Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
The Role of D-Dimers in the Initial Evaluation of COVID-19
Authors Baroiu L , Lese AC , Stefanopol IA , Iancu A , Dumitru C , Ciubara AB , Bujoreanu FC , Baroiu N , Ciubara A, Nechifor A, Anghel L , Tatu AL
Received 18 January 2022
Accepted for publication 8 March 2022
Published 31 March 2022 Volume 2022:18 Pages 323—335
DOI https://doi.org/10.2147/TCRM.S357214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Liliana Baroiu,1,* Ana Cristina Lese,2 Ioana Anca Stefanopol,3,* Alina Iancu,3,4,* Caterina Dumitru,4,5,* Alexandru Bogdan Ciubara,3,* Florin Ciprian Bujoreanu,6 Nicusor Baroiu,7,* Anamaria Ciubara,1,* Alexandru Nechifor,1,4,* Lucretia Anghel,1,* Alin Laurentiu Tatu1,4,6
1Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University of Galați, Galati, Romania; 2“George Enescu” National University of Arts, Faculty of Visual Arts and Design, Iasi, Romania; 3Departament of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University of Galați, Galati, Romania; 4Multidisciplinary Integrated Center of Dermatological Interface Research Center (MICDIR), “Dunărea de Jos” University of Galați, Galati, Romania; 5Pharmaceutical Sciences Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University of Galați, Galati, Romania; 6Dermatology Department, “Sf. Parascheva” Infectious Diseases Clinical Hospital, Galati, Romania; 7Manufacturing Engineering Department, Faculty of Engineering, “Dunărea de Jos” University of Galati, Galati, Romania
*These authors contributed equally to this work
Correspondence: Ana Cristina Lese, “George Enescu” National University of Arts, Faculty of Visual Arts and Design, No. 29, str. Cuza Voda, Iasi, 700040, Romania, Tel +40232212549, Fax +40232212551, Email [email protected] Florin Ciprian Bujoreanu, “Sf. Parascheva” Infectious Diseases Clinical Hospital, No. 343, str., Traian, Galati, 800179, Romania, Tel +40236334477, Fax +40236467752, Email [email protected]
Purpose: The COVID-19 pandemic was noted for the high degree of contagion and the large number of cases, as well as for the various clinical forms, from asymptomatic towards rapid evolution to death. The hospitals limited care capacity imposed the need to identify some markers of unfavorable evolution. The purpose of our study is to identify the parameters correlated with COVID-19 unfavorable evolution and to draw the profile of the patient at risk of unfavorable evolution. This set of parameters will help the doctor in deciding whether to hospitalize a patient and in choosing the treatment.
Patients and Methods: We performed a prospective, observational, actively controlled study on 849 patients with COVID-19, hospitalized in the Second Clinic of “Sf. Cuv. Parascheva” Infectious Diseases Clinical Hospital Galati, Romania, between 1.03.2020– 30.11.2020.
Results: The parameters statistically significant modified at the admission of the patients with COVID-19 unfavorable evolution were age, oxygen saturation, D-dimers, creatine kinase (CK), troponin, erythrocytes sedimentation rate (ESR), leukocytes, lymphocytes, neutrophils, platelets, hemoglobin (Hb), aspartate transaminase (AST), total and direct bilirubin (TBIL, DBIL), urea, creatinine, serum glucose. Strong correlations were observed between the unfavorable evolution and the admission values of D-dimers, AST, TBIL and between D-dimers and AST, which suggests that D-dimers levels can be considered predictive for the alteration of liver function and for the negative prognosis of the patient.
Conclusion: Coagulation disorders and acute respiratory failure are the prevailing causes of death from COVID-19. Together with other parameters that constitute the risk profile for severe COVID-19 evolution, the D-dimers dosing at admission proved to be extremely useful in the management of COVID-19.
Keywords: COVID-19, D-dimer, aspartate transaminase, total bilirubin, unfavorable evolution marker
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Introduction
On 28.11.2021, at the time of writing this article, 261,373,214 COVID-19 cases were registered in the world of which 5,212,953 deaths during the pandemic’s 5 waves with various strains of SARS-CoV-2, the last and deadliest of them being the Omicron strain because of its high contagiousness and multiple mutations on the spike protein.1
SARS-CoV-2 infection affects the whole body even if predominant are respiratory symptoms. Coagulation abnormalities such as hypercoagulation, thrombocytopenia, venous thrombosis (TV) and disseminated intravascular coagulation (DIC) have been observed in approximately 60–70% of hospitalized patients. The autopsies showed that in almost 58% of patients the cause of death was a pulmonary embolism or venous thrombosis, while DIC was reported in 70% of the patients who died from COVID-19.2–4 In the current practice the D-dimers value proves to be useful in the diagnosis and prediction of deep vein thrombosis recurrence; it is also an early marker for disseminated intravascular coagulation. In COVID-19, the average value of D-dimers is 0.9 mg/L, with increased values in 36% of the patients.5,6
The D-dimers level positively correlates with the severity of the disease, and is inversely proportional to the survival. Some case series note D-dimers values> 3 mg/L in 85% of the patients who died post COVID-194, others D-dimers values> 1 mg/L in 81% of the patients who died post COVID-19, and only 24% of D-dimers survivors> 1 mg/L.7,8
The average level of D-dimers is 2.4 mg/L in critical cases, as compared to 0.5 mg/L in mild cases.9,10
In addition, in COVID-19 non-survivors, a steady progressive increase in D-dimers is noted, compared with survivors in whom the D-dimers value remains stable or improves. Therefore, D-dimers can be considered an important prognostic factor in COVID-19 and correlates with unfavorable evolution and higher mortality.4,9
Liver injury is also important in COVID-19 and is considered to be a prognostic factor for patient progression. The factors involved in causing liver damage are: the SARS-CoV-2 direct attack, drug toxicity of the COVID-19 therapy, acute inflammatory changes and hypoxia.10
Abnormal levels of aspartate transaminase (AST)/alanine transaminase (ALT) can be attributed to muscular, hepatocyte and myocardial lesions. AST/ALT values in COVID19 are slightly elevated (between 1–2 normal values) in approximately 60% of patients; moderate (2 to 5 times higher than normal) in about 30% of patients; and increased by more than 5 times the normal value in approximately 10% of patients.11,12 Strikingly increased transaminases have also been reported, such as ALT = 7590 U/L and AST = 1445 U/L, in a patient with a severe form of COVID-19.13
Clinical studies found correlations between the severity of COVID-19 and its unfavorable evolution and the degree of liver damage.10,13–15
Coagulation disorders and microthrombus formation are also responsible for some of the dermatological lesions in the COVID-19.16,17
Another study of critically ill patients with COVID-19 showed dermatological manifestations of hypercoagulability such as significant ischemia of the limbs with plantar plaques and acral cyanosis.18–21
Coagulation disorders along with inflammatory mechanisms have also been implicated in the onset of neurological manifestations in COVID-19, such as cerebral hemorrhage or ischemia, cerebral venous thrombosis, demyelinating and neurodegenerative lesions, taste, smell and visual dysfunctioning and consciousness disorders.22
Materials and Methods
A prospective, observational, actively controlled study was performed on 849 COVID-19 patients (67 children and 782 adults), admitted to “Sf. Parascheva” Infectious Diseases Clinic Hospital in Galati, between 1.03.2020–30.11.2020. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of “Sf. Cuv.Parascheva” Infectious Diseases Clinical Hospital Galati, with number 22 and date of approval 02/26/2021. Written informed consent has been obtained from all the patients involved in the study, to publish this paper.
The inclusion criteria were: adults or children with moderate or severe forms of COVID-19, confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) of the nasopharyngeal swab, patients who had written informed consent for participation in the study, staff in full knowledge, themselves or their parents or legal guardian in the case of children.
Criteria for exclusion from the study were: inability of the patient to sign informed consent, refusal to participate in the study, pregnancy and lactation, and outliers (patients with extreme results in routine laboratory tests: leukocytes, hemoglobin, bilirubin, AST, ALT, serum creatinine).
Patients were monitored during hospitalization and one month after discharge were evaluated by telephone or in the day ward of the hospital.
Study sublots: the 849 patients were divided into 2 groups: group A (N = 807) of the favorable evolution patients consisted of discharged patients in good general condition, while group B (N = 42) of the unfavorable evolution patients included the patients who died or were transferred to intensive care.
It is worth mentioning that not all patients had complete data available.
The endpoint of the study was the total number of deaths and patients transferred to intensive care.
Patient data extracted from the observation sheets were analyzed using IBM Statistics V.24 * SPSS, INC., Chicago, Il, USA, and Excel 2019. Gross descriptive statistical parameters were calculated for all variables, at which this analysis was useful. For continuous numerical variables were calculated: minimum and maximum value, mean value and standard deviation (SD), then simple bivariate correlations were performed, with the calculation of the Pearson correlation coefficient (r - linear correlation coefficient) and the determining factor (R2). For the categorical values were calculated: frequency, median and mode and then they were entered in the contingency tables and the non-parametric chi-square test was applied (χ2). Comparisons were made between the group of patients with COVID-19 and favorable outcome (group A) compared to the group with COVID-19 with unfavorable outcome (group B). For each of the statistical tests used, a level of statistical significance was considered for values below 0.05 (with p-index calculated at both ends).
Results
The characteristics of the main scalar variables were analyzed, as they were detected at the group level. Thus, it can be observed that:
- The average age of patients is 50.21 years, to which a standard deviation (SD) of ± 20.05 years is associated, and the median value is 51 years. The Gaussian curve is deviated to the right, with a maximum incidental peak around 40 years (Figure 1). The average age of the group of unfavorable evolution patients (73.22 years) is statistically significantly higher (p<0.0001) than that of the group of favorable evolution patients (49.01 years) (Table 1).
 |
Table 1 The Comparative Values of COVID-19 Characteristics with Favorable Evolution versus COVID-19 with Unfavorable Evolution |
 |
Figure 1 Distribution by age, total group of patients. |
- Predominantly female sex- 460 women (54.18%), in the total group. In the group of favorable evolution patients, women predominate (55.14%) and in the group of unfavorable evolution patients, men predominate (64.28%) (a statistically significant difference: p = 0.0212) (Table 2).
 |
Table 2 Categorial Variables Characteristics of the COVID-19 Favorable Evolution Patients Compared to the COVID-19 Unfavorable Evolution Patients |
- Predominantly environment - urban origin - 72.90% patients in the total group, 72.61% of the patients in the group of favorable evolution patients, and 76.19% in the group of unfavorable evolution patients (Table 2).
- Charlson score, of cumulative comorbidities - average-3.16, minimum-0, maximum-11, the score 0–3 points predominates in the favorable evolution group, and the score 4–6 points predominates in the unfavorable evolution group (Table 2).
- Curb 65 score, of pneumonia severity - average-2.34, minimum-0, maximum-3, the score 1 point predominates in the favorable evolution group, and the score 2 points predominates in the unfavorable evolution group (Table 2).
- Nutrition status at the start of hospitalization, body mass index – average-28.40, minimum-14.42, maximum-58.59, which means without statistically significant differences between the two groups under study (Table 1).
-The average number of days of hospitalization was 11.06 days, with an associated SD of ± 7622 days, the median being 10 days. Statistical indices define the homogeneous distribution with a Gaussian curve deviated to the left. As the maximum incidence, 10–13 days hospitalizations are noted (Figure 2). The average length of hospitalization was, statistically, significantly higher in the favorable evolution group who performed the entire treatment until the significant improvement of health, in our clinic, compared to those with unfavorable evolution who either decompensated suddenly and died in our clinic, or needed further intensive care hospitalization (p<0.0001) (Table 1).
 |
Figure 2 Distribution according to the hospitalization duration of the total patients group. |
A series of biochemical parameters, at hospitalization, indicating the degree of liver damage, were analyzed, from a descriptive point of view, by tracking the values of liver markers (AST, ALT, total and direct bilirubin (DBIL), simultaneously with the D-dimers values and other biochemical parameters (Table 1):
- An average value of D-dimers was 841.22 ng/mL, with an SD of 1160.85 ng/mL, a median value of 473.73 ng/mL, a minimum value of 52 ng/mL and a maximum value over 10,000 ng/mL in the total group. The average value of D-dimers in the unfavorable evolution group (2057 ng/mL) was, statistically, significantly higher than the average D-dimers in the favorable evolution group (784 ng/mL; p<0.0001). The COVID-19 favorable evolution patients had D-dimers values between 1500–5000 ng/mL in a proportion of 7.9% and values between 5000–10,000 ng/mL in a proportion of 1.4% as opposed to unfavorable evolution patients whose D-dimers values were between 1500–5000 ng/mL in a proportion of 28.6%, and between 5000–10,000 ng/mL in a proportion of 7.1%.
- The AST has an average value of 34.91 U/L, associating an SD of 29.64 U/L, while the ALT is characterized by an average value of 40.67 U/L and an SD of 44.08 U/L, in the total group. The average values of AST and ALT in the favorable evolution group fall within the normal values range of the analysis, and those in the unfavorable evolution group exceed the maximum limit of normal values. It is also worth mentioning that the average of AST in the unfavorable evolution group is, statistically, significantly higher than in the favorable evolution group, probably justified due to cell lysis, more important products of SARS-CoV-2, simultaneously in liver, striated muscles and myocardium, in patients with severe forms of COVID-19. The unfavorable evolution patients had modified AST values in a percentage of 64.3%, compared to 27.1% in those with favorable evolution.
- The values of total bilirubin are defined by an average value of 0.55 mg/dl (with SD of 0.46 mg/dl), and the direct bilirubin with an average value of 0.23 mg/dl (with SD of 0.67 mg/dl), in the total group.
The mean values of the total bilirubin in both groups of patients fall within the normal values range of the analysis, however, being, statistically, significantly higher with the unfavorable evolution group. The mean values of direct bilirubin are in the normal values range of the analysis in the favorable evolution group and exceed the maximum limit of normal values in the unfavorable evolution group. The mean values of direct bilirubin are, statistically, significantly higher in the unfavorable evolution group compared to the favorable evolution group. The unfavorable evolution patients had 14.3% modified values of TBIL as compared to 4% in those with favorable evolution.
Therefore, the causal relationship existing between the AST and ALT values was followed, respectively the D-dimers values obtained from the blood analyses of the patients. Obviously and punctually, it can be seen that there are a number of relationships of positive interdependence, directly proportional, but with different degrees of statistical power. Therefore, the correlations between the D-dimers determined values were analyzed and the existence of positive correlation with AST, statistically strong, were noticed. Consequently, a tendency of variation of the AST and ALT values related to those of the D-dimers (D-dimer values [y], the values of the hepatic indices on the [x] axis) is obvious. We will discuss them in detail below (Figure 3).
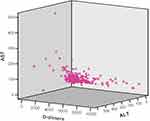 |
Figure 3 The correlation between the D-dimers, ALT and AST values at the beginning of hospitalization of patients. |
By corroborating the existing information in the statistical analysis table below, that of bivariate correlations with Pearson index, it is possible to admit a series of conclusions regarding the incidence of unfavorable evolution patients, by reference to a series of biochemical parameters characteristics (AST, ALT, D-dimers, TBIL, DBIL). Thus, the values of the p index can be followed by statistical analysis at two stages (for an index p <0.05 we will have a statistically strong significance, while the values of p <0.01 will indicate extremely strong statistically significant meanings). The existence of the following correlations was found (Table 3):
 |
Table 3 Pearson Correlations Between D-Dimers, AST, ALT, DBIL and TBIL Values |
- From a statistical point of view, there was an extremely strong correlation between D-dimers values and AST (r=+0.127; p=0.001), detected at the start of the hospitalization.
- The same type of strong correlation from a statistical point of view was detected between the values of AST and ALT (r= +0.718; p=0.001);
-A significant correlation we observed between TBIL and DBIL (r= +0.119; p=0.002) and between DBIL and ALT (r= +0.09; p=0.021).
The same type of extremely strong correlation from a statistical point of view was detected between the patients’ evolutions during hospitalization, respectively the mean values of D-dimers decrease from 1269±105 to 966±83 (p=0.001) and mean values of ALT increase from 52.53±3.74 to 75.81±5.50 (p=0.003) (Figures 4 and 5).
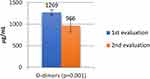 |
Figure 4 The evolution of D-dimers values during of hospitalization of patients. |
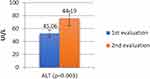 |
Figure 5 The evolution of ALT values during of hospitalization of patients. |
In patients with unfavorable evolution we noticed the following differences (Table 1):
-the mean values of D-dimers were significantly higher (2057 vs 784 µg/mL; p=0.001) (Figure 4);
- the mean values of AST were significantly higher (53.23 vs 33.98 UI/L; p=0.001) (Figure 6);
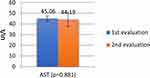 |
Figure 6 The evolution of AST values during of hospitalization of patients. |
- the mean values of TBIL were significantly higher (0.85 vs 0.53 UI/L; p=0.001) (Figure 7).
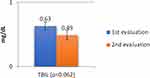 |
Figure 7 The evolution of TBIL values during of hospitalization of patients. |
The comparative analysis of the two groups of patients also included:
-the value of creatine kinase (CK) - its average in the unfavorable evolution group (287U/L) was statistically significantly higher than the average in the favorable evolution group (142 U/L), p = 0.0071 (Table 1).
-the value of troponin- its average in the unfavorable evolution group (666.49 ng/mL) was statistically significantly higher than the average in the favorable evolution group (20.73 ng/mL), p = 0.0016 (Table 1).
-The comparative study of hemoleukograms revealed a higher, average number of total leukocytes and neutrophils, a lower average number of lymphocytes and platelets, as well as mean values of hemoglobin, statistically significant, in the group with unfavorable evolution compared to the group with favorable evolution (Table 1).
-The average values of serum urea and creatinine were statistically significant, higher, in the unfavorable evolution group as compared to the favorable evolution group. There have been cases of acute renal failure but the median values are within normal values in both groups (Table 1).
- The average values of glycemia was statistically significant, higher, in the unfavorable evolution group as compared to the favorable evolution group. There have been cases of hyperglycaemia and decompensation of pre-existing diabetes in both groups (Table 1).
-The average values of the erythrocyte sedimentation rate were statistically significantly higher in the unfavorable evolution group (56.31mm/h) as compared to the favorable evolution group (36.64 mm/h), p<0.0001 (Table 1).
-The average oxygen saturation values at admission were, statistically, significantly lower in the unfavorable evolution group (72.28%) as compared to the favorable evolution group (93.49%), p<0.0001. The fact should be mentioned that in the unfavorable evolution group, there were patients who needed intensive care hospitalization, or, because there were no places in intensive care, they received care in our infectious diseases section (Table 1).
-From the radiological images point of view, in both groups, the lesions accentuating the pulmonary interstitium predominated at the admission (Table 2).
Multivariate analysis, by linear regression line, showed that D-dimers values are influenced by biochemically determined parameters at admission (Table 4):
 |
Table 4 Regression Liniar Model Summary. Dependent Variable D-Dimers |
- Model 1 highlights the fact that AST is a good predictor of D-dimer growth
(y = 623 + 7.25 AST; p = 0.001);
- Model 2 highlights the fact that both AS and ALT are good predictors of D-dimer determinism (y = 524 + 12.4 AST - 4.1 ALT; p = 0.022);
- Model 7 highlights the fact that AST, ALT, TBIL, DBIL, CK, leukocytes (* 1000/microL) and hemoglobin are good predictors in the determinism of D-dimers (y = 2222 + 12.0 AST - 3.7 ALT +138 TBIL +38.4 DBIL + 0.02 CK + 26.8 le 137 hemoglobin; p = 0.001);
- Model 8 highlights the fact that AST, ALT, TBIL, DBIL, CK, leukocytes (* 1000/microL), hemoglobin and troponin are good predictors in the determinism of D-dimers (y = 2067 + 12.4 AST - 3.7 ALT +128 TBIL +0.002 DBIL + 0.02 CK + 27.3 leucocytes–127 hemoglobin + 0.24 troponin; p = 0.001);
Discussion
COVID-19 is a potentially severe systemic infection. The pulmonary and hepatic tropism of SARS-CoV-2 and the prothrombotic physiopathological mechanisms justify to carefully monitoring the respiratory, hepatic, coagulation and renal functions and the glycemic balance, as well as the early therapeutic interventions to prevent the complications.
Clinical studies support the monitoring of D-dimers in COVID-19 patients and describe its correlation with the unfavorable evolution of the cases with increased D-dimers values.
A study of 2377 adult patients hospitalized with COVID-19 was performed on patients with D-dimers > 2000 ng/mL and observed a risk of critical illness of 66%, thrombotic event of 37.8%, acute kidney damage of 58.3% and of death of 47% (the critical illness being considered hospitalization in intensive care, mechanical ventilation, or death).23–25
A meta-analysis of 29 clinical trials involving 4328 hospitalized patients with COVID-19 found a higher average of D-dimers levels, at admission), in severe patients than in non-severe patients, as well as in patients who required intensive care and those who died. The conclusion of the study was that elevated levels of D-dimers at a the beginning of hospitalization, in patients with SARS-CoV-2 infection were associated with an increased risk of severe disease progression and mortality.26–28
Another meta-analysis of 16 clinical trials showed higher levels of D-dimers in patients with severe disease and death from COVID-19 and noted the usefulness of anticoagulant therapy with lower mortality in patients treated with anticoagulants than those without anticoagulant therapy.27,29
Xu et al observed in postmortem liver biopsies microvesicular steatosis and mild lobular and portal inflammatory activity.30 Other biopsy studies described thrombosis, fibrous thickening of the vascular wall and luminal ectasia, suggesting that endothelial lesion and coagulation dysfunction are involved in the pathogenesis of liver failure in COVID-19.10 Cai et al observed SARS-CoV-2 viral particles in hepatocyte’s cytoplasm, and described the following hepatocyte changes: mitochondrial swelling cell membrane damage and endoplasmic reticulum dilation.31
Increased AST and ALT levels in adults are correlated in clinical trials with an unfavorable prognosis of the patient compared to children, where ALT, AST levels are usually below twice the normal value. Severe liver damage is very rare in children.11,32–34
In most cases COVID-19, TBIL is normal or slightly increased.11,35–37
Clinical studies demonstrated the correlation between the severity of COVID-19, its negative prognosis and the degree of liver damage.13
Our study statistically demonstrates that high blood values of D-dimers can predict the liver damage and that the values of AST and total bilirubin are accurate parameters correlated with an unfavorable evolution.
Other parameters with statistically significant higher values in the unfavorable evolution group compared to those with favorable evolution were: oxygen saturation, erythrocyte sedimentation rate, hemoleukogram changes, hyperglycemia, elevated values of serum CK, troponin, urea and creatinine.
Our study also notes the unfavorable evolution of patients with the mean age over 70 years, predominantly men, with multiple comorbidities (Charlson score between 4 and 6) and with severe pneumonia (CURB-65 score equal to 2). Elderly patients with multiple comorbidities have an increased risk of infection in general and COVID-19 in particular.38,39 Demonstration of severe prognosis in this patient profile, elderly with multiple comorbidities requires the establishment of a set of personalized prophylactic measures, such as priority vaccination, hygiene measures and increased isolation, easy and quick access to specific antiviral therapy, mandatory measures to improve the prognosis of these patients.40
Conclusion
Given the endothelial aggression and prothrombotic mechanisms triggered by SARS-CoV-2, and the statistical results obtained in our study, we can say that the increased value of the D-dimers may be predictive for abnormal liver functional parameters and for a severe evolution.
The profile of the patient at risk with COVID-19 severe evolution resulting from our study is: patient over 70 years of age, male, with multiple comorbidities (Charlson score between 4 and 6), with severe pneumonia (CURB-65 score equal to 2) with high values of D-dimers, AST, ESR, CK, troponin, total and direct bilirubin, urea, creatinine, glycemia, total leukocytes, neutrophils and low values of oxygen saturation, hemoglobin, platelets and lymphocytes at admission.
Defining this profile of a patient at risk with COVID-19 severe evolution argues the need for repeated monitoring of these parameters and their use in the patient’s hospitalization decision and the choice of therapy. These parameters along with clinical and imaging parameters help in assessing the severity of the case, in choosing antiviral therapy or the immunomodulatory therapy, in choosing doses of anticoagulant medication, in modulating hepatoprotective therapy and in the management of the glycemic and renal balance.
Acknowledgments
The present study was academically supported by the Multidisciplinary Integrated Center of Dermatological Interface Research Center MICDIR.
Funding
The present study was academically supported by the “Dunarea de Jos” University of Galati, Romania.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Report coronavirus cases. Available from: https://www.worldometers.info/coronavirus/.
2. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi:10.7326/M20-2003
3. Klok FA, Kruip MJ, Van der Meer NJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi:10.1016/j.thromres.2020.04.013
4. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi:10.1111/jth.14768
5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
6. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
7. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi:10.1016/S0140-6736(20)30566-3
8. Cha MH, Regueiro M, Sandhu DS. Gastrointestinal and hepatic manifestations of COVID-19: a comprehensive review. World J Gastroenterol. 2020;26(19):2323–2332. doi:10.3748/wjg.v26.i19.2323
9. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
10. Baroiu L, Dumitru C, Iancu A, et al. COVID-19 impact on the liver. World J Clin Cases. 2021;9(16):3814–3825. doi:10.12998/wjcc.v9.i16.3814
11. Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. cohort. Hepatology. 2020;72(3):807–817. doi:10.1002/hep.31404
12. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72(1):287–304. doi:10.1002/hep.31281
13. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. bioRxiv. 2020. doi:10.1101/2020.02.03.931766
14. Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52(4):584–599. doi:10.1111/apt.15916
15. Baroiu L, Dumea E, Năstase F, et al. Assessment of depression in patients with COVID-19. Brain. 2021;12(2):254–264. doi:10.18662/brain/12.2/204
16. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):700. doi:10.1016/j.jaad.2020.04.018
17. Tatu AL, Nadasdy T, Bujoreanu FC. Inflammation and vascular injury as the basis of COVID-19 skin changes: preliminary analysis of 23 patients from the literature [Letter]. Clin Cosmet Investig Dermatol. 2021;14:185–186.
18. Zhang Y, Cao W, Xiao M. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. ZhonghuaXue Ye XueZaZhi. 2020;41:E006.
19. AlSamman M, Caggiula A, Ganguli S, Misak M, Pourmand A. Non-respiratory presentations of COVID-19, a clinical review. Am J Emerg Med. 2020;38(11):2444–2454. doi:10.1016/j.ajem.2020.09.054
20. Tatu AL, Baroiu L, Fotea S, et al. A working hypothesis on vesicular lesions related to COVID-19 infection, koebner phenomena Type V, and a short review of related data. Clin Cosmet Investig Dermatol. 2021;14:419–423.
21. Tatu AL, Nadasdy T, Bujoreanu FC. Familial clustering of COVID-19 skin manifestations. Dermatol Ther. 2020;33(6):e14181.
22. Singh V, Allawadhi P, Khurana A, Banothu AK, Bharani KK. Critical neurological features of COVID-19: role of imaging methods and biosensors for effective diagnosis. Sens Int. 2021;2:100098. doi:10.1016/j.sintl.2021.100098
23. Berger JS, Kunichoff D, Adhikari S, et al. Prevalence and outcomes of D-Dimer elevation in hospitalized patients with COVID-19. Arterioscler ThrombVasc Biol. 2020;40(10):2539–2547. doi:10.1161/ATVBAHA.120.314872
24. Lupu MN, Miulescu M, Stefanopol IA, et al. Effect of 2,6-diisopropylphenol and 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy) propane as anesthetic. Rev Chim. 2019;70(5):1888–1892. doi:10.37358/RC.19.5.7239
25. Baroiu N, Berbinschi S, Teodor VG, Susac F, Oancea N. The complementary graphical method used for profiling side mill for generation of helical surface. IOP Conf Ser Mater Sci Eng. 2017;227:012013. doi:10.1088/1757-899X/227/1/012013
26. Nugroho J, Wardhana A, Maghfirah I, et al. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients: a meta-analysis. Int J Lab Hematol. 2021;43(1):110–115. doi:10.1111/ijlh.13336
27. Vidali S, Morosetti D, Cossu E, et al. D-dimer as an indicator of prognosis in SARS-CoV-2 infection: a systematic review. ERJ Open Res. 2020;6(2):00260–2020. doi:10.1183/23120541.00260-2020
28. StefanopolI A, Miulescu M, Baroiu L, Anghele AD, Danila DM, Tiron Z. An unusual case of Meckel diverticulitis misdiagnosed as an infected urachal cyst. Medicina-Lithuania. 2021;57(5):495.
29. Baroiu N, Baroiu L, Teodor VG, Ciocan TL. Graphical method for profiling the side mill which generate helical flute of tungsten carbide dental cross cut bur. Rev Rom Mater. 2018;48(1):131–139.
30. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi:10.1016/S2213-2600(20)30076-X
31. Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi:10.1016/j.jhep.2020.04.006
32. Lu X, Zhang L, Du H, et al. Chinese pediatric novel coronavirus study team. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi:10.1056/NEJMc2005073
33. D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi:10.1002/lt.25756
34. Berbinschi S, Baroiu N, Teodor V, Oancea N. Profiling method of side mill for threading screw for dental implants. Adv Mater Res. 2014;837:22. doi:10.4028/www.scientific.net/AMR.837.22
35. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW. The northwell COVID-19 research consortium. presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi:10.1001/jama.2020.6775
36. Ferm S, Fisher C, Pakala T, et al. Analysis of gastrointestinal and hepatic manifestations of SARS-CoV-2 infection in 892 patients in queens, NY. Clin Gastroenterol Hepatol. 2020;18:2378–2379.
37. Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a Multi-center Cohort Study. Gastroenterology. 2020;159(2):765–767. doi:10.1053/j.gastro.2020.04.045
38. Marin M, Petropolou M, Baroiu L, Chirosca AC, Anghel L, Luca L. Schizophrenia and the family burden during the pandemic. Brain. 2020;11(3–1):89–97. doi:10.18662/brain/11.3Sup1/125
39. Baroiu L, Beznea A, PleseaCondratovici C, et al. Comparative effectiveness of vancomycin and metronidazole for the initial episode of nonsevere clostridium difficile infection. Rev Chim. 2019;70(10):3741–3745. doi:10.37358/RC.19.10.7637
40. Khurana A, Allawadhi P, Khurana I, et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38:101142. doi:10.1016/j.nantod.2021.101142
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
