Back to Journals » Neuropsychiatric Disease and Treatment » Volume 14
The role of COMT gene Val108/158Met polymorphism in suicidal behavior: systematic review and updated meta-analysis
Authors González-Castro TB , Hernández-Díaz Y, Juárez-Rojop IE , López-Narváez ML, Tovilla-Zárate CA , Ramirez-Bello J , Pérez-Hernández N, Genis-Mendoza AD , Fresan A , Guzmán-Priego CG
Received 25 April 2018
Accepted for publication 12 July 2018
Published 28 September 2018 Volume 2018:14 Pages 2485—2496
DOI https://doi.org/10.2147/NDT.S172243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Thelma Beatriz González-Castro,1 Yazmín Hernández-Díaz,1 Isela Esther Juárez-Rojop,2 María Lilia López-Narváez,3 Carlos Alfonso Tovilla-Zárate,4 Julian Ramírez-Bello,5 Nonanzit Pérez-Hernández,6 Alma Delia Genis-Mendoza,7 Ana Fresan,8 Crystell Guadalupe Guzmán-Priego2
1Multidisciplinary Academic Division of Jalpa de Méndez, Juarez Autonomous University of Tabasco, Jalpa de Méndez, Tabasco, Mexico; 2Multidisciplinary Academic Division of Health Sciences, Juarez Autonomous University of Tabasco, Villahermosa, Tabasco, Mexico; 3General Hospital of Yajalon, Ministry of Health, Yajalon, Chiapas, Mexico; 4Multidisciplinary Academic Division of Comalcalco, Juarez Autonomous University of Tabasco, Comalcalco, Tabasco, Mexico; 5Research Unit, Juárez Hospital of Mexico, Ministry of Health, Mexico City, Mexico; 6Department of Molecular Biology, National Institute of Cardiology, Mexico City, Mexico; 7Psychiatric Care Services, National Institute of Genomic Medicine (INMEGEN), Health Secretary, Ministry of Health, Mexico City, Mexico; 8Sub-direction of Clinical Research, Children’s Psychiatric Hospital “Dr. Juan N. Navarro”, Mexico City, Mexico
Background: It is accepted that there is a genetic factor that influences the risk of suicidal behavior. The catechol-O-methyltransferase (COMT) gene, especially the Val108/158Met polymorphism, has been associated with suicide; however, no conclusive outcome has been attained. Therefore, the aim of the present study was to assess the role of COMT Val108/158Met in suicidal behavior throughout an updated meta-analysis.
Methods: We performed an online search using PubMed and Web of Science (up to March 2017). Our systematic review included case-control studies of individuals who attempted suicide and completed suicide. We tested allelic, homozygous, heterozygous, dominant, and recessive inheritance models. The meta-analysis was performed in accordance with the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results: The meta-analysis comprised 17 studies, which included 3,282 cases and 3,774 controls, and showed that when evaluating the overall population, the Val108/158Met polymorphism of COMT was not associated with suicidal behavior in any of the inheritance models; however, the subanalyses showed that this polymorphism exhibits a risk factor in males and a protective effect in females. Additionally, it conveyed a risk factor in Asian populations when using the allelic (OR 1.25; CI: 1.04–1.51) and recessive models (OR 1.32; CI: 1.03–1.68).
Conclusion: Our updated meta-analysis suggests a possible association between COMT Val108/158Met and suicidal behavior in Asian populations. However, in view of the small number of studies, these results should be considered exploratory. We recommend that more studies be performed with larger samples.
Keywords: suicide, epidemiology, mental health, risk factors
Introduction
Suicidal behavior (SB) is a complex phenotype with biological, genetic, and environmental risk factors involved.1,2 Several arguments show that psychiatric disturbances are major contributing factors in SB; however, genetic predisposition has been strongly considered to be a contributory factor in SB,3,4 and evidence indicates that SB is modulated by a number of gene variants.2,4 The majority of investigations have focused on genes that codify proteins of different neurotransmitter systems, such as dopamine transporter, serotonin transporter, the two isoforms of tryptophan hydroxylase, the serotonin receptors family, and catechol-O-methyltransferase (COMT).2,5,6
The COMT gene has been repeatedly explored in SB. COMT is one of the major enzymes involved in catecholamine degradation. Its gene is located on chromosome 22q11.1-11.2, and more than 4,000 polymorphisms have been identified (https://www.genecards.org/).7–12 Human COMT contains a common functional polymorphism, G>A substitution in exon 4, which changes the amino acid at codon 108 (Val108Met) in soluble COMT or position 158 (Val158Met) in the membrane-bound COMT protein.13–16 It has been observed that the COMT enzyme that contains valine has a relatively higher activity than the COMT enzyme that contains methionine.17–21 This allele seems to be codominant with heterozygote enzyme activity following midway between the homozygous alleles.13–16
During the last few decades, many studies have analyzed the role of COMT and SB, with contradictory results. For instance, Ohara et al22 reported, in 1996, that there was no association between COMT and suicide, while the group of Pivac et al23 reported, in 2011, an association between COMT Val/Val genotype and suicide in nonalcoholic suicide completers. In addition, other studies have shown that the genotype that encodes the more active COMT enzyme (Val/Val) is more frequent in suicide attempters than in healthy subjects used as controls.24 However, other reports have found no overall difference in allele/genotype frequency distribution between cases and controls but have found that the Met allele was more frequent among violent suicide attempters.25 Several studies suggest that the Val108/158Met polymorphism of the COMT gene plays a role in numerous psychiatric disorders26–32 but have not obtained conclusive results about a relationship between SB and the COMT gene. Therefore, meta-analytic techniques that summarize all data available and assess sample size effects as well as publication bias can provide results with a major statistical power.7,14,33–36 Up to 2011, three meta-analyses had evaluated the association between the COMT gene and suicide;7,33,37 however, five more recently published studies have contributed more information to understand the complexity of the genetic background of SB. Given the importance of the COMT gene in SB, we performed an updated meta-analysis and systematic review to further explore the hypothesis of the genetic predisposition of the Val108/158Met COMT polymorphism in SB.
Materials and methods
The systematic review and meta-analysis were conducted in light of the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses. This study had been previously registered in PROSPERO (PROSPERO 2017 CRD42017070229). As no human participants or animals had been recruited, ethical approval was not required.
Literature search strategy
To identify relevant articles, we performed an online search through PubMed and Web of Science, up to March 2017, using as keywords “COMT gene AND suicide,” “rs4680 AND suicide,” and “Val108/158Met AND suicide.”
Inclusion/exclusion criteria
Relevant studies had to fulfill the following criteria: 1) studies with a case-control or cohort design; 2) studies evaluating the association between rs4680 COMT variant and suicide; 3) studies published in English and in peer-reviewed journals; 4) the genotype frequency distribution had to be cited or could be calculated; and 5) studies containing sufficient information to calculate ORs. The articles were excluded when 1) they were found to contain overlapping data, 2) the number of null and wild genotypes could not be ascertained, and 3) family members had been included in the case group because their analyses were based on linkage consideration.
Data extraction
The general information extracted from the articles included the following: first author, year of publication, country (area) of origin, study design, source of control groups (case-control studies), sample size, genotyping methods, diagnostics, methods used for the diagnostics matching variables, genotype and allele frequency distributions, and outcome findings. This key information was extracted in consensus by Hernández-Díaz and González-Castro.
Statistical analysis
The meta-analysis was carried out with the use of the Comprehensive Meta-Analysis Version 2.0 software (Biostat Inc., Englewood, NJ, USA). The analysis was performed using five genetic models (allele, dominant, recessive, homozygous, and heterozygous). For each model, the OR and 95% CI were calculated, and the results are presented as the random effects model of meta-analysis. The presence of heterogeneity was indicated when the P-value of the Q test <0.1 or I2>50%. The OR estimates for each study were used to analyze the fixed effects model (the Mantel–Haenszel method) when there was no evidence of statistical heterogeneity. Otherwise, the random effects model (the DerSimonian and Laird method) was considered. The Galbraith plot and sensitivity analysis were used to search for published studies with heterogeneity, and the meta-analysis was performed again after these published studies were excluded. The χ2 test was used to define whether the gene frequency distribution was in Hardy Weinberg Equilibrium (HWE); the studies with a P<0.5 were considered out of the HWE; therefore, they were excluded from the meta-analysis. Subgroup analyses based on location and ethnicity (Asian and European), gender (males and females), and individuals with suicide attempt (SA) were also completed. Finally, a meta-regression based on age was performed.
The power of the study was calculated as previously reported elsewhere.38 Using an effect size d=0.20 and 14 studies with 140 patients per group, we obtained a power of 0.99.
Quality assessment
The quality assessment of each study was performed using the Newcastle–Ottawa Quality Assessment Scale (NOS). A NOS >6 was considered a high-quality study. Any disparity about the NOS scores was resolved in consultation with a third reviewer.
Publication bias and sensitivity analyses
In addition, sensitivity and publication bias of the studies were evaluated. The sensitivity analysis was done by eliminating one study at a time. Begg’s test and Egger’s test were used to evaluate publication bias of the included studies; P<0.5 was considered statistically significant.
Results
Studies included
Ninety-three studies were first identified; after reviewing titles, abstracts, and compliance with the inclusion criteria, 17 studies were enrolled in this meta-analysis. A flowchart describing the inclusion/exclusion of the individual studies is displayed as Figure 1. The included studies were published between 1998 and 2016. Of these 17 studies, six evaluated European populations, six evaluated Asian descendants, three used a mixed population, and two evaluated American individuals. The genotype and frequency distributions are presented in Table 1. The studies of Schosser et al,32 Baud et al,24 and Sun et al,36 were excluded from all the analyses because their control groups presented a HWE P-value <0.05.
Overall findings
In the overall population, after discarding the studies of Nedic et al,11 and Ono et al,21 (because the sensitivity and heterogeneity analyses indicated that they favored the presence of heterogeneity between studies), the association between the Val108/158Met COMT polymorphism and susceptibility to suicide was evaluated in 3,282 cases and 3,774 controls. No significant association was observed in any of the comparisons: allele model (OR =1.05; 95% CI =0.95–1.18; Z P-value =0.29), homozygous (OR =1.15, 95% CI =0.94–1.42, Z P-value =0.16), heterozygous (OR =1.06, 95% CI =0.91–1.24, Z P-value =0.43), dominant (OR =1.15, 95% CI =0.97–1.37, Z P-value =0.10), and recessive model (OR =1.10, 95% CI =0.95–1.28, Z P-value =0.18). The Egger’s test and Begg’s funnel did not evidence publication bias; Figure 1. Our results are presented in Table 2.
  | Table 2 Meta-analysis results comparing inherence models between cases and controls |
Regarding the meta-regression based on age, the overall population analysis revealed a point estimate slope of 0.0183 and a P-value of 0.222 (Figure 2).
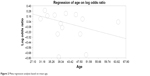  | Figure 2 Meta-regression analysis based on mean age. |
Subgroup analysis
Asian populations
Five studies were included to investigate the association between the Val108/158Met COMT polymorphism and SB in Asian populations. We used allelic, homozygous, heterozygous, dominant, and recessive models and did not find any association; however, when we discarded the studies that favored heterogeneity, we found a slight association in the allelic (OR =1.25, 95% CI =1.04–1.51, Z P-value =0.01) and recessive (OR =1.32, 95% CI =1.03–1.68, Z P-value =0.02) models; Figure 3 and Table 2.
European populations
Subsequently, using five articles, we performed an analysis of European populations. In the absence of heterogeneity, no statistical association was observed in any of the models used: allele (OR =1.00; 95% CI =0.86–1.17; Z P-value =0.95), homozygous (OR =1.01, 95% CI =0.74–1.37, Z P-value =0.93), heterozygous (OR =0.86, 95% CI =0.67–1.11, Z P-value =0.25), dominant (OR =1.06, 95% CI =0.81–1.40, Z P-value =0.64), and recessive (OR =0.94, 95% CI =0.73–1.21, Z P-value =0.65) models; Figure 1 and Table 2.
Suicide attempters
Finally, to better comprehend the role of the COMT108/158 polymorphism, we performed a subgroup analysis using 11 studies that had evaluated suicide attempters. We still found the same negative results, even when we excluded one study that supported heterogeneity: allelic (OR =1.11; 95% CI =0.99–1.23; Z P-value =0.05), homozygous (OR =1.22; 95% CI =0.97–1.55; Z P-value =0.08), heterozygous (OR =1.03; 95% CI =0.86–1.23; Z P-value =0.71), dominant (OR =1.19; 95% CI =0.98–1.44; Z P-value =0.07), and recessive (OR =1.12; 95% CI =0.96–1.31; Z P-value =0.13) models; Figure 3 and Table 2.
Male group analysis
Because of the possible influence that gender could have over the psychopathology of suicide, we evaluated the role of the COMT polymorphism in males. The analysis revealed an association when heterogeneity was absent in the allelic (OR =1.29; 95% CI =1.04–1.60; Z P-value =0.01), homozygous (OR =1.54; 95% CI =1.04–2.28; Z P-value =0.02), heterozygous (OR =1.67; 95% CI =1.17–2.38; Z P-value =0.001), and dominant (OR =1.66; 95% CI =1.18–2.31; Z P-value =0.001) models; Table 2 and Figure 4.
  | Figure 4 Forest plot in the (A) male group in the allelic model, (B) male group in the homozygous model, (C) female group in the dominant model, (D) female group in the recessive model. |
Female group analysis
Similarly, we explored the involvement of the COMT gene variants in suicide in females. Here too, we found an association when heterogeneity was absent in the allelic (OR =0.74; 95% CI =0.59–0.92; Z P-value =0.001), homozygous (OR =0.57; 95% CI =0.37–0.87; Z P-value =0.01), heterozygous (OR =0.64; 95% CI =0.43–0.95; Z P-value =0.02), dominant (OR =0.60; 95% CI =0.41–0.88; Z P-value =0.001), and recessive (OR =0.71; 95% CI =0.51–0.98; Z P-value =0.03) models; Table 2 and Figure 4.
Sensitivity analysis
We conducted a sensitivity analysis to evaluate the stability of our results by removing each study at a time. However, no obvious changes were observed, and this confirmed that our results were stable under the five genetic models for the COMT Val108/158Met polymorphism.
Discussion
Some studies support the idea that there are neurobiological and genetic risks to developing SB, but the results have been inconsistent. The inconclusive results might be due to the use of small samples and differences in ethnicity.13,39 Therefore, our aim was to further understand the effect of the COMT polymorphism in healthy individuals and suicide attempters by undertaking a systematic review and an updated meta-analysis. The current meta-analysis investigated the effect of the COMT Val108/158Met polymorphism in SB in global and subgroup analyses divided into ethnicities (Asians and Europeans), suicide attempters, and gender.
The global analysis showed no effect of the COMT polymorphism and SB in the overall population (using 14 high-quality studies); even though we used five different models, we did not observe any statistical association. On the contrary, in 2007, a meta-analysis that evaluated six studies showed a significant association between the COMT Val108/158Met polymorphism and SB; however, when one study at a time was removed from the analysis, the relationship between COMT and SB was no longer significant.14
Furthermore, when we divided the populations by location and ethnicity (Asians and Europeans), we observed an association between the Val108/158 polymorphism and SB in Asian individuals, but not in Europeans; however, it is noteworthy that statistical significance was seen only after we excluded heterogeneity and only after three studies remained; therefore, this sample size was too small to give conclusive results. We therefore recommend that more studies be undertaken in order to reach a conclusion about this relationship. The discrepancy observed among the outcomes can be explained as follows: the frequency distribution of the Val and Met alleles might be dependent on ethnicity, genetic architecture, as well as the combination of behavioral and environmental risk factors, assignment of a higher or lower risk of developing SB to a particular location or sample.17,25
Furthermore, we performed, with the five models previously indicated, another subanalysis using researches that studied only suicide attempters and observed no association. However, it has been seen that the etiology of SB is multifactorial, including biological and genetic factors that differ in each particular SB (risk of suicide, suicide ideation, SA, or completed suicide); therefore, to reach definitive conclusions, it is necessary to take this multifactorial characteristic into consideration.14,40
The negative association we observed in the global analysis agrees with the meta-analyses by Tovilla-Zárate et al33 and Calati et al,7 who also obtained negative results in the pooled ORs in the overall populations. On the other hand, the study of Sadeghiyeh et al,41 published in 2017, showed that the COMT 158G/A (COMT Val158Met) polymorphism was associated with suicide susceptibility only in females.7,14,33 Following this sense, we performed an analysis by gender; the evaluation of males using the five genetic models proposed previously revealed that Val could be a risk factor for suicide in males. Regarding the group of females, we observed a protective effect of the Val allele of COMT polymorphism, which is similar to the findings observed by Sadeghiyeh et al,41 namely, a risk effect of the Met allele in homozygous and recessive models in the female group. However, we recommend increasing the sample size in features studies in order to increase the power to detect small effects of the polymorphism.
We emphasize that the main strength of our study in comparison with previous meta-analyses published is the number of cases and controls included. Kia-Keting et al14 used 519 cases and 933 controls, Calati et al,7 1,324 cases and 1,415 controls, and Tovilla-Zárate et al,33 2,723 cases and 1,886 controls. An additional meta-analysis by Sadeghiyeh et al41 involved 2,353 suicide attempters and 2,593 controls. Meanwhile, we compared 3,282 cases and 3,774 controls. Therefore, our study has more power to detect the small effects of the polymorphism.
Our study has some limitations. First, despite the sample size we used (a large number of cases and controls), our sample is not as large as the ones evaluated in other reports that have studied psychiatric disorders such as schizophrenia or bipolar disorder.42–44 Second, we did not analyze the endophenotypes of SB. Hence, because of the available data, we could evaluate only SA, apart from SB. This is an important limitation because it has been reported that SA, suicide ideation, and death by suicide could have differences in the etiology. Third, we did not analyze environmental or biological factors that influence SB, although analyses of all variants should be performed.21,45
Conclusion
To sum up, our outcomes revealed a possible association of the COMT Val108/158 polymorphism with SB. In males, COMT Val108/158 increased the risk of SB, whereas in females, COMT Val108/158 exhibited a protective factor against SB. Also, COMT Val108/158 could be a risk factor in Asian individuals. Because of the limitation of the study, we recommend that more studies be undertaken using larger samples.
Acknowledgments
We would like to thank the authors who provided additional information on their studies. This research received no specific grant from any funding agency and commercial or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Baldessarini RJ, Hennen J. Genetics of suicide: an overview. Harv Rev Psychiatry. 2004;12(1):1–13. | ||
Mcguffin P, Perroud N, Uher R, et al. The genetics of affective disorder and suicide. Eur Psychiatry. 2010;25(5):275–277. | ||
Voracek M, Loibl LM. Genetics of suicide: a systematic review of twin studies. Wien Klin Wochenschr. 2007;119(15–16):463–475. | ||
Bondy B, Buettner A, Zill P. Genetics of suicide. Mol Psychiatry. 2006;11(4):336–351. | ||
Brezo J, Klempan T, Turecki G. The genetics of suicide: a critical review of molecular studies. Psychiatr Clin North Am. 2008;31(2):179–203. | ||
Souery D, Oswald P, Linkowski P, Mendlewicz J. Molecular genetics in the analysis of suicide. Ann Med. 2003;35(3):191–196. | ||
Calati R, Porcelli S, Giegling I, et al. Catechol-o-methyltransferase gene modulation on suicidal behavior and personality traits: review, meta-analysis and association study. J Psychiatr Res. 2011;45(3):309–321. | ||
Chen CK, Lin SK, Chiang SC, Su LW, Wang LJ. Polymorphisms of COMT Val158Met and DAT1 3′-UTR VNTR in illicit drug use and drug-related psychiatric disorders. Subst Use Misuse. 2014;49(11):1385–1391. | ||
Ikeda M, Kitajima T, Iwata N, Ozaki N. [Molecular genetics of mood disorders]. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22(5):137–143. Japanese. | ||
Nedic Erjavec G, Nenadic Sviglin K, Nikolac Perkovic M, Muck-Seler D, Jovanovic T, Pivac N. Association of gene polymorphisms encoding dopaminergic system components and platelet MAO-B activity with alcohol dependence and alcohol dependence-related phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:321–327. | ||
Nedic G, Nikolac M, Sviglin KN, Muck-Seler D, Borovecki F, Pivac N. Association study of a functional catechol-O-methyltransferase (COMT) Val108/158Met polymorphism and suicide attempts in patients with alcohol dependence. Int J Neuropsychopharmacol. 2011;14(3):377–388. | ||
Nolan KA, Volavka J, Czobor P, et al. Suicidal behavior in patients with schizophrenia is related to COMT polymorphism. Psychiatr Genet. 2000;10(3):117–124. | ||
De Luca V, Tharmalingam S, Müller DJ, Wong G, de Bartolomeis A, Kennedy JL. Gene-gene interaction between MAOA and COMT in suicidal behavior: analysis in schizophrenia. Brain Res. 2006;30(1):26–30. | ||
Kia-Keating BM, Glatt SJ, Tsuang MT. Meta-analyses suggest association between COMT, but not HTR1B, alleles, and suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1048–1053. | ||
Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signalling. Prog Neurobiol. 2010;92(2):112–133. | ||
Ozalp E. [The genetics of suicidal behavior]. Turk Psikiyatri Derg. 2009;20(1):85–93. Turkish. | ||
Cheng WW, Jia CX, Pan YF, Zhao SY, Jia GY, Hu MH, Mh H. [The relationship between gene polymorphism of catechol-O-methyltransferase and survival of oral pesticides suicide attempters]. Zhonghua Nei Ke Za Zhi. 2006;45(5):403–405. Chinese. | ||
De Luca V, Strauss J, Kennedy JL. Power based association analysis (PBAT) of serotonergic and noradrenergic polymorphisms in bipolar patients with suicidal behaviour. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):197–203. | ||
Jia CX, Zhao ZT, Hu MH, Gao LJ, Wang XT. [A paired case-control study on related factors to attempted suicide]. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(5):339–343. | ||
Ohara K, Nagai M, Suzuki Y, Ohara K. Low activity allele of catechol-o-methyltransferase gene and Japanese unipolar depression. Neuroreport. 1998;9(7):1305–1308. | ||
Ono H, Shirakawa O, Nushida H, Ueno Y, Maeda K. Association between catechol-O-methyltransferase functional polymorphism and male suicide completers. Neuropsychopharmacology. 2004;29(7):1374–1377. | ||
Ohara K, Nakamura Y, Xie DW, et al. Polymorphisms of dopamine D2-like (D2, D3, and D4) receptors in schizophrenia. Biol Psychiatry. 1996;40(12):1209–1217. | ||
Pivac N, Pregelj P, Nikolac M, et al. The association between catechol-O-methyl-transferase Val108/158Met polymorphism and suicide. Genes Brain Behav. 2011;10(5):565–569. | ||
Baud P, Courtet P, Perroud N, Jollant F, Buresi C, Malafosse A. Catechol-O-methyltransferase polymorphism (COMT) in suicide attempters: a possible gender effect on anger traits. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1042–1047. | ||
Rujescu D, Giegling I, Gietl A, Hartmann AM, Möller HJ. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol Psychiatry. 2003;54(1):34–39. | ||
De Luca V, Tharmalingam S, Sicard T, Kennedy JL. Gene-gene interaction between MAOA and COMT in suicidal behavior. Neurosci Lett. 2005;383(1–2):151–154. | ||
Lee HY, Kim YK. Gender effect of catechol-O-methyltransferase Val158Met polymorphism on suicidal behavior. Neuropsychobiology. 2011;63(3):177–182. | ||
Pasi S, Singh PK, Pandey RK, Dikshit PC, Jiloha RC, Rao VR. Evaluation of psychiatric and genetic risk factors among primary relatives of suicide completers in Delhi NCR region, India. Psychiatry Res. 2015;229(3):933–939. | ||
Perroud N, Jaussent I, Guillaume S, et al. COMT but not serotonin-related genes modulates the influence of childhood abuse on anger traits. Genes Brain Behav. 2010;9(2):193–202. | ||
Pinna M, Manchia M, Oppo R, et al. Clinical and biological predictors of response to electroconvulsive therapy (ECT): a review. Neurosci Lett. 2018;669:32–42. | ||
Russ MJ, Lachman HM, Kashdan T, Saito T, Bajmakovic-Kacila S. Analysis of catechol-O-methyltransferase and 5-hydroxytryptamine transporter polymorphisms in patients at risk for suicide. Psychiatry Res. 2000;93(1):73–78. | ||
Schosser A, Calati R, Serretti A, et al. The impact of COMT gene polymorphisms on suicidality in treatment resistant major depressive disorder – a European multicenter study. Eur Neuropsychopharmacol. 2012;22(4):259–266. | ||
Tovilla-Zárate C, Juárez-Rojop I, Ramón-Frias T, et al. No association between COMT val158met polymorphism and suicidal behavior: meta-analysis and new data. BMC Psychiatry. 2011;11(1):11–151. | ||
Schosser A, Serretti A, Souery D, et al. European Group for the Study of Resistant Depression (GSRD) – where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol. 2012;22(7):453–468. | ||
Soyka M, Zill P, Koller G, Samochowiec A, Grzywacz A, Preuss UW. Val158Met COMT polymorphism and risk of aggression in alcohol dependence. Addict Biol. 2015;20(1):197–204. | ||
Sun SH, Hu X, Zhang JY, Qiu HM, Liu X, Jia CX. The COMT rs4680 polymorphism and suicide attempt in rural Shandong, China. Psychiatr Genet. 2016;26(4):166–171. | ||
Kia-Keating BM, Glatt SJ, Tsuang MT. Meta-analyses suggest association between COMT, but not HTR1B, alleles, and suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(8):1048–1053. | ||
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: John Wiley & Sons; 2011. | ||
Du L, Merali Z, Poulter MO, Palkovits M, Faludi G, Anisman H. Catechol-O-methyltransferase Val158Met polymorphism and altered COMT gene expression in the prefrontal cortex of suicide brains. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:178–183. | ||
Blum K, Chen A, Chen TJ, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model. 2008;5:24. | ||
Sadeghiyeh T, Hosseini Biouki F, Mazaheri M, Zare-Shehneh M, Neamatzadeh H, Poursharif Z. Association between Catechol-O-Methyltransferase Val158Met (158G/A) Polymorphism and Suicide Susceptibility: A Meta-analysis. J Res Health Sci. 2017;17(2):e00383. | ||
González-Castro TB, Tovilla-Zárate CA. Meta-analysis: a tool for clinical and experimental research in psychiatry. Nord J Psychiatry. 2014;68(4):243–250. | ||
Ou J, Li M, Xiao X. The schizophrenia susceptibility gene ZNF804A confers risk of major mood disorders. World J Biol Psychiatry. 2017;18(7):557–562. | ||
Rao S, Yao Y, Ryan J, et al. Genetic association of rs1344706 in ZNF804A with bipolar disorder and schizophrenia susceptibility in Chinese populations. Sci Rep. 2017;7:41140. | ||
Liou YJ, Tsai SJ, Hong CJ, Wang YC, Lai IC. Association analysis of a functional catechol-o-methyltransferase gene polymorphism in schizophrenic patients in Taiwan. Neuropsychobiology. 2001;43(1):11–14. | ||
Antypa N, Souery D, Tomasini M, et al. Clinical and genetic factors associated with suicide in mood disorder patients. Eur Arch Psychiatry Clin Neurosci. 2016;266(2):181–193. | ||
Zalsman G, Huang YY, Oquendo MA, et al. No association of COMT Val158Met polymorphism with suicidal behavior or CSF monoamine metabolites in mood disorders. Arch Suicide Res. 2008;12(4):327–335. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



