Back to Journals » Clinical Interventions in Aging » Volume 10
The role of citicoline in cognitive impairment: pharmacological characteristics, possible advantages, and doubts for an old drug with new perspectives
Authors Gareri P, Castagna A, Cotroneo AM, Putignano S, De Sarro G, Bruni AC
Received 4 May 2015
Accepted for publication 1 July 2015
Published 3 September 2015 Volume 2015:10 Pages 1421—1429
DOI https://doi.org/10.2147/CIA.S87886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Pietro Gareri,1 Alberto Castagna,1 Antonino Maria Cotroneo,2 Salvatore Putignano,3 Giovambattista De Sarro,4 Amalia Cecilia Bruni1
1Centro Regionale di Neurogenetica, ASP Catanzaro, Lamezia Terme, Catanzaro, 2ASL2 Turin, Turin, 3Elderly Assistance Unit, Naples, 4Department of Health Sciences, School of Medicine, University Magna Græcia of Catanzaro, Catanzaro, Italy
Background: Citicoline is able to potentiate neuroplasticity and is a natural precursor of phospholipid synthesis, or rather serves as a choline source in the metabolic pathways for biosynthesis of acetylcholine. Several studies have shown that it can have beneficial effects both in degenerative and in vascular cognitive decline. The aim of the present study was to review the pharmacokinetics and pharmacodynamics of this drug and its role in cognitive impairment according to the present medical literature.
Methods: A MEDLINE® search was made using the following key words: citicoline, pharmacokinetics, pharmacodynamics, elderly, cognitive impairment, vascular dementia, and Alzheimer’s disease. Recent studies on the possible role of citicoline in increasing sirtuin 1 (SIRT1) expression were assessed. Some personal studies were also considered, such as the VITA study and the IDEALE study.
Results: Administered by both oral and intravenous routes, citicoline is converted into two major circulating metabolites, cytidine and choline. It is metabolized in the gut wall and liver. Pharmacokinetic studies suggested that it is well absorbed and highly bioavailable with oral dosing. A number of studies have clearly shown the possible role of citicoline in cognitive impairment of diverse etiology. It can also modulate the activity/expression of some protein kinases involved in neuronal death and increases SIRT1 expression in the central nervous system. The VITA study and the IDEALE study suggested that both parenteral and oral citicoline are effective and safe. Other studies have clearly demonstrated citicoline’s effects on several cognitive domains. Conversely, some studies did not point out any evidence of efficacy of this drug.
Conclusion: Citicoline appears to be a promising agent to improve cognitive impairment, especially of vascular origin. In fact, so far it appears as a drug with the ability to promote “safe” neuroprotection, capable of enhancing endogenous protective. Large clinical trials are needed to confirm its benefits.
Keywords: citicoline, cognitive impairment, elderly, neurodegeneration, neuroprotective effects, sirtuins
Corrigendum for this paper has been published
Introduction
CDP-choline (cytidine-5′-diphosphate choline) is an endogenous compound normally produced by the body. When it is introduced as a drug, it can be called citicoline. Both in animals and in human beings, citicoline has been shown to possess proved neuroprotective properties.1–3 In clinical practice, a number of different studies have clearly shown that citicoline is effective in cognitive impairment of diverse etiology, cerebrovascular disease, head trauma, glaucoma, amblyopia, and Parkinson’s disease (PD).
In this review, we are going to focus on some important points as follows:
- citicoline’s main characteristics. Where the present study starts from;
- evidence for citicoline-related neuroprotection and its role in cognitive impairment;
- personal experience: the IDEALE study and VITA study.
A number of evidences show that CDP-choline provides modest but consistent improvement in memory and behavior in cognitively impaired patients.1–3 One of the most critical points regards the relatively short term of clinical controlled observations, which in all studies but one lasted for no more than 3 months, too short a time for getting the best effects of citicoline; 6 months is the reported recommendation for getting the best outcomes.
However, citicoline is one of the most frequently prescribed drugs for cognitive impairment in several European countries.1–3
Figure 1 shows the chemical structure of this drug. It is composed of ribose, pyrophosphate, cytosine (a nitrogenous base), and choline.
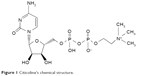  | Figure 1 Citicoline’s chemical structure. |
Citicoline inhibits apoptosis associated with cerebral ischemia and several models of neurodegeneration.1–3 It is also able to potentiate neuroplasticity and is a natural precursor of phospholipid synthesis, chiefly phosphatidylcholine, or rather serves as a choline source in the metabolic pathways for biosynthesis of acetylcholine.1–3 On a molecular basis, CDP-choline activates the biosynthesis of structural phospholipids in the neuronal membranes and increases cerebral metabolism, noradrenaline, and dopamine levels in the central nervous system (CNS).1 Furthermore, it is able to prevent the loss of cardiolipin, an exclusive inner mitochondrial phospholipid enriched with unsaturated fatty acids, which is essential for mitochondrial electron transport.1 All these evidences can explain why this drug can show neuroprotective effects in situations of hypoxia and ischemia through the restoration of mitochondrial ATPase and membrane Na+/K+ ATPase activity and the inhibition of phospholipase A2 activity. It also accelerates the reabsorption of cerebral edema in various experimental models, and since many years, it has been shown to improve learning and memory performances in animal models of brain aging (old rats with long-term memory impairment due to selective hippocampal damage, proved benefits from a dietary supplementation of CDP-choline).1,3 In particular, animal studies suggest that exogenously administered CDP-choline may protect cell membranes by accelerating resynthesis of phospholipids, thus resulting in rapid repair of injured cell surface and mitochondrial membranes. CDP-choline may also attenuate the progression of ischemic cell damage by suppressing the release of free fatty acids.2 Several studies have shown that it can have beneficial effects both in degenerative and in vascular cognitive decline.4–9
The aim of the present study was to review the pharmacokinetics and pharmacodynamics of this drug and its role in cognitive impairment according to the present medical literature.
Methods
A MEDLINE® search was made using the following key words: citicoline, pharmacokinetics, pharmacodynamics, elderly, cognitive impairment, vascular dementia, and Alzheimer’s disease (AD).
We tried to focus the possible role of citicoline and why it should work in cognitive impairment. Pharmacokinetic and pharmacodynamic studies were also reported in order to better show the possible effects of chronic administration of this drug. Furthermore, recent studies have shown the very new perspectives, that is, the probable role of citicoline in increasing sirtuin 1 (SIRT1 = silent information regulator) expression, a protein with neuroprotective effects.
Evidences from some personal studies were also considered, such as the VITA study10 and the IDEALE study.11 Finally, we have selected some studies in which citicoline has been shown to fail, trying to give possible explanations, especially when it was wrongly used; an appropriate research was also made on all the studies on its tolerability and safety.
Citicoline’s main pharmacological characteristics
Notes on citicoline pathway
CDP-choline is a water-soluble compound with >90% bioavailability. Exogenous CDP-choline (administered by both oral and intravenous routes) is hydrolyzed, absorbed as cytidine and choline, and resynthesized in the brain by cytidine triphosphatephosphocholine (CTP)-cytidylyltransferase.6
In particular, in human beings, plasma cytidine is transformed in the brain to uridine phosphate, which in turn will be converted into cytidine triphosphate at the neuronal level; plasma levels peak in a biphasic manner, at 1 hour after ingestion followed by a second peak at 24 hours postdosing. It is also metabolized in the gut wall and liver.6
Figure 2 reports the metabolic pathways of citicoline.
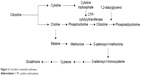  | Figure 2 Citicoline’s metabolic pathways. |
When labeled CDP-choline is administered by either the oral or parenteral route, radioactivity is eliminated very slowly by the urinary or fecal routes and in expired CO2. In particular, urinary excretion occurs through two phases: the first one lasting ~36 hours (rapid decrease of excretion rate), and a second phase (slow excretion rate).1,6,12
Evidences on the effectiveness of citicoline
Overall evidence of benefit of CDP-choline on memory function and behavior in at least the short-to-medium term was shown in a review by Fioravanti and Yanagi, where the drug was well-tolerated.2 Fourteen studies were included in this review; seven of the included studies observed the subjects for a period between 20 and 30 days, one study was of 6 weeks duration, five studies over 2 months and 3 months, and one study was prolonged up to 12 months of observation.2 However, the crucial point is the duration of citicoline administration; in fact, we think that the protection of cell membranes, neuroplasticity, the increase in acetylcholine biosynthesis, and in short, the important neuroprotective effects of this drug, require its chronic administration (at least six months for the best outcomes). So far, citicoline appears as a drug with the ability to promote “safe” neuroprotection, capable of enhancing endogenous protective pathways at the same time as preparing the scenario for plasticity. The possible mechanisms involved regard the improvement of cellular functions mainly aimed to preserve membrane function and integrity. This may occur by controlling excitotoxicity and maintaining cellular adenosine 5′-triphosphate levels.3
By far, citicoline has been shown to work in vascular cognitive impairment, vascular dementia, and AD, especially when associated with significant cerebrovascular disease.5 On the other hand, stroke can double the risk of dementia; a trial lasting 12 months in patients with first-ever ischemic stroke showed that citicoline prevented cognitive decline after stroke, with significant improvements in temporal orientation, attention, and executive functions.5
The VITA study and the IDEALE study also showed substantial benefits in vascular cognitive impairment.10,11 Other studies have clearly demonstrated citicoline’s effects on several cognitive domains.8 Indeed:
- it improves both the immediate and the delayed recall of words and objects;
- it ameliorates short- and long-term memory, attention, and perceptual-motor ability, as well as behavioral and emotional control;
- it improves verbal memory functioning in older individuals with relatively inefficient memory.
A multicentric, double-blind, crossover, randomized, comparative study vs placebo was sponsored by the Marketing Authorization Holder (MAH) Wyeth Lederle S.p.A. in 1998.2 This study was performed in 509 patients. Its aim was to assess the efficacy of CDP-choline 1,000 mg administered intramuscularly once a day in patients suffering from mild-to-moderate vascular dementia. The study showed significant differences in favor of active treatment over the placebo on delayed recall and memory index. Six 21-day cycles of treatment, with 21 days free of drug in between, three of these cycles with active treatment, and three with placebo treatment were carried on.
A better efficacy was shown in a statistically significant proportion on Clinician’s Interview-Based Impression of Change (CIBIC) scale (P<0.0001). Advantages over placebo were also found on the emotional subscale of the Gottfries-Bråne-Steen Scale (P=0.006). As regards the safety concerns, no statistical differences were found in rates or types of adverse events in the patients treated vs placebo.2 The possible roles of CDP-choline were also assessed in patients affected by AD; however, one of the most significant limitations of these studies was their short duration.
The effects of CDP-choline was studied in 19 AD patients (mean age 66.21±1.48 years); citicoline was administered at a dosage of 1,000 mg/day for 30 days and was effective in significantly improving cognitive functions, in particular in early-onset AD patients (EOAD).13 The authors concluded that the therapeutic effects of CDP-choline may be mediated by the enhancement of cholinergic neural transmission, activation of repair mechanism, regulation of several immunological responses, and attenuation of hypoperfusion patterns in cerebral blood flow.13
Caamaño et al14 investigated the effects of CDP-choline on cognition at a dosage of oral 1,000 mg/day for 1 month in 20 patients, mean age 66.7±6.73 years. Cognition was assessed by the Mini-Mental State Examination (MMSE), whereas blood flow velocities were measured by transcranial Doppler ultrasonography. Increased blood flow velocity was registered; they postulated that citicoline’s cholinergic effects influence cytokine production, and immunogenic and/or neurotrophic effects at the microvascular niche may partially account for its benefits in these individuals.14
On the other hand, we also know that excess of histamine influences some ethiopathogenic events in AD; CDP-choline was able to reduce the basal levels of blood histamine in both EOAD and late-onset Alzheimer’s disease (LOAD).15
Cacabelos et al16 showed that the administration of CDP-choline 1,000 mg/day for 3 months in AD and multi-infarct dementia improved mental performance (assessed by MMSE and Hamilton Rating Scale for Depression). The possible explanations given by the authors were that CDP-choline can improve vascular risk factors and stabilize immune function.
CDP-choline was also studied in a double-blind, placebo-controlled, randomized trial involving 30 patients with apolipoprotein E (APOE) genotyped AD. They received CDP-choline 1,000 mg orally or placebo for 12 weeks. Improvement in cognitive performance and cerebral blood perfusion in these patients (measured by transcranial Doppler recording) was found, and the drug was quite well-tolerated.17 An open-label, randomized study was performed in 347 patients in order to assess the safety of citicoline long-term administration and its possible efficacy in preventing poststroke cognitive decline in patients with first-ever ischemic stroke vs usual treatment.5 All the subjects were selected 6 weeks after suffering a qualifying stroke and randomized by age, sex, education, and stroke type into parallel arms of citicoline (1 g/day) for 12 months vs no citicoline (control group). Mean age was 67.2 years; patients were 186 men (56.6%) and 161 women (46.4%), their mean education was 5.7 years. Only 172 patients (49.6%) received citicoline for 12 months, whereas controls were 175. All the patients underwent neuropsychological evaluation at 1 month, 6 months, and 1 year after stroke. The neurocognitive domains studied were attention, executive functions, memory, language, spatial perception, motor speed, and temporal orientation. Only 37 subjects (10.7%) discontinued treatment (10.5% citicoline vs 10.9% control) at 6 months, 30 (8.6%) due to death (16 [9.3%] citicoline vs 14 [8.0%] controls, P=0.740) and seven lost to follow-up or incorrect treatment. Only four patients (2.3%) had adverse events from citicoline, but this did not require its discontinuation. Citicoline-treated patients showed better outcome in attention–executive functions (P=0.027 at 6 months; P=0.007 at 12 months) and temporal orientation (P=0.042 at 6 months; P=0.045 at 12 months) during the follow-up. Moreover, citicoline group showed a better functional outcome (modified Rankin scale ≤2) at 12 months (57.3% vs 48.7%) without statistically significant differences (P=0.186). The authors concluded that citicoline treatment for 12 months in patients with first-ever ischemic stroke is safe and probably effective in improving poststroke cognitive decline.5
Neuroprotective effects
One of the most recent findings on citicoline derives from its neuroprotective effect linked to sirtuins. Sirtuins are highly conserved NAD-dependent protein deacetylases that are able to regulate aging in lower organisms and possess important roles in centenarians18 and in age-related diseases in higher organisms.19,20
In mammals, there are seven sirtuins, SIRT1–7, which are expressed in different tissues with different cellular localizations and different enzymatic activities.19,20 SIRT1 and SIRT2 have been studied mostly in terms of neurodegenerative diseases and seem to have opposite effects. Recent evidences showed that SIRT1 is an important regulator of adult hippocampal neural stem cells (aNSCs)/adult neural progenitor cells (aNPCs) self-renewal and a potential mediator of the effect of metabolic changes.21 SIRT3 also seems to have neuroprotective effects against excitotoxic injury in mouse cortical neurons, but data are still poor.22 The human SIRT3 gene contains an intronic variable number tandem repeat (VNTR) enhancer whose variability is correlated with life span.18 Another study pointed out that SIRT3 gene expression is a link between inherited mitochondrial DNA variants and oxidative stress. The data highlighted a link between SIRT3, mitochondrial DNA variability, and mitochondrial functionality, three fundamental components of the cellular stress response.23
SIRT1 has been extensively studied in the context of several neurodegenerative diseases such as AD, PD, and Huntington’s disease (HD). Some interesting studies have also been done regarding amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Wallerian degeneration, prion diseases, and cerebral ischemia.24 Since SIRT1’s effect in almost all the aforementioned diseases is shown to be protective in different animal and cell culture models, we can postulate that any drug able to activate SIRT1 might be beneficial in more than one disease type.
SIRT1’s effects were investigated both in A and tau models of AD. By using APPswe, PSEN1dE9 model of AD, it was shown that overexpression of SIRT1 reduced Aβ plaques and improved learning and memory,25 whereas deletion of SIRT1 in the same model showed the opposite effect. Furthermore, SIRT1 deacetylates retinoic acid receptor-β (RAR-β), can activate the transcription of “the good secretase”, ADAM10 (the most important enzyme with α-secretase activity for proteolytic processing of the amyloid precursor protein), and increases its RNA and protein levels.24 This leads to upregulated amyloid protein precursor (APP) processing by α-secretase, resulting in reduced production of Aβ.26 SIRT1 deficiency is also associated with increased levels of phosphorylated tau in neurons.27
Overexpression of SIRT1 reduced Aβ production and Aβ plaques, whereas deleting SIRT1 increased Aβ levels.26
Besides animal models, many in vitro models have widely shown the protective effects of SIRT1.24,28 SIRT1 protects against microglia-dependent Aβ toxicity through inhibiting NF-kB (nuclear factor kappa-light-chain-enhancer of activated B-cells) signaling. NK-kB is a protein complex that controls DNA transcription and exerts several functions, but it has also been implicated in synaptic plasticity and memory.29
α-synuclein aggregates are reduced in the brains of mice with SIRT1 overexpression and increased by SIRT1 deletion. It was shown that SIRT1 deacetylates heat shock factor 1 (HSF1) and increases HSP70 RNA and protein levels, but only in the brains of mice with A53T and SIRT1 expressions. In other words, SIRT1 responds to α-synuclein aggregation-induced stress by activating molecular chaperones to protect against the disease.30
As regards SIRT2, and in contrast to SIRT1, there is actually no study conducted on the role and effect of SIRT2 in AD, except the SIRT2 polymorphism rs10410544 as a novel minor genetic risk factor for AD in the APOE ε4-negative Caucasian population.31 However, the research that has been conducted on SIRT2 regarding neurodegeneration so far demonstrated a general negative effect of SIRT2 in PD and HD.19 Moreover, the inhibition of SIRT2 is neuroprotective. This indicates that SIRT1 and SIRT2 work through different targets and pathways. On the other hand, these two enzymes reside in different subcellular locations and have different targets that have been identified so far. It remains to examine the effects of SIRT2 on AD models and eventually analyze whether the inhibition of SIRT2 could prevent amyloid plaque or neurofibrillary tangle formation.19
Therefore, activating SIRT1 could be beneficial against these diseases. On the other hand, deletion of SIRT2 seems to be protective against PD.26
Hurtado et al recently showed that CDP-choline increases SIRT1 protein expression in rat brain, in cultured neurons, and in circulating blood mononuclear cells, an effect that is strongly involved in the neuroprotective actions of this drug.32
In experimental models of stroke, sirtinol, a specific inhibitor of SIRT1, was shown to abolish the neuroprotective effect of CDP-choline at a concentration previously shown to be effective;33 furthermore, it does not have any effect on infarct volume in the absence of CDP-choline. To emphasize its neuroprotective effects, CDP-choline-induced reduction in infarct volume is totally abolished in the absence of SIRT1. Sirt1-/- mice display larger infarct volumes than their wild-type counterparts after being subjected to brain ischemia, thus suggesting the remarkable neuroprotective action of endogenous SIRT1 in stroke.34
Moreover, CDP-choline and the SIRT1 activator resveratrol have a potent synergistic effect leading up to 60% reductions in the infarct volume after middle cerebral artery occlusion (MCAO), when used together at doses individually subeffective of these drugs.32 As aforementioned, the neuroprotective effects following the use of CDP-choline might be explained through the prevention of fatty acid release, the stimulation of phosphatidylcholine synthesis, the preservation of cardiolipin and sphingomyelin levels, the increase in glutathione synthesis and glutathione reductase activity, the restoration of Na+/K+-ATPase activity, and the antiapoptotic effects.3
Other possible neuroprotective effects are linked to citicoline’s possible modulation of activity/expression of some protein kinases involved in neuronal death, namely, mitochondrial activated protein kinase (MAP kinase) in the postischemic brain and extracellular signal-regulated kinase 1/2 (ERK1/2) in the rat retina after kainic acid (KA) treatment.35
The effects of CDP-choline on intracellular mechanisms of signal transduction confirm its key role in recovery after ischemic stroke.
Personal experience
The IDEALE study
The “Studio di intervento nel decadimento vascolare lieve” (IDEALE study) was an open-label, multicenter, Italian study, the aim of which was to assess the effectiveness and safety of oral citicoline (1 g/day) in elderly people with mild vascular cognitive impairment.
This study was at first performed in 387 elderly patients selected from six Italian regions (Calabria, Campania, Lazio, Liguria, Piedmont, and Veneto). People included in the study were subjects aged ≥65 years, MMSE ≥21, or subjective memory complaints but no evidence of deficits on MMSE and both evidencing vascular lesions on neuroradiology. Those with probable AD and other dementias were excluded. Subjects underwent brain computed tomography (CT) or magnetic resonance imaging (MRI) of the brain, plasma dosage of vitamin B12, folate, and thyroid hormones (thyroid stimulating hormone, free triiodothyronine, free tetraiodothyronine, thyroid peroxidase antibodies, and thyroglobulin antibodies). Functional dependence was investigated by scores on the Activities of Daily Living (ADL) and Instrumental Activities of Daily Living (IADL) Scales. Mood was investigated by the Geriatric Depression Scale (GDS) and behavioral disorders by the Neuropsychiatric Inventory (NPI) Scale. Comorbidity was assessed using the Cumulative Illness Rating Scale (CIRS), a test assessing the number and severity of diseases in individual patients. All the patients gave their written informed consent.11
A total of 349 patients met our criteria and completed the study. They were assigned to open-label treatment with oral citicoline 500 mg twice a day in a fasting state or to no treatment (controls). Therefore, the treatment group included 265 patients, being 122 men and 143 women of mean age 79.9±7.8 (range: 65–94) years. The control group included 84 patients, 36 men, and 48 women of mean age 78.9±7.01 (range: 67–90) years.11
An assessment was made at baseline (T0), after 3 months (T1), and after 9 months (T2, in other words, 6 months after T1). The main outcomes were changes in MMSE, ADL, and IADL scores in the study group compared with the controls. Side effects were also investigated.
Statistical analysis was performed through the Student’s t-test or the chi-square test. Repeated measures analysis of variance were used to assess the difference in changes at the baseline, T1, and T2. Significant differences were assumed to be present at P<0.05. All analyses were performed using the Statistical Package for the Social Sciences software program version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).11
The MMSE score in the treated group remained essentially unchanged over time (22.4±4 at T0; 22.7±4 at T1; 22.9±4 at T2). A mild improvement of 0.5 points on average was found during the 9 months of the study. Importantly, the untreated group showed a decline in MMSE score over the 9 months (21.5 at T0; 20.4 at T1; 19.6 at T2; -1.9 points between T0 and T2). Furthermore, a significant difference in MMSE scores was found between the treatment and control groups at T1 (P<0.0001) and T2 (P<0.0001) time points, but not between T0 and T1 or between T0 and T2 in the active group. No differences were found for ADL and IADL scores between the two groups.11 A slight difference in GDS score was found between the study and control groups (P=0.06), probably depending on the citicoline-induced increase in noradrenaline and dopamine levels in the brain. No significant adverse events were recorded over time.11
The IDEALE study was one of the few trials conducted for a period longer than 6 months. A number of studies have shown that citicoline’s bioavailability is very good following oral administration.12,36,37 With regard to its mechanism of action, we suggest that the most pronounced benefits of treatment with citicoline are probably due to the activation of biosynthesis of phospholipids in neuronal membranes, the increase in brain metabolism, and the neuroprotective effects during hypoxia and ischemia. Furthermore, they are most likely to be accrued with prolonged use. This is confirmed by the positive results in our treated group and by the decrease in MMSE scores in the control group at only 9 months, after starting the study.
The VITA study
The VITA study was aimed to evaluate the safety, tolerability, and efficacy of citicoline administered intravenously 2 g/day in geriatric syndrome with a complex clinical picture of confusion on a retrospective and observational basis.10
The study involved ten centers throughout the country after performing geriatric home visits. Patients over the age of 65 who could not be hospitalized, with moderate-to-severe neurological deficits due to cerebral ischemia, were enrolled.14,17,38,39 The following scales were administered: the National Institute of Health Stroke Scale (NIHSS),40,41 Rankin Scale (modified version), and Barthel Index.42 The enrolled patients needed to have NIHSS 8–14/>15, Rankin Scale 4–5, and Barthel Index 40–20/<20. The study lasted 6 months and was divided into three phases. During the first phase that lasted 4 months, patients were enrolled and treated with 2 g of citicoline through a slow intravenous infusion in saline 500 mL for 5 days, to be repeated for 5 more days in the case of nonresponders. In the second phase (T2), after clinical reassessment and verification of side effects and tolerability, (Step A) treatment with intramuscular citicoline 1,000 mg was administered and it was repeated for 21 days (Step B) after 7 days interruption. During the third and final phase (T3), the results were evaluated.10
A total of 272 patients were enrolled, and 197 of them were recruited and completed treatment; of them, 55 (20%) were given the treatment scheduled during Stage T1, Step B. Seventy-five patients (27%) dropped out of the study, of whom five (1.8%) refused to continue treatment, and 70 (25%) gave up the study protocol or dropped out for other reasons. The average age of the enrolled sample was 81.5 years; five subjects (2.5%) aged 60–69 years, 78 subjects (39.6%) aged 70–79 years, 106 subjects (53.8%) aged 80–89 years, and eight subjects (4.1%) over 90 years old. The results were compared with a control group whose mean age was 86.7 years; it included eight men (54%) and seven women (46%). The control group was treated with intravenous administration of saline (500 mL) and glucose 5% (500 mL).10
Citicoline was shown to be effective in the complex geriatric syndrome complicated by confusion due to the worsening of general health conditions for the NIHSS during T1–T2, the Rankin Scale during T1–T2–T3, and ADL Scale during T1–T2. There was no significance in IADL scores. More specifically, in subacute ischemic cerebrovascular disease, administration of citicoline at the intravenous dose of 2 g in 500 mL of saline for 5 or 10 days has proven to be effective in improving functional independence and in reducing the burden of care. After 5 days (80% of cases), 10 days (20% of cases; T2), or 2 months (T3) since the beginning of treatment, there was an improvement in key measures of performance. This was more evident in the younger old-age groups. The most important and significant positive impact was evident in the results of the oldest age groups.
No major side effects were recorded in any phase of the study.10 In conclusion, the results must cautiously consider the poor sample size, the short follow-up, and its features of being a retrospective and observational study.
Poor results following citicoline use
The Citicoline Brain Injury Treatment Trial (COBRIT) was a Phase III, double-blind, randomized, clinical trial conducted between 2007 and 2011 among 1,213 patients at eight US level 1 trauma centers, in order to investigate the effects of citicoline vs placebo in patients with traumatic brain injury (TBI), classified as complicated mild, moderate, or severe.43 The study lasted 90 days, and the patients were administered oral citicoline (2 g) or placebo. Main outcomes were related to functional and cognitive status and examination of the long-term maintenance of treatment effects. The citicoline and placebo groups did not differ significantly at the 90-day evaluation (global odds ratio [OR], 0.98 [95% CI, 0.83–1.15]). No significant treatment effect was pointed out in the two severity subgroups (global OR, 1.14 [95% CI, 0.88–1.49] and 0.89 [95% CI, 0.72–1.49] for moderate/severe and complicated mild TBI, respectively).43 At the 180-day evaluation, the citicoline and placebo groups did not differ significantly with respect to the primary outcome (global OR, 0.87 [95% CI, 0.72–1.04]). In conclusion, among patients with TBI, the use of citicoline compared with placebo for 90 days did not result in improvement in functional and cognitive status.43
Furthermore, citicoline was not successful in another large trial, including poststroke patients.44 It was a randomized, double-blind, placebo-controlled, sequential trial performed in patients with moderate-to-severe acute ischemic stroke admitted at university hospitals in Germany, Portugal, and Spain.44 Patients were randomly assigned in a 1:1 ratio to receive citicoline or placebo within 24 hours after the onset of symptoms (1 g every 12 hours intravenously) during the first 3 days and orally thereafter for a total of 6 weeks (500 mg oral tablets twice a day given every 12 hours). The primary outcome was recovery at 90 days measured by NIHSS ≤1, modified Rankin score ≤1, and Barthel Index ≥95. Safety endpoints included symptomatic intracranial hemorrhage in patients treated with recombinant tissue plasminogen activator, neurological deterioration, and mortality. A total of 2,298 patients were enrolled in the study from 2006 to 2011. Of the 2,298 patients who gave informed consent and underwent randomization, 1,148 were assigned to citicoline and 1,150 to placebo. The trial was stopped for futility at the third interim analysis on the basis of complete data from 2,078 patients. Global recovery was similar in both the groups (P=0.364), and no significant differences were reported in the safety variables or in the rate of adverse events. The ICTUS trial showed that citicoline is not effective in the treatment of moderate-to-severe acute ischemic stroke.44 However, in both the studies, the time of administration was short. We postulate that citicoline’s neuroprotective effects should need a longer time of administration.
Overview of safety
In terms of safety, choline has a low level of toxicological concern; moreover, the administration of choline with cytidine in the form of CDP-choline lowers the toxicity index by an additional 20-fold.45 Occasional digestive intolerance and occasional excitability or restlessness has been reported in the first few days of treatment (especially following parenteral administration).6
Self-limiting headache, tingling sensation, and numbness have been occasionally reported.46 No clinically significant ECG and EEG abnormalities were ever registered after citicoline administration. In 2,817 patients of all ages, but with a predominance of patients aged between 60 and 80 years old who had different neurological conditions, only 5.01% of the patients experienced side effects, mostly digestive intolerance. However, in no case, treatment had to be interrupted.36
Importantly, using citicoline and levodopa together allows a significant reduction of the levodopa dose, thus minimizing side effects of levodopa treatment.37
Conclusion
CDP-choline (also named citicoline) has shown to possess beneficial physiological actions on cellular function. CDP-choline and its hydrolysis products play important roles in phospholipid synthesis and neuronal repair. Considering the great amount of published studies and assessing the pros and cons, it appears to be effective in cognitive impairment of any kind, especially of vascular origin. This result was also widely shown in our personal experience regarding the VITA study and the IDEALE study.
The evidence is definitely strong, although limited by the duration of studies, so it would be appropriate to perform more long-term studies. It can be assumed that long-term treatment is safe orally, intramuscularly, and intravenously (500 mg–2 g/day) because serious side effects have never been reported. Furthermore, neither ECG nor EEG changes nor significant systemic cholinergic effects have ever been reported.
Importantly, citicoline has been recently shown to increase SIRT1 protein expression and this is strongly involved in its neuroprotective actions.
Some studies have shown poor results following citicoline administration, even if this could be due to its short time of administration.
Also, it would be interesting to study whether the use of citicoline in association with cholinesterase inhibitors may help in delaying the progression of AD. It might be the possible target of future studies.
Disclosure
The authors report no conflicts of interest in this work.
References
Secades JJ, Frontera G. CDP-choline: pharmacological and clinical review. Methods Find Exp Clin Pharmacol. 1995;17(suppl B):1–54. | ||
Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev. 2005;18(2):CD000269. | ||
Hurtado O, Lizasoain I, Moro MÁ. Neuroprotection and recovery: recent data at the bench on citicoline. Stroke. 2011;42(suppl1):S33–S35. | ||
Grieb P. Neuroprotective properties of citicoline: facts, doubts and unresolved issues. CNS Drugs. 2014;28(3):185–193. | ||
Alvarez-Sabín J, Ortega G, Jacas C, et al. Long-term treatment with citicoline may improve poststroke vascular cognitive impairment. Cerebrovasc Dis. 2013;35(2):146–154. | ||
Secades JJ. Citicoline: pharmacological and clinical review, 2010 update. Rev Neurol. 2011;52(suppl 2):S1–S62. | ||
Alvarez-Sabin J, Roman GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke. 2011;42:S40–S43. | ||
García-Cobos R, Frank-García A, Gutiérrez-Fernández M, Díez-Tejedor E. Citicoline, use in cognitive decline: vascular and degenerative. J Neurol Sci. 2010;299(1–2):188–192. | ||
Fioravanti M, Buckley AE. Citicoline (Cognizin) in the treatment of cognitive impairment. Clin Interv Aging. 2006;1(3):247–251. | ||
Putignano S, Gareri P, Castagna A, et al. Retrospective and observational study to assess the efficacy of citicoline in elderly patients suffering from stupor related to complex geriatric syndrome. Clin Interv Aging. 2012;7:113–118. | ||
Cotroneo AM, Castagna A, Putignano S, et al. Effectiveness and safety of citicoline in mild vascular cognitive impairment: the IDEALE study. Clin Interv Aging. 2013;8:131–137. | ||
Secades JJ, Lorenzo JL. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006;28(suppl B):1–56. | ||
Franco-Maside A, Caamaño J, Gómez MJ, Cacabelos R. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16(8):597–607. | ||
Caamaño J, Gómez MJ, Franco A, Cacabelos R. Effects of CDP-choline on cognition and cerebral hemodynamics in patients with Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16(3):211–218. | ||
Fernández-Novoa L, Alvarez XA, Franco-Maside A, Caamaño J, Cacabelos R. CDP-choline-induced blood histamine changes in Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16(4):279–284. | ||
Cacabelos R, Alvarez XA, Franco-Maside A, Fernández-Novoa L, Caamaño J. Effect of CDP-choline on cognition and immune function in Alzheimer’s disease and multi-infarct dementia. Ann N Y Acad Sci. 1993;695:321–323. | ||
Alvarez XA, Mouzo R, Pichel V, et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find Exp Clin Pharmacol. 1999;21(9):633–644. | ||
Bellizzi D, Dato S, Cavalcante P, et al. Characterization of a bidirectional promoter shared between two human genes related to aging: SIRT3 and PSMD13. Genomics. 2007;89(1):143–150. | ||
Donmez G. Sirtuins as possible targets in neurodegenerative diseases. Curr Drug Targets. 2013;14:644–647. | ||
Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. | ||
Ma CY, Yao MJ, Zhai QW, Jiao JW, Yuan XB, Poo MM. SIRT1 suppresses self-renewal of adult hippocampal neural stem cells. Development. 2014;141:4697–4709. | ||
Kim SH, Lu HF, Alano CC. Neuronal SIRT3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS One. 2011;6:e14731. | ||
D’Aquila P, Rose G, Panno ML, Passarino G, Bellizzi D. SIRT3 gene expression: a link between inherited mitochondrial DNA variants and oxidative stress. Gene. 2012;497(2):323–329. | ||
Zhang F, Wang S, Gan L, et al. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95:373–395. | ||
Donmez G, Wang D, Cohen D, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. | ||
Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med. 2013;5:344–352. | ||
Min SW, Cho SH, Zhou Y, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. | ||
Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. TIPS. 2012;33(9):494–501. | ||
Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kB in hippocampal synaptic plasticity. Synapse. 2010;35(2):151–159. | ||
Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 protects against α-synuclein aggregation by activating molecular chaperones. J Neurosci. 2012;32(1):124–132. | ||
Polito L, Kehoe PG, Davin A, et al. The SIRT2 polymorphism rs10410544 and risk of Alzheimer’s disease in two Caucasian case control cohorts. Alzheimers Dement. 2013;9(4):392–399. | ||
Hurtado O, Hernández-Jiménez M, Zarruk JG, et al. Citicoline (CDP-choline) increases Sirtuin1 expression concomitant to neuroprotection in experimental stroke. J Neurochem. 2013;126:816–819. | ||
Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family and NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. | ||
Hernández-Jiménez M, Hurtado O, Cuartero MI, et al. Silent information regulator 1 (SIRT1) protects the brain against cerebral ischemic damage. Stroke. 2013;44(8):2333–2337. | ||
Krupinski J, Slevin M, Badimon L. Citicoline inhibits MAP kinase signalling pathways after focal cerebral ischaemia. Neurochem Res. 2005;30(8):1067–1073. | ||
Lozano-Fernandez R. Efficacy and safety of oral CDP-choline. Drug surveillance study in 2,817 cases. Arzneimittelforschung. 1983;33(7A):1073–1080. | ||
Secades JJ. CDP-choline: update and review of its pharmacology and clinical use. Methods Find Exp Clin Pharmacol. 2002; 24(suppl B):1–53. | ||
Cacabelos R, Caamaño J, Gómez MJ, Fernández-Novoa L, Franco-Maside A, Alvarez XA. Therapeutic effects of CDP-choline in Alzheimer’s disease. Cognition, brain mapping, cerebrovascular hemodynamics, and immune factors. Ann N Y Acad Sci. 1996; 777:399–403. | ||
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. | ||
Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25(11):2220–2226. | ||
Kasner SE, Chalela JA, Luciano JM, et al. Reliability and validity of estimating the NIH stroke scale score from medical records. Stroke. 1999;30(8):1534–1537. | ||
Sulter G, Steen C, De Keyser J. Use of the Barthel Index and modified Rankin Scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. | ||
Zafonte RD, Bagiella E, Ansel BM, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: citicoline brain injury treatment trial (COBRIT). JAMA. 2012;308(19):1993–2000. | ||
Dávalos A, Alvarez-Sabín J, Castillo J, et al; International Citicoline Trial on acUte Stroke (ICTUS) trial investigators. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet. 2012;380(9839):349–357. | ||
D’Orlando KJ, Sandage BW Jr. Citicoline (CDP-choline): mechanism of action and effects in ischemic brain injury. Neurol Res. 1995;17:281–284. | ||
Dinsdale JR, Griffiths GK, Castelló J, Maddock J, Ortiz JA, Aylward M. CDP-choline: repeated oral dose tolerance studies in adult healthy volunteers. Arzneimittelforschung. 1983;33(7A):1061–1065. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
