Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
The role of empagliflozin in the management of type 2 diabetes by patient profile
Authors Hedrington M, Davis S
Received 4 March 2015
Accepted for publication 2 April 2015
Published 5 May 2015 Volume 2015:11 Pages 739—749
DOI https://doi.org/10.2147/TCRM.S71762
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Maka S Hedrington, Stephen N Davis
Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
Abstract: Current recommendations for the management of type 2 diabetes mellitus (T2DM) include patient-centered approach, ie, targeting glycemic control based on patient and disease characteristics. Ten different classes of oral and injectable anti-hyperglycemic agents have been developed for T2DM, including the newest class – sodium–glucose cotransporter 2 (SGLT2) inhibitors. Four members of the class with comparable glycemic efficacy and side effects have gained approval in the US and the rest of the world. This review covers empagliflozin – third approved SGLT2 inhibitor in the US. The drug has shown rapid absorption reaching peak levels in ~2 hours and an elimination half-life of ~13 hours. Empagliflozin is a highly selective SGLT2 inhibitor with 2600-fold higher affinity for SGLT2 compared with SGLT1. Oral administration results in a dose-dependent inhibition of the transporters with increased urinary glucose excretion and resultant reduction in plasma glucose. Its efficacy and safety have been shown in a number of studies conducted in many countries. Across the trials, significant improvements in primary and secondary efficacy end points have been demonstrated, including reductions in HbA1c (~-0.8%), fasting plasma glucose (~-2 mmol/L), body weight (~-2 kg), and blood pressure (systolic -4 mmHg and diastolic -2 mmHg). Similar to other SGLT2 inhibitors, empagliflozin does not increase the risk for hypoglycemia, and the most commonly reported side effects are urinary and genital tract infections. Although empagliflozin can be used as the first-line monotherapy, its current place in the treatment of T2DM appears to be as an add-on to other oral anti-hyperglycemic agent(s) or insulin at any stage of the disease.
Keywords: anti-hyperglycemic agents, diabetes, glucose, SGLT2
Introduction to the management issues in the type 2 diabetes mellitus
There are over 100 different drug formulations approved by the US Food and Drug Administration (FDA) for use in type 2 diabetes mellitus (T2DM), and yet, challenges in the management of the disease remain. The issues are usually associated with insufficient glycemic control and/or side effects of oral or injectable medications. Currently, six mechanisms targeted by oral agents offer lowering of blood glucose: (1) increased insulin production (sulfonylureas, meglitinides), (2) increased insulin sensitivity and reduced glucose production (biguanides, thiazolidinediones [TZD]), (3) inhibited breakdown of carbohydrates (a-glucosidase inhibitors), (4) increased insulin release and reduced glucose production (dipeptidyl peptidase-4 inhibitors), (4) inhibited renal glucose reabsorption (sodium–glucose cotransporter 2 [SGLT2] inhibitors), (5) modulation of the hypothalamic regulation of metabolism and increased insulin sensitivity (dopamine-2 agonists), and (6) an unknown primary physiological action (bile acid sequestrants). Injectable treatment options for T2DM include insulin and insulin analogs, amylin mimetics with slowing of gastric emptying time and inhibition of glucagon production, and glucagon-like peptide-1 (GLP-1) receptor agonists that increase insulin release and inhibit glucagon secretion.1,2
Key side effects of the above agents include hypoglycemia – insulin and sulfonylureas; gastrointestinal side effects (nausea, vomiting, diarrhea, abdominal cramping) – biguanides, α-glucosidase inhibitors, GLP-1 receptor agonists, and amylin mimetics; and weight gain – insulin, sulfonylureas, meglitinides, and TZDs.1,3,4
Inadequate glycemic efficacy has also limited the widespread use of α-glucosidase inhibitors, amylin mimetics, bile acid sequestrants, and dopamine-2 agonists.4
Overview of the clinical aspects of the main patient profiles in diabetes and treatment considerations
The American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) are calling for a more patient-centered approach for diabetes care.1,2 ADA and EASD recommend choosing a target HbA1c based on patient and disease characteristics.1 For example, tighter glycemic control with target HbA1c <6.5% is recommended for newly diagnosed patients with a longer life expectancy, with low risks of hypoglycemia or other side effects, who do not have comorbidities or vascular complications, who are highly motivated, and who have social support readily available. For individuals newly diagnosed with T2DM, metformin remains the drug of choice, unless contraindicated or not tolerated (GI side effects). Although, SGLT2 inhibitors are also approved as an initial monotherapy, they are currently mostly used as second- or third-line agents.5
A newer approach is being considered for individuals who are newly diagnosed with T2DM with HbA1c ≥9%. Since the chance of achieving near-normal glycemia with one agent is very low, ADA recommends starting dual combination therapy with metformin and a second agent.1 Based on patient and disease characteristics, insulin may also be initiated and, in fact, may be the best option in this patient category.
In individuals with T2DM who were started on metformin monotherapy but were unable to achieve target HbA1c within 3 months, addition of a second anti-hyperglycemic agent is recommended.2 The 2015 position statement of ADA and EASD suggests considering SGLT2 inhibitors as reasonable options for addition to metformin.1 When triple combinations are required, initiation of insulin should be considered; otherwise, agents with the complementary mechanisms of action, eg, metformin + an SGLT2 inhibitor + a TZD6 or a sulfonylurea, should be chosen.7
In all, ~80% of T2DM patients are either overweight or obese.8 In this patient group, as a common practice, medications with weight neutrality or reduction are favored (metformin, SGLT2 inhibitors, GLP-1 receptor agonists, and amylin mimetics).
Review of pharmacology, mode of action, pharmacokinetics of empagliflozin
Introduction to SGLT2 inhibitors and the mode of action
SGLT2 inhibitors are the newest class of anti-diabetes drugs currently available on the market. The class targets the highest capacity subtype of sodium-coupled transporters (ie, SGLT2) that are responsible for the reabsorption of filtered glucose in the proximal tubules of kidneys (Figure 1).9 As a result, excess glucose is eliminated from the body, which can lead to an HbA1c reduction of ~1%.10 Although SGLT2 normally transports ~90% of the filtered glucose, its inhibition results in excretion of only 30%–50% of glucose (Figure 2), and therefore, the risk of hypoglycemia is low. An important advantage of SGLT2 inhibitors is the insulin-independent mechanism of action, which allows the class to be effective at any stage during the progression of T2DM. Additional benefits include weight loss (~−2 kg) and reduction in blood pressure (BP) (systolic: ~−3 mmHg and diastolic: ~−2 mmHg).10,11
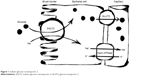  | Figure 1 Sodium–glucose cotransporter 2. |
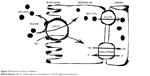  | Figure 2 Mechanism of SGLT2 inhibition. |
Currently, four SGLT2 inhibitors have gained approval from the US FDA, the European Medicines Agency, Therapeutic Goods Administration of Australia, and Pharmaceuticals and Medical Devices Agency of Japan: dapagliflozin (Farxiga™, marketed as Forxiga™ outside the US), canagliflozin (Invokana™), ipragliflozin (Suglat™), and empagliflozin (Jardiance™). The latter has been developed and marketed by Boehringer Ingelheim Pharmaceuticals in collaboration with Eli Lilly and Company. Empagliflozin was approved by FDA on August 1, 2014 as an addition to diet and exercise to improve glycemic control in T2DM adults.12 Recommended starting dose is 10 mg/day. If greater glycemic control is required and 10 mg is well tolerated, the dose can be increased to 25 mg/day.13
Chemistry
Empagliflozin (C23H27ClO7; molecular weight: 450.9) is a highly selective inhibitor of SGLT2 with a half maximal SGLT2 inhibitory concentration (IC50) of 3.1 nM, which is >2,600-fold higher compared with the IC50 for SGLT1 (8,300 nM).14
Pharmacodynamics
Pharmacodynamic (PD) properties of a single dose of empagliflozin (from 0.5 mg to 800 mg) were investigated in healthy males during two separate trials in Germany15 and Japan.16 The studies demonstrated that empagliflozin significantly inhibited glucose reabsorption (maximum effect – 100 mg dose: 50% vs placebo −0.6%) and increased urinary glucose excretion (UGE) in a dose-dependent manner: from 20 g/24 hours with the 1 mg dose to ~76 g/24 hours with the 100 mg vs ~0.05 g/24 hours with placebo. The inhibition of the resultant glucosuria continued for up to 72 hours.16 There was a linear relationship between empagliflozin exposure and UGE. However, time to reach maximum rate of UGE was not dose dependent and was similar in all groups (5–7 hours).
Heise et al studied PD parameters of a single dose of empagliflozin (10 mg, 25 mg, or 100 mg) following 4 weeks of treatment in 78 T2DM individuals (mean HbA1c ~7.2%, duration of diabetes ~6.3 years) in a randomized, double-blind, placebo-controlled manner (Table 1).17 The participants were being treated with lifestyle modification, monotherapy, or dual therapy that did not include TZDs. After an acute single-dose empagliflozin administration, mean cumulative UGE was inhibited by 36% (with 10 mg), 42% (with 25 mg), and 45% (with 100 mg). The study also demonstrated that the acute effects of the drug on UGE did not change during the 4-week study. At the end of 28 days, trial reductions in fasting plasma glucose (FPG) levels (−1.6 mmol/L in the 100 mg group, P<0.001) were also significantly compared to the placebo (−0.2 mmol/L). Similar results in individuals with T2DM (mean HbA1c ≤8.5%, duration of diabetes ~6.3 years) were demonstrated in another randomized, placebo-controlled trial conducted in Germany (Table 1).18
Pharmacokinetics
Empagliflozin has been demonstrated to have rapid absorption (~2 hours) across a range of doses (1–100 mg).16 Half-life (t1/2) of the drug ranged from 7.8 hours to 11.7 hours, and the total clearance ranged from 140 mL/min to 172 mL/min. Exposure to empagliflozin was also shown to be dose dependent, the Cmax increasing from 37 nmol/L following a 1 mg dose to 2,980 nmol/L with a 100-mg dose. Empagliflozin remained measurable in urine for up to 72 hours following administration. It was estimated that ~22% of the drug was excreted in the urine.
Pharmacokinetic (PK) parameters in T2DM were studied in a randomized, double-blind, placebo-controlled, parallel-group trial in 78 individuals in three centers of Germany.17 Empagliflozin was rapidly absorbed, reaching tmax in 1.5 hours with all doses. Similar to the study in healthy individuals, drug exposure was dose proportional: Cmax increased from 309 nmol/L in the 10 mg group to 2,630 nmol/L in the 100 mg group; AUC0–24 ranged from 1,550 nmol h/L (10 mg) to 15,900 nmol h/L (100 mg). Renal clearance of empagliflozin over 72 hours was ~13% with all doses. The study also measured PK parameters after 4 weeks of treatment (steady state) and reported only a 10% difference compared to the acute administration studies: Cmax 2,390 nmol/L, AUC0–24 18,700 nmol h/L, and renal clearance 17% in the 100 mg group. Similar results were also obtained in another randomized, placebo-controlled trial of empagliflozin in T2DM individuals that investigated multiple doses of the drug administered over 8 days.18
A number of studies have investigated the PK interactions of empagliflozin with drugs typically used in combination therapy for T2DM. These studies have demonstrated that empagliflozin does not alter PK properties of metformin,15 sitagliptin,19 linagliptin,20 ramipril, digoxin, verapamil, and diuretics.21
Clinical efficacy
To investigate the clinical efficacy, empagliflozin has been studied either alone or in combination with other oral anti-hyperglycemic agents or as add-on to insulin in individuals with inadequately controlled T2DM. Several of these studies have been followed by extension trials, data from which are currently only available as abstracts.
Monotherapy
The key objective of monotherapy studies was to evaluate the effects of different therapeutic doses of empagliflozin on glycemic control as measured by reductions in HbA1c and FPG and to provide additional data on body weight and BP.
A randomized, double-blind, placebo-controlled phase 2 trial investigated empagliflozin (5 mg, 10 mg, 25 mg, and 50 mg doses) monotherapy for 12 weeks in 547 individuals with T2DM in Japan (Table 1).22 Drug-naïve participants underwent a 2-week placebo run-in period; for individuals on oral anti-hyperglycemic agents, placebo run-in was preceded by a 4-week washout period. Mean HbA1c of all participants was ~8%, FPG was ~8.7 mmol/L, and BMI ranged from18 kg/m2 to 40 kg/m2. The study demonstrated that HbA1c was significantly reduced in all empagliflozin groups and the differences from the placebo ranged from −0.7% with the 5 mg dose to −0.9% with the 50 mg dose (P<0.001). In addition, more patients in the empagliflozin groups (28%–35%, P<0.001) achieved HbA1c <7% by the end of treatment compared to the placebo (3%). Other efficacy end points, including FPG (difference vs placebo: −1.5 mmol/L to −2 mmol/L, P<0.001), body weight (−1.6 kg to – 2.2 kg, P<0.001), and systolic BP (−1.5 mmHg to 4 mmHg, P<0.001), were also significantly improved (P<0.02) with all doses (5–50 mg). The initial 12-week study was followed by a 40-week extension trial23 during which improvements in glycemic control, body weight, and BP were maintained and remained significantly greater in the empagliflozin groups compared to the placebo.
Another large phase 2b trial in T2DM individuals (n=408) with mean HbA1c ~8% and BMI 28 kg/m2 compared 5 mg, 10 mg, and 25 mg empagliflozin with placebo and open-label metformin (Table 1).24 The participants were either treatment-naïve or on monotherapy that did not include TZDs, GLP-1 receptor agonists, or insulin. After 12 weeks of treatment, HbA1c was significantly reduced in a dose-dependent manner (5 mg: −0.4%, 10 mg: −0.5%, 25 mg: −0.6%, P<0.0001) compared to the slight increase in the placebo group (0.09%). Maximum changes from baseline in other efficacy end points with the 25 mg dose were as follows: FPG −1.7 mmol/L (vs +0.04 mmol/L in the placebo group, P<0.0001) and body weight −2 kg (vs −0.7 kg in the placebo group, P<0.0001).
Drug-naïve T2DM individuals (HbA1c ~8%, BMI ~28 kg/m2, disease duration ~5 years) were also studied in a multicenter, randomized, placebo-controlled phase 3 trial to investigate the effects of 24 weeks treatment with 10 mg (n=224) and 25 mg (n=224) doses of empagliflozin in comparison with placebo (n=228) and sitagliptin as an active comparator (n=223) (Table 1).25 At week 24, compared with the placebo, changes in HbA1c from baseline were −0.7% in the 10 mg group, −0.9% in the 25 mg group, and −0.7% in the sitagliptin group (P<0.001).
Effects of 4-week treatment with empagliflozin (10 mg or 25 mg dose) on postprandial glucose (PPG) have been studied in Japanese T2DM individuals (HbA1c ~8%, BMI ~24 kg/m2, disease duration ~5–10 years) in a randomized, double-blind, placebo-controlled, parallel-group trial (Table 1).26 At the end of the treatment period, PPG was significantly improved in the 25 mg group (−6.8 mmol h/L vs −1 mmol h/L in the placebo group, P<0.001); FPG (−2.2 mmol/L, P<0.001) was also significantly reduced compared to the placebo (−0.3 mmol/L).
Combination therapy
The sections below focus on phase 3 trials that investigated 10 mg or 25 mg empagliflozin doses to assess therapeutic efficacy of the drug as add-on to other oral agents or insulin.
Add-on to metformin
T2DM patients (HbA1c ~8%, BMI ~29 kg/m2, disease duration ~5–10 years) inadequately controlled with metformin were randomized in a double-blind manner to receive 10 mg or 25 mg of empagliflozin or placebo for 24 weeks (EMPA-REG MET) (Table 1).27 The reductions in HbA1c were significantly greater in both empagliflozin groups (10 mg: −0.7% and 25 mg: −0.8%, P<0.001) compared to placebo (−0.1%). Improvements in secondary end points following 25 mg empagliflozin included FPG −1.2 mmol/L (placebo: +0.4 mmol/L, P<0.001), body weight −2.5 kg (placebo: −0.5 kg, P<0.001), systolic BP −5 mmHg (placebo: −0.4 mmHg, P<0.001), and diastolic BP −1.6 mmHg (placebo: 0 mmHg, P<0.05).
Similar results were demonstrated in a large multinational (16 countries), dose-ranging, double-blind, placebo-controlled phase 2 trial in 495 individuals with T2DM (HbA1c ~8%, FPG ~9.7 mmol/L, BMI ~31 kg/m2).11 The participants received empagliflozin (from 1 mg to 50 mg), placebo, or open-label sitagliptin (100 mg/day) added to metformin (≥1,500 mg or maximum tolerated dose) for 12 weeks. The trial demonstrated that, except for the 1 mg dose group, change from the baseline in HbA1c was significant with sitagliptin (−0.5%) and all doses of empagliflozin (~−0.5% with 10 mg, 25 mg, and 50 mg, P<0.001) compared to placebo (+0.2%). The percent of patients who achieved HbA1c <7% by the end of treatment was ~37% with 10 mg, 25 mg, and 50 mg doses (P≤0.01) vs 15% with placebo and 34% with sitagliptin. The study also measured differences in FPG (maximum effect with 50 mg: −1.6 mmol/L vs +0.3 mmol/L in the placebo group, P≤0.0001, and −0.7 mmol/L in the sitagliptin group, P≤0.01 vs placebo) and body weight (maximum effect with 50 mg: −2.9 kg vs −1.2 kg in the placebo group, P≤0.0001, and −0.8 kg in the sitagliptin group). There was a non-statistical reduction in systolic and diastolic BP (50 mg group – systolic: −3 mmHg and diastolic: −2 mmHg vs −2.2 mmHg and −1 mmHg in the placebo group).
The 12-week study was followed by a randomized open-label 78-week extension trial conducted in 21 countries.28 In all, 78% from the initial study continued the extension trial. The trial included six treatment groups: empagliflozin 10 mg or 25 mg monotherapy, metformin (active comparator), metformin and empagliflozin 10 mg or 25 mg, and metformin with sitagliptin. At week 78, compared to the baseline of week 1, HbA1c was improved equally with empagliflozin 25 mg monotherapy (−0.5%) and add-on to metformin (−0.6%). The 25 mg empagliflozin group combination with the metformin group also achieved highest reductions in FPG (−1.8 mmol/L), body weight (−4 kg), waist circumference (−2.4 cm), systolic BP (−3 mmHg), and diastolic BP (−2 mmHg). In addition, more patients achieved HbA1c <7% by the end of the treatment (45%) compared to the other groups.
Triple combination therapy
The effects of empagliflozin (10 mg or 25 mg) as add-on to metformin and a sulfonylurea were investigated in a large (n=666) 24-week randomized, double-blind, placebo-controlled trial,7 followed by a ≥52-week extension trial.29 At the end of the first treatment period, HbA1c was significantly reduced in both empagliflozin groups (−0.8%, P<0.001) compared to the placebo group (−0.2%) and more patients achieved HbA1c <7% (10 mg: 26%, 25 mg: 32% vs placebo: 9%, P<0.001). There were significant improvements in key secondary end points: FPG (−1.3 mmol/L in both empagliflozin groups vs +0.3 mmol/L in the placebo group, P<0.001), 2-hour PPG (−2 mmol/L in both empagliflozin groups vs −0.1 mmol/L in the placebo group, P=0.003), body weight (10 mg: −2.2 kg, 25 mg: −2.4 kg vs placebo: −0.4 kg, P<0.001), waist circumference (−1.5 cm in both empagliflozin groups vs −0.3 in the placebo group, P=0.003), and systolic BP (−4 mmHg in both empagliflozin groups vs −0.3 mmHg in the placebo group, P=0.003). In all, 71% of the initial participants entered into the extension trial.29 At week 76, differences in the efficacy end points vs those of the placebo group were maintained in HbA1c (−0.7%, P<0.001), body weight (~−1.7 kg, P<0.001), and systolic BP (−2 mmHg, P<0.05).
A similar design study (24 weeks randomized, placebo controlled) investigated the effects of 10 mg and 25 mg empagliflozin as add-on therapy with metformin and/or pioglitazone (Table 1).6 After 24 weeks, reductions in HbA1c were significantly greater in the empagliflozin groups (change from baseline in 10 mg: −6%, 25 mg: −7%, P<0.001) compared to the placebo group (−1%). In all, 36% of participants in the 10 mg group and 48% in the 25 mg group (vs 12% in the placebo group) achieved HbA1c <7% at week 24 (P<0.001). Interestingly, glycemic efficacy was shown to be similar (~−0.7%) in groups receiving dual (empagliflozin with pioglitazone) and triple (empagliflozin + pioglitazone + metformin) combination therapies. The study also demonstrated significant improvements in FPG at week 24 (−0.9 mmol/L in the 10 mg group and −1.2 mmol/L in the 25 mg group vs increase in the placebo group +0.4 mmol/L, P<0.001). Body weight and waist circumference were reduced in the empagliflozin groups (10 mg: −1.6 kg and −1.7 cm, 25 mg: −1.5 kg and 0.9 cm, respectively, P<0.001) and increased in the placebo group (0.3 kg and 0.2 cm, respectively). Systolic and diastolic BPs were also significantly reduced in both empagliflozin groups (10 mg: systolic −3 mmHg and diastolic −2 mmHg, 25 mg: systolic −4 mmHg and diastolic −2 mmHg, P≤0.01) compared to the slight increase in the placebo group (systolic: 0.7 mmHg, diastolic: 0.3 mmHg). In all, 61% participants of the first trial completed the 52-week extension trial in a double-blind manner.30 The extension trial demonstrated that changes in the efficacy end points (HbA1c: −0.7%, P<0.001; body weight: ~−2 kg, P<0.001; systolic BP: −4 mmHg, P<0.01; diastolic BP: −2 mmHg, P<0.01) can be maintained for at least 76 weeks.
Add-on to insulin
Rosenstock et al investigated empagliflozin in combination with insulin in two trials (Table 1).31,32 The first trial studied addition of empagliflozin (10 mg or 25 mg) to basal insulin in 494 T2DM individuals31 and demonstrated significant improvements in glycemic control as well as secondary efficacy end points. HbA1c at week 78 was reduced by 0.5% in the 10 mg group and 0.6% in the 25 mg group compared to reduction of 0.02% in the placebo group (P<0.001); FPG was reduced by −0.6 mmol/L in the 10 mg group and −2.5 mmol/L in the 25 mg group vs increase in the placebo group (0.2 mmol/L) (P<0.001). Weight was reduced significantly in the empagliflozin groups (10 mg: −2.2 kg, 25 mg: −2 kg, P<0.001) vs a 0.7-kg weight gain in the placebo. Systolic BP was also reduced in the empagliflozin groups (10 mg: −4 mmHg, P<0.01; 25 mg: −2 mmHg) and slightly increased in the placebo group (0.1 mmHg). In addition, at week 78 compared to the baseline, insulin dosage was significantly reduced in the empagliflozin groups (10 mg: −1.2 IU, 25 mg: −0.5 IU, P<0.01) compared to an increase in the placebo group (+5.5 IU). The second trial evaluated empagliflozin (10 mg or 25 mg) added to multiple daily injections of insulin in 563 obese, inadequately controlled (HbA1c ~8.3%) T2DM individuals.32 Changes from baseline were assessed at weeks 18 and 52: HbA1c in the 10 mg group was −0.9%, 25 mg group was −1% vs −0.5% in the placebo group at week 18 (P<0.001). The glycemic control was further improved by week 52 with HbA1c reduced by −1.2% in the 10 mg group and –1.3% in the 25 mg group vs −0.8% in the placebo group. In addition, insulin doses in both empagliflozin groups were significantly reduced (−9 to −11 IU/day, P<0.01). Compared to the placebo group, body weight was also reduced in the empagliflozin groups (−2.4 kg to −2.5 kg, P<0.001).
Pooled analysis
Pooled analysis of the efficacy data from four phase 3 trials (n=2,477 T2DM participants) demonstrated improved HbA1c (−0.8% in the 25 mg group vs −0.08 in the placebo group), body weight (−2.3 kg in the 25 mg group vs 0.2 kg gain in the placebo group), and reduction in BP (systolic 4 mmHg and diastolic 2 mmHg in the 25 mg group vs 0.5 mmHg and 0.6 mmHg, respectively, in the placebo group) at 24 weeks compared to baseline.33
Phase 3 clinical trials have investigated empagliflozin as a monotherapy or in combination with other anti-diabetes agent in over 14,000 individuals with T2DM.34 All trials demonstrated improved glycemic control. Additional benefits included low risk of hypoglycemia and reductions in body weight and BP. Similar to other SGLT2 inhibitors, empagliflozin increased risk of mild-to-moderate genital and urinary tract infections.
Comorbidities
Renal impairment
Empagliflozin (10 mg or 25 mg) as add-on to existing therapy was studied in T2DM individuals with impaired renal function: stage 2 (eGFR (estimated glomerular filtration rate) 60 mL/min/1.73 m2–90 mL/min/1.73 m2) or 3 (eGFR 30 mL/min/1.73 m2–60 mL/min/1.73 m2) chronic kidney disease.35 The study demonstrated that compared to the baseline, at 24 weeks, HbA1c was reduced in both stages of chronic kidney disease: −0.7% and −0.4%, respectively, in the 25 mg group (P<0.0001).
Liver impairment
PK and PD properties of 50 mg empagliflozin were studied in T2DM individuals with mild (Child-Pugh Class A, 5–6 points), moderate (Child-Pugh Class B, 7–9 points), or severe (Child-Pugh Class C, 10–15 points) hepatic impairment compared to T2DM controls (with normal hepatic function) in an open-label parallel-group study.36 The study demonstrated increased empagliflozin exposure (mild: 13,800 nmol×h/L, moderate: 16,100 nmol×h/L, and severe impairment: 19,000 nmol×h/L vs normal hepatic function: 10,800 nmol×h/L) with increasing hepatic impairment. The study also assessed PD parameters and showed similar mean UGE between the groups (normal: 43 g/24 hours, mild: 36 g/24 hours, moderate: 38 g/24 hours, and severe: 40 g/24 hours).
Hypertension
A large, double-blind, randomized, placebo-controlled, multinational phase 3 trial has investigated the effects of 12-week treatment with empagliflozin (10 mg or 25 mg) in 825 individuals with T2DM and hypertension (mean systolic BP: 130–159 mmHg, mean diastolic BP: 80–99 mmHg).37 The study demonstrated significant improvements in glycemic control as well as BP. The change from the baseline in HbA1c at week 12 was −0.6% (vs placebo) with the 10 mg dose and −0.7% with the 25 mg dose (P<0.001). Mean 24-hour systolic BP was reduced by −3.4 mmHg with the 10 mg dose and −4.2 mmHg with the 25 mg dose (P<0.001). Changes in mean diastolic BP were −1.4 mmHg and −1.7 mmHg with 10 mg and 25 mg doses, respectively (P<0.001).
Safety and tolerability
Across the initial clinical studies as well as longer-term extension trials, empagliflozin has been shown to be safe and generally well tolerated.15–20,22–38 Empagliflozin safety was assessed in a systemic review and meta-analysis of 10 randomized controlled trials in 6,203 T2DM participants.38 The study demonstrated that treatment with empagliflozin did not increase the risk of hypoglycemia. The total number of hypoglycemic events was 195 in the 25 mg empagliflozin group and 186 in the placebo group. Majority of these episodes occurred in trials that investigated patients who were also treated with agents known to increase risk of hypoglycemia (sulfonylurea, insulin).31,38 There were only eight participants (out of 3,165) who experienced severe hypoglycemia – requiring assistance by another person. Liakos et al also analyzed the risk of urinary and genital tract infections in the empagliflozin-treated individuals. While the risk of urinary tract infections was not increased, there were more episodes of genital tract infection (mainly in male participants) in the empagliflozin groups compared to the placebo group.38
Another review of phase 3 clinical trials reported effects of empagliflozin on plasma low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides.33 LDL was increased in empagliflozin 10 mg (3.1 mg/dL) and 25 mg (3.9 mg/dL) groups compared to the placebo group (0.8 mg/dL). HDL was also increased by 2.7 mg/dL in both empagliflozin groups vs there was no change in the placebo group. There was a reduction in triglycerides in the 10 mg (−9.7 mg/dL) and 25 mg (−1.8 mg/dL) groups vs increase in the placebo group (+2.7 mg/dL). The clinical significance of these results has yet to be determined.
Ongoing trials
FDA approval of empagliflozin included a requirement for postmarketing studies that are currently continuing, and the results are not yet available.12
EMPA-REG OUTCOME™ is an ongoing randomized, placebo-controlled, cardiovascular outcome trial of empagliflozin that will determine the long-term cardiovascular safety of the drug and potential benefits on macrovascular and microvascular outcomes (ClinicalTrials.gov identifier: NCT01131676). The trial is being conducted in 592 clinical sites where 7,034 patients with T2DM on background anti-hyperglycemic therapy have been randomized and treated with 10 mg or 25 mg empagliflozin or placebo.39
PK and PD properties of empagliflozin are currently being studied in children and adolescents aged 10 to less than 18 years with T2DM to identify a safe and effective dose of the drug in this patient category (ClinicalTrials.gov identifier: NCT02121483).
The efficacy and safety of empagliflozin (10 mg/day or 25 mg/day) in African–American T2DM individuals with hypertension is being investigated in a randomized, double-blind, placebo-controlled, parallel-group manner (ClinicalTrials.gov identifier: NCT02182830). Primary efficacy end point is a reduction in HbA1c at week 24 with secondary end points of decreases in systolic and diastolic BPs at 12 weeks and 24 weeks.
The safety and efficacy of the drug are also being studied in Japanese elderly (aged 65 and over) patients with T2DM (ClinicalTrials.gov identifier: NCT02367131).
Specific patient profiles suited to the treatment with empagliflozin
Because of the favorable interaction with other anti-hyperglycemic agents and the unique mechanism of action that does not involve insulin, empagliflozin can be used as a monotherapy or as part of combination treatment in most patient categories with any stage of T2DM. The following paragraphs describe examples of specific patient profiles that would be suited to the treatment with empagliflozin.
Patient profile #1
Jane is a 52-year-old female with ~10-year history of T2DM. She is 5′3″ and weighs 190 lb; her most recent HbA1c was 9.1% and BP 162/92 mmHg. She is currently taking metformin 1,000 mg bid and glipizide 20 mg/day for diabetes and enalapril 20 mg/day for hypertension.
Patient profile #2
Tom is an overweight 62-year-old African–American man who was diagnosed with T2DM 16 years ago. He finds it difficult to adhere to diet and exercise recommendations. His clinical and laboratory parameters are as follows: BP 156/84 mmHg, BMI 37 kg/m2, HbA1c 8.4%, and FPG 132 mg/dL. He was started on insulin 2 years ago, currently taking insulin glargine 48 IU/day.
Patient profile #3
Saul is a 49-year-old man who has a 5-year history of T2DM. Initially managed with lifestyle modification, he was started on metformin 2 years ago, presently taking 2,000 mg/day. He also takes ramipril 5 mg/day for hypertension. His BP is 138/82 mmHg, BMI 36 kg/m2, HbA1c 7.3%, FPG 129 mg/dL, and 2-hour PPG 166 mg/dL, and urine albumin is within normal limits.
Patient profile #4
JN is a 59-year-old woman with T2DM diagnosed in 1998, hypertension, hyperlipidemia, and asthma. She was initially treated with metformin and lifestyle modification and was switched to insulin ~5 years ago. She is treated with Humalog 75/25, 31 units before breakfast and 27 units before dinner. Her other medications include lisinopril 30 mg/day, fluvastatin 20 mg at bedtime, fluticasone MDI 4 puffs/day, and levalbuterol two inhalations every 5 hours. Because of the exacerbation of asthma, she requires prednisone therapy. Physical examination and lab results include weight 299 lb, height 5′4″, BP 131/81 mmHg, HbA1c 7.1%, FPG 131 mg/dL, LDL cholesterol 114 mg/dL, and HDL cholesterol 49 mg/dL.
Patient profile #5
Linda is a 44-year-old Hispanic obese female diagnosed with T2DM 7 years ago. She is currently taking metformin 1,500 mg/day and glimepiride 4 mg/day. Her most recent HbA1c was 7.9%, FPG was 131 mg/dL, and BP was 129/78 mmHg.
Conclusion and place in therapy
In adult T2DM individuals, empagliflozin is characterized by rapid absorption reaching peak levels in ~2 hours,15 dose proportional exposure, inhibition of glucose reabsorption, and increase in UGE. Empagliflozin produces clinically meaningful improvements in HbA1c and FPG without increasing risk of hypoglycemia. The drug is also associated with significant reductions in body weight and systolic BP. Empagliflozin can be used as a monotherapy or in combination with one or two other oral agents or insulin. Although FDA has approved empagliflozin as the first-line therapy, according to the position statement of ADA and EASD, SGLT2 inhibitors are more likely to be used in combination with metformin and/or other medications.1
Glycemic efficacy of empagliflozin is comparable to other approved members of the class. Reductions in HbA1c have been shown to be ~−0.9% with canagliflozin,40 ~0.8% with dapagliflozin,41 and ~1% with ipragliflozin.42 Weight loss also appears to be similar to other SGLT2 inhibitors: canagliflozin ~−2.9 kg,40 dapagliflozin ~ −2.6 kg,43 and ipragliflozin ~−2 kg.42 Most common side effects associated with empagliflozin were genital and urinary tract infections, which were also reported in trials with dapagliflozin and canagliflozin.44,45
In conclusion, empagliflozin taken as a single medication or as add-on to other common anti-hyperglycemic agents (including insulin) is safe, well tolerated, and effective and has additional clinical benefits of weight loss and reductions in BP. Ongoing cardiovascular outcome studies will reveal long-term safety and efficacy of the drug.39
Disclosure
The authors have no financial conflicts of interest to declare. The manuscript was written in the absence of any commercial or financial relationships.
References
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379. | ||
Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912. | ||
Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2009;52(1):17–30. | ||
Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(5):410–417. | ||
Kovacs CS, Seshiah V, Swallow R, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16(2):147–158. | ||
Häring HU, Merker L, Seewaldt-Becker E, et al; EMPA-REG METSU Trial Investigators. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11): 3396–3404. | ||
Nelson JM, Dufraux K, Cook PF. The relationship between glycemic control and falls in older adults. J Am Geriatr Soc. 2007;55(12): 2041–2044. | ||
Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261(1):32–43. | ||
Vasilakou D, Karagiannis T, Athanasiadou E, et al. Sodium–glucose cotransporter 2 inhibitors for type 2 diabetes a systematic review and meta-analysis. Ann Intern Med. 2013;159(4):262–274. | ||
Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15(12):1154–1160. | ||
US Food and Drug Administration. FDA approves Jardiance to treat type 2 diabetes [press release]. Silver Spring, MD: US Food and Drug Administration; 2014 [August 1]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm407637.htm. Accessed February 25, 2015. | ||
Jardiance® (empagliflozin) [prescribing information]. Ingelheim: Licensed from: Boehringer Ingelheim International GmbH; 2014. | ||
Grempler R, Thomas L, Eckhardt M, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14(1):83–90. | ||
Komoroski B, Vachharajani N, Boulton D, et al. Empagliflozin (BI 10773), a potent and selective SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Drug Dev. 2013;2(2): 152–161. | ||
Sarashina A, Koiwai K, Seman LJ, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28(3):213–219. | ||
Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613–621. | ||
Heise T, Seman L, Macha S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther. 2013;4(2):331–345. | ||
Brand T, Macha S, Mattheus M, Pinnetti S, Woerle HJ. Pharmacokinetics of empagliflozin, a sodium glucose cotransporter-2 (SGLT-2) inhibitor, coadministered with sitagliptin in healthy volunteers. Adv Ther. 2012;29(10):889–899. | ||
Friedrich C, Metzmann K, Rose P, Mattheus M, Pinnetti S, Woerle HJ. A randomized, open-label, crossover study to evaluate the pharmacokinetics of empagliflozin and linagliptin after coadministration in healthy male volunteers. Clin Ther. 2013;35(1):A33–A42. | ||
Scott LJ. Empagliflozin: a review of its use in patients with type 2 diabetes mellitus (vol 74, pg 1769, 2014). Drugs. 2015;75(1):141–141. | ||
Kadowaki T, Haneda M, Inagaki N, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther. 2014;31(6): 621–638. | ||
Woerle HJ, Kadowaki T, Haneda M, et al. Safety and efficacy of empagliflozin monotherapy in a 52-week study in Japanese patients with type 2 diabetes mellitus. Poster No. 930 presented at: 49th Annual Meeting of the European Association for the Study of Diabetes; September 23–27, 2013; Barcelona. | ||
Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721–728. | ||
Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO Trial Investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013; 1(3):208–219. | ||
Nishimura R, Tanaka Y, Koiwai K, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14(1):11. | ||
Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014; 37(6):1650–1659. | ||
Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36(12):4015–4021. | ||
Ridderstråle M, Svaerd R, Zeller C, et al. Empagliflozin (EMPA) for ≥76 weeks as add-on to metformin plus sulfonylurea (SU) in patients with type 2 diabetes (T2DM). Diabetes. 2014;63(suppl 1):A280. [abstract no.1077-P]. | ||
Kovacs CS, Seshiah V, Merker L, et al. Empagliflozin (EMPA) for ≥76 weeks as add-on to pioglitazone with or without metformin in patients with type 2 diabetes (T2DM). Diabetes. 2014;63(suppl 1):A273. [abstract no.1055-P]. | ||
Rosenstock J, Jelaska A, Wang F, Kim G, Broedl UC, Woerle HJ. Empagliflozin as add-on to basal insulin for 78 weeks improves glycaemic control with weight loss in insulin-treated type 2 diabetes mellitus. Diabetologia. 2013;56:S372–S372. | ||
Rosenstock J, Jelaska A, Frappin G, et al; EMPA-REG MDI Trial Investigators. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37(7):1815–1823. | ||
Hach T, Gerich J, Salsali A, et al. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials. Poster No: 943 presented at: the European Association for the Study of Diabetes (EASD); September 23–27, 2013; Barcelona. | ||
Dailey G. Empagliflozin for the treatment of type 2 diabetes mellitus: an overview of safety and efficacy based on phase 3 trials. J Diabetes. Epub 2015 Feb 11. | ||
Barnett AH, Mithal A, Manassie J, et al; EMPA-REG RENAL Trial Investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2(5):369–384. | ||
Macha S, Rose P, Mattheus M, et al. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16(2): 118–123. | ||
Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420–428. | ||
Liakos A, Karagiannis T, Athanasiadou E, et al. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(10):984–993. | ||
Zinman B, Inzucchi SE, Lachin JM, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol. 2014;13:102. | ||
Stenlöf K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013; 15(4):372–382. | ||
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93(1): 397–404. | ||
Hedrington MS, Davis SN. Ipragliflozin, a sodium–glucose cotransporter 2 inhibitor, in the treatment of type 2 diabetes. Expert Opin Drug Metab Toxicol. 2015;11(4):613–623. | ||
Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med. 2015;32(4):531–541. | ||
Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin. 2012;28(7):1173–1178. | ||
Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375(9733):2223–2233. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

