP2, Runx2 and Smad4; Figure 8B). The results showed that the expression of ALP, Smad4, OPN and Runx2 decreased after Smad4 was blocked, whereas the secretion and expression of BMP2 did not change. The results (Figure 7) also showed that ECM mineralization and Smad4 expression as detected by immunocytochemistry decreased after Smad4 was blocked, possibly because the inhibitor was a Smad4 inhibitor, which can inhibit other types of Smad4 and indirectly act on BMP2 to reduce its expression. Furthermore, the expression of Smad4 and Runx2 decreased after Smad4 inhibition. Regarding the upstream events of Smad4/Runx2 signaling activation induced by Ta NPs, we focused on BMP2. There is abundant evidence supporting a correlation between BMP2 and Smad4/Runx2 signaling.21,27 BMP2 is located upstream of Smad4/Runx2 signaling.29
Our results indicate that Smad4 and Runx2 crosstalk is a positive regulator of BMSC osteoblastic differentiation mediated through BMP2 in response to Ta NPs. Our study advances our understanding of the mechanisms underlying BMSC osteogenic differentiation induced by Ta NPs. The identification of Smad4 as an important component in the BMP2/Smad4/Runx2 pathway (Figure 9) suggests the possible involvement of other signaling networks.
In this paper we have reported that tantalum nanoparticles have good osteogenic properties and have briefly discussed the potential mechanism. However, it is not clear whether these beneficial properties are due to the basic properties of tantalum or to the structure of tantalum nanoparticles. We will explore this issue in our subsequent research.
Conclusion
In this study, the osteogenic induction of BMSCs by Ta NPs was studied for the first time. The results showed that Ta NPs can enter BMSCs, activate the BMP2/Smad4/Runx2 signaling pathway, promote the osteogenic differentiation of BMSCs and promote bone formation. However, the detailed mechanisms remain unclear and require further study. In conclusion, this study suggests that Ta NPs may be a potential candidate material for bone regeneration and can serve as a reference for future studies of Ta NPs in bone regeneration.
Acknowledgments
This project was funded by the National Natural Science Foundation of China (No. 81870786), China Postdoctoral Science Foundation (No. 2018M633086), Guangdong Medical Research Foundation (No. A2016274), Science Foundation of Southern Medical University (No. PY2018N093) and President’s Foundation of Nanfang Hospital, Southern Medical University (No. 2018C011).
Author Contributions
All authors contributed to conception and design, acquisition of data, or analysis and interpretation of data, drafting or revising the article; provided reagents/materials/analysis tools; approved the final manuscript and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vieira S, Vial S, Reis RL, et al. Nanoparticles for bone tissue engineering. Biotechnol Prog. 2017;33(3):590–611. doi:10.1002/btpr.2469
2. Walmsley GG, Mcardle A, Tevlin R, et al. Nanotechnology in bone tissue engineering. Nanomedicine. 2015;11(5):1253–1263. doi:10.1016/j.nano.2015.02.013
3. Zhang X, Zhang C, Lin Y, et al. Nanocomposite membranes enhance bone regeneration through restoring physiological electric microenvironment. ACS Nano. 2016;10(8):7279–7286. doi:10.1021/acsnano.6b02247
4. Li J, Lee WY, Wu T, et al. Near-infrared light-triggered release of small molecules for controlled differentiation and long-term tracking of stem cells in vivo using upconversion nanoparticles. Biomaterials. 2016;110:1–10. doi:10.1016/j.biomaterials.2016.09.011
5. Kang H, Wong DSH, Yan X, et al. Remote control of multimodal nanoscale ligand oscillations regulates stem cell adhesion and differentiation. ACS Nano. 2017;11(10):9636–9649. doi:10.1021/acsnano.7b02857
6. Wong DS, Li J, Yan X, et al. Magnetically tuning tether mobility of integrin ligand regulates adhesion, spreading, and differentiation of stem cells. Nano Lett. 2017;17(3):1685–1695. doi:10.1021/acs.nanolett.6b04958
7. Shi LY, Wang A, Zang FZ, et al. Tantalum-coated pedicle screws enhance implant integration. Colloids Surf B Biointerfaces 2017;160:22–32. doi:10.1016/j.colsurfb.2017.08.059
8. Gokhuldass M, Nikita O, Mcmahon MT, et al. Porous tantalum and tantalum oxide nanoparticles for regenerative medicine. Acta Neurobiol Exp. 2014;74:188–196.
9. Hu X, Wang Y, Xu M. Study of the cell responses in tantalum carbide nanoparticles-enriched polysaccharide composite hydrogel. Int J Biol Macromol. 2019;135:501–511. doi:10.1016/j.ijbiomac.2019.05.191
10. Horandghadim N, Khalil-Allafi J. Characterization of hydroxyapatite-tantalum pentoxide nanocomposite coating applied by electrophoretic deposition on Nitinol superelastic alloy. Ceram Int. 2019;45(8):10448–10460. doi:10.1016/j.ceramint.2019.02.105
11. Zhu H, Ji X, Guan H, et al. Tantalum nanoparticles reinforced polyetheretherketone shows enhanced bone formation. Mater Sci Eng C Mater Biol Appl 2019;101:232–242. doi:10.1016/j.msec.2019.03.091
12. Elango J, Robinson J, Zhang J, et al. Collagen peptide upregulates osteoblastogenesis from bone marrow mesenchymal stem cells through MAPK- Runx2. Cells. 2019;8(5):E446. doi:10.3390/cells8050446
13. Chu C, Wei S, Wang Y, et al. Extracellular vesicle and mesenchymal stem cells in bone regeneration: recent progress and perspectives. J Biomed Mater Res A. 2019;107(1):243–250. doi:10.1002/jbm.a.36518
14. Li G, Song Y, Shi M, et al. Mechanisms of Cdc42-mediated rat MSC differentiation on micro/nano-textured topography. Acta Biomater 2017;49:235–246. doi:10.1016/j.actbio.2016.11.057
15. Huang J, Wang D, Chen J, et al. Osteogenic differentiation of bone marrow mesenchymal stem cells by magnetic nanoparticle composite scaffolds under a pulsed electromagnetic field. Saud Pharm J 2017;25(4):575–579. doi:10.1016/j.jsps.2017.04.026
16. Zhu Y, Zhang K, Zhao R, et al. Bone regeneration with micro/nano hybrid-structured biphasic calcium phosphate bioceramics at segmental bone defect and the induced immunoregulation of MSCs. Biomaterials. 2017;147:133–144. doi:10.1016/j.biomaterials.2017.09.018
17. Rashkow JT, Lalwani G, Sitharaman B. In vitro bioactivity of one- and two-dimensional nanoparticle-incorporated bone tissue engineering scaffolds. Tissue Eng Part A. 2018;24(7–8):641–652. doi:10.1089/ten.tea.2017.0117
18. Blazquez-Medela AM, Jumabay M, Bostrom KI. Beyond the bone: bone morphogenetic protein signaling in adipose tissue. Obes Rev. 2019;20(5):648–658. doi:10.1111/obr.12822
19. Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 2016;12(4):203–221. doi:10.1038/nrendo.2016.12
20. Blair HC, Larrouture QC, Li Y, et al. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng Part B. 2017;23(3):268–280. doi:10.1089/ten.teb.2016.0454
21. Ma XY, Feng YF, Wang TS, et al. Involvement of FAK-mediated BMP-2/SMAD pathway in mediating osteoblast adhesion and differentiation on nano-HA/chitosan composite coated titanium implant under diabetic conditions. Biomater Sci. 2017;6(1):225–238. doi:10.1039/C7BM00652G
22. Miyazono KI, Ohno Y, Wada H, et al. Structural basis for receptor-regulated SMAD recognition by MAN1. Nucleic Acids Res. 2018;46(22):12139–12153. doi:10.1093/nar/gky925
23. Zhang S, Takaku M, Zou L, et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature. 2017;551(7678):105–109. doi:10.1038/nature24283
24. Ma X, Fan C, Wang Y, et al. MiR-137 knockdown promotes the osteogenic differentiation of human adipose-derived stem cells via the LSD1/BMP2/SMAD4 signaling network. J Cell Physiol. 2019;235(2):909–919. doi:10.1002/jcp.29006
25. Urata M, Kokabu S, Matsubara T, et al. A peptide that blocks the interaction of NF-kappaB p65 subunit with Smad4 enhances BMP2-induced osteogenesis. J Cell Physiol. 2018;233(9):7356–7366. doi:10.1002/jcp.26571
26. Moon YJ, Yun CY, Choi H, et al. Smad4 controls bone homeostasis through regulation of osteoblast/osteocyte viability. Exp Mol Med. 2016;48(9):e256. doi:10.1038/emm.2016.75
27. Liang W, Mn L, Li X, et al. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med. 2012;30(4):889–895. doi:10.3892/ijmm.2012.1079
28. Wang Q, Zhou C, Li X, et al. TGF-beta1 promotes gap junctions formation in chondrocytes via Smad3/Smad4 signalling. Cell Prolif. 2019;52(2):e12544. doi:10.1111/cpr.12544
29. Lia L, Sapkotaa M, Gao M, et al. Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Eur J Pharmacol. 2017;815:202–209. doi:10.1016/j.ejphar.2017.09.015
30. Salazar VS, Zarkadis N, Huang L, et al. Postnatal ablation of osteoblast Smad4 enhances proliferative responses to canonical Wnt signaling through interactions with beta-catenin. J Cell Sci. 2013;126(24):5598–5609. doi:10.1242/jcs.132233
31. Park JS, Kim M, Song NJ, et al. A reciprocal role of the Smad4-Taz axis in osteogenesis and adipogenesis of mesenchymal stem cells. Stem Cells. 2019;37(3):368–381. doi:10.1002/stem.2949
32. Luo Y, Cao X, Chen J, et al. MicroRNA-224 suppresses osteoblast differentiation by inhibiting SMAD4. J Cell Physiol. 2018;233(10):6929–6937. doi:10.1002/jcp.26596
33. Valenti MT, Deiana M, Cheri S, et al. Physical exercise modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p expression in progenitor cells promoting osteogenesis. Cells. 2019;8(7):e742. doi:10.3390/cells8070742
34. Yan J, Li J, Hu J, et al. Smad4 deficiency impairs chondrocyte hypertrophy via the Runx2 transcription factor in mouse skeletal development. J Biol Chem. 2018;293(24):9162–9175. doi:10.1074/jbc.RA118.001825
35. Li H, Ji Q, Chen X, et al. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J Biomed Mater Res A. 2017;105(1):265–273. doi:10.1002/jbm.a.35900
36. Liu Y, Liu Y, Zheng C, et al. Ru nanoparticles coated with gamma-Fe2O3 promoting and monitoring the differentiation of human mesenchymal stem cells via MRI tracking. Colloids Surf B Biointerfaces. 2018;170:701–711. doi:10.1016/j.colsurfb.2018.05.041
37. Scarpa E, Janeczek AA, Hailes A, et al. Polymersome nanoparticles for delivery of Wnt-activating small molecules. Nanomedicine. 2018;14(4):1267–1277. doi:10.1016/j.nano.2018.02.014
38. Zhu Z, Xie Q, Huang Y, et al. Aucubin suppresses Titanium particles‑mediated apoptosis of MC3T3-E1 cells and facilitates osteogenesis by affecting the BMP2/Smads/RunX2 signaling pathway. Mol Med Rep. 2018;18(3):2561–2570. doi:10.3892/mmr.2018.9286
39. Fan J, Guo M, Im CS, et al. Enhanced mandibular bone repair by combined treatment of bone morphogenetic protein 2 and small-molecule phenamil. Tissue Eng Part A. 2017;23(5–6):195–207. doi:10.1089/ten.tea.2016.0308
40. Kang C, Wei L, Song B, et al. Involvement of autophagy in tantalum nanoparticle-induced osteoblast proliferation. Int J Nanomedicine. 2017;12:4323–4333. doi:10.2147/IJN
41. Dobbenga S, Fratila-Apachitei LE, Zadpoor AA. Nanopattern-induced osteogenic differentiation of stem cells - a systematic review. Acta Biomater. 2016;46:3–14. doi:10.1016/j.actbio.2016.09.031
42. Heo DN, Ko W-K, Bae MS, et al. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J Mater Chem B. 2014;2(11):1584–1593. doi:10.1039/C3TB21246G
43. Wang J, Wang M, Chen F, et al. Nano-hydroxyapatite coating promotes porous calcium phosphate ceramic-induced osteogenesis via BMP/Smad signaling pathway. Int J Nanomedicine. 2019;14:7987–8000. doi:10.2147/IJN.S216182
44. Wang C, Xiao F, Wang C, et al. Gremlin2 suppression increases the BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2 signaling pathway. J Cell Biochem. 2017;118(2):286–297. doi:10.1002/jcb.v118.2
45. Kim H-Y, Park S-Y, Choung S-Y. Enhancing effects of myricetin on the osteogenic differentiation of human periodontal ligament stem cells via BMP 2/Smad and ERK/JNK/p38 mitogen activated protein kinase signaling pathway. Eur J Pharmacol. 2018;834:84–91. doi:10.1016/j.ejphar.2018.07.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.