Back to Journals » Journal of Inflammation Research » Volume 15
The Role of Bone Morphogenetic Protein 4 in Microglial Polarization in the Process of Neuropathic Pain
Authors Liu C, Sun Q, Xu J, Shen W, Li H , Yang L
Received 12 January 2022
Accepted for publication 27 April 2022
Published 3 May 2022 Volume 2022:15 Pages 2803—2817
DOI https://doi.org/10.2147/JIR.S356531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Changqing Liu,1,2,* Qi Sun,1,2,* Junmei Xu,1,2 Weiyun Shen,1,2 Hui Li,1,2 Lin Yang1,2
1Department of Anesthesiology, Second Xiangya Hospital, Central South University, Changsha, Hunan Province, People’s Republic of China; 2Hunan Province Center for Clinical Anesthesia and Anesthesiology, Research Institute of Central South University, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Li, Department of Anesthesiology, Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China, Fax +86 85295970, Email [email protected] Lin Yang, Department of Anesthesiology, Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China, Fax +86 85295970, Email [email protected]
Background: Neuropathic pain (NP) is known to be highly correlated with microglial polarization, of which the regulatory mechanism remains to be elucidated. Here, the aim of this study is to further investigate the relationship between bone morphogenetic protein 4 (BMP4) and microglial polarization in the process of NP.
Methods: Firstly, normal adult rats received intrathecal BMP4 administration to assess BMP4ʹs effect on microglial polarization. Secondly, a BMP4 antagonist – Noggin – was applied to a rat NP model achieved by L5 spinal nerve ligation (SNL) to investigate whether antagonizing BMP4 signaling could alleviate allodynia by reversing the imbalance of the M1/M2 polarization ratio. In both experiments, Von-Frey filaments were used to test the changes in the paw withdrawal threshold (PWT), and Western blotting, immuno-fluorescence, PCR and flow cytometry were further performed to investigate microglial activity and the expression patterns of M1 and M2 markers, respectively.
Results: Firstly, BMP4 administration induced a significant PWT decrease and microglial activation in normal rats; Western blotting, PCR and flow cytometry further revealed that M1 markers including CD16, MHCII, and TNF-α showed a marked elevation after BMP4 application; while M2 markers, such as Arg-1, CD204 and IL-4, peaked at an early stage (P1 or P4) and then fell to the Sham level on P7, leading to a persistent imbalance of the M1/M2 ratio throughout the 1st week. Secondly, Noggin treatment significantly relieved allodynia and microglial activation in SNL rats. Moreover, Noggin persistently downregulated the M1 marker levels and simultaneously induced a late-stage elevation of M2 markers expressions, thereby reversing the imbalance of the M1/M2 polarization ratio.
Conclusion: Our results indicate that BMP4 has the ability to induce microglial polarization. Antagonizing BMP4 signaling can relieve pain behavior via mitigating microglial activation and reversing the imbalance of the M1/M2 polarization ratio in the process of NP.
Keywords: bone morphogenetic protein-4, Noggin, microglia, polarization, neuropathic pain
Introduction
Chronic pain, especially the neuropathic pain (NP), has become an increasing health problem, and the treatment remains challenging.1 It2,3 has been now well demonstrated that neuron-glia (especially microglia and astrocyte) reactions play a critical role in the initiation and maintenance of NP. Microglia are the resident immune cells in the central nervous system (CNS). Once triggered by noxious insults, microglia will be initially activated3–5 and polarized into two main subsets: the “classical activation” M1 type and the “alternative activation” M2 type.6 In general, M1 microglia, characterized by the secretion of pro-inflammatory cytokines, can further induce astrocyte activation and aggravate pain severity; In comparison, M2 microglia exert neuroprotection and pain relief mainly through the expression of anti-inflammatory factors. Importantly, an impressive body of studies6–8 has revealed that NP is characterized with excessive M1 polarization, together with inadequate or gradually dissipated M2 polarization. This imbalance of M1/M2 ratio will increase the inflammatory response in the CNS and finally contribute to the central sensitization. Besides, comprehensive microglial inhibitor without distinguishing their states may not be an optimal choice due to the compromise of the M2 protective functions.2 Therefore, therapeutic strategies targeting the switching of the pattern of microglial polarization should be recommended.
Bone morphogenetic proteins (BMPs) belong to the transforming growth factor β (TGF-β) superfamily and contain at least 20 members.9 Among these, BMP4 has been demonstrated to play a diverse role in regulating glial activity including oligodendrocyte inhibition and astrocyte activation following spinal cord injury.10,11 Nevertheless, the relationship between BMP4 and microglial activity still remains to be elucidated. A previous study12 has shown that the specific receptors of BMP4 are expressed in microglia in normal adult spinal cord, suggesting BMP4ʹs potential role in microglial activity. Moreover, one of our recent research projects13 has already demonstrated that endogenous BMP4 is constantly upregulated in the spinal cord during the early phase of NP, which is in coincidence with the time window of microglial activation; Besides, intrathecal administration of exogenous BMP4 appears to be sufficient to induce allodynia in normal adult rats. Based on these, we hypothesize that BMP4 might have the ability to regulate microglial polarization, thereby playing a role in the pathological process of NP initiation.
In this study, we aim to investigate the effect of BMP4 on microglial polarization and pain behavior in normal rats. Furthermore, we test whether Noggin–an antagonist of BMP4–could alleviate allodynia in NP rats by switching the imbalance of the M1/M2 ratio. These findings may provide an understanding of the role of BMP4 in microglial polarization, which could help to unravel the molecular basis of NP.
Method
Animals
Young adult male Sprague-Dawley rats (8 weeks old, 180–200 g) were purchased from Silaike Jingda Experimental Animal Co., Ltd (Changsha, Hunan, CHN). Random groups of 4–5 rats were placed in one cage each and housed in a temperature (22–25°C) and humidity (40–50%) controlled and 12/12h light/dark cycle room. Animals were provided with food and water freely and were allowed to acclimatize to the circumstances for 1 week before the experiments started. All procedures and practices were approved by the Institutional Animal Care and Use Committee of the Central South University (approval number: 2021198) and were conducted according to the Health Guidelines of National Institutes for the Use and Care of Laboratory Animals.
Animal Grouping
Sample size and selections for the concentrations of exogenous BMP4 and Noggin were decided according to previous researches13,14 and our pre-experimental data (not included in the present study). Totally, 194 rats were enrolled and 14 of them were excluded due to the failure to meet the standard after accepting SNL or intrathecal injections (seen as below). In order to test the exogenous effect of BMP4 on microglial polarization, 90 rats were randomly divided into two groups: ①Sham group (n=45): rats received 10 μL sterile phosphate buffer solutions (PBS), twice a day; ②BMP4 group (n=45): rats received 10 ng of BMP4 (120–05ET, PeproTech, NJ, US) dissolved in 10 μL sterile PBS, twice a day. In order to assess the effect of antagonizing BMP4 signaling on SNL-induced allodynia and microglial polarization, another 90 rats were divided into three groups: ①Sham group (n=18): rats went through L5 spinal nerve exposure (but not ligated), followed by intrathecal administration of 10μL sterile PBS, twice a day; ②SNL group (n=36): rats went through SNL, followed by intrathecal administration of 10 μL sterile PBS, twice a day; ③SNL+NOG group (n=36): rats went through SNL, followed by intrathecal administration of 100 ng of Noggin (120–10C, PeproTech) dissolved in 10 μL sterile PBS, twice a day.
Nine rats in each group were used for behavior test in the consecutive 7 days following surgical procedure; while other animals were euthanized at postoperative day 1 (P1), P4 and P7 after intrathecal injections. Then, the lumbar enlargements of the spinal cord were harvested for further analysis.
Behavioral Testing
The paw withdrawal threshold (PWT) was tested for each rat using Von-Frey filaments (Stoelting, IL, US) according to our previous research.13 All tests were conducted at a fixed time (15:00–17:00) and recorded by a researcher who was blinded to the group assignments. Each rat was placed separately in a wire mesh cage and left undisturbed for 30 minutes for acclimatization. Then, the plantar surface of the rat’s hindlimb was pricked with filaments decreasing in weight from 15.0 g to 0.4 g. A positive sign was identified as a rapid shaking, pulling back or licking of the hindlimb within 5 seconds. Then, the up and down method was applied to determine the 50% PWT.
Intrathecal Catheterization
Intrathecal catheterization was conducted according to Martin’s research.15 Briefly, the rats were anesthetized with pentobarbital (30 mg/kg, Intraperitoneal) and placed in a prone position. A sterile PE-10 tube (Smith, OH, US) was inserted through the L4-5 intervertebral space to the lumbar enlargement. Then, an intradermal tunnel was built to fix the catheter at the midline of neck, parallel to the ears. Clear cerebrospinal fluid drainage through the tube was considered to be a sign of successful catheterization. Three days later, the catheter location was reconfirmed with a lidocaine test before accepting further interventions. During the experiments, 6 rats were excluded: four were withdrawal due to the significant decline of PWT (catheter-induced nerve injury), and another two were excluded because of the failure to show immediate hind limb paralysis after lidocaine application (catheter displacement).
Spinal Nerve Ligation (SNL)
SNL was performed according to the method described by Chung et al.16 Briefly, the rats were anesthetized and placed in a prone position, followed by the removal of the left L6 transverse process and its neighboring articular processes. Then, the L5 spinal nerve was isolated and tightly ligated using a 6–0 silk thread. Eight rats were excluded from the experiment: Five of them failed to show allodynia (the L5 spinal nerve ligation might not be tight enough); the other three developed evident left hindlimb paralysis (the neighboring L4 spinal nerve was likely to be injured).
Western Blotting
Rats (n=3 for each group at each time point) were deeply anesthetized with overdose of pentobarbital (100 mg/kg, Intraperitoneal) and sacrificed. Lumbar enlargements of the spinal cord were harvested, homogenized in a mixture of RIPA lysis buffer containing proteinase inhibitor (Roche, Mannheim, GER), and centrifuged at 4°C at 12,000 rpm for 15 min in order to collect the supernatants. Then, the protein concentrations were tested using the bicinchoninic acid (BCA) method. After that, each sample, containing 20 μg protein, was loaded into 8% or 10% SDS-polyacrylamide gels for electrophoresis, then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA, US). The membrane was blocked with 5% non-fat dried milk for 1 hour and immunoblotted overnight at 4°C with primary antibodies, including rabbit anti-CD11b (DF7553, 1:1000, Affinity, OH, US), anti-CD16 (DF7007, 1:1000, Affinity), anti-ARG1 (DF6657, 1:500, Affinity) and anti-GAPDH (60004-1-Ig, 1:6000, Proteintech, IL, US), followed by HRP-conjugated goat anti-rabbit IgG (SA00001-2, 1:6000, Proteintech) for 1 hour at room temperature. Each membrane was washed three times and processed with Luminol Reagent (Millipore, MA, US) and exposed to an MP-ECL film in a dark room. All of the films were then scanned and analyzed with Image-Pro Plus 6.0 (Rockville, MD, US).
Immunofluorescence
Rats (n=3 for each group at each time point) were deeply anesthetized and then perfused transcardially with 200 mL cold saline followed by 300 mL 4% cold paraformaldehyde (PFA). Next, lumbar enlargements were collected and fixed in PFA for 8 hours and cryoprotected in 30% sucrose. Samples were further embedded in OCT medium (Torrance, CA, USA) and cut into 8-μm-thick sections. All sections were rinsed in 0.2%Triton X-100 mixed with 5% donkey serum for 40 min at room temperature and then incubated overnight with primary antibodies at 4°C at the following dilutions: mouse anti-CD11b (SM262PS, 1:50, OriGene, MD, US), rabbit anti-CD16 (1:50, Affinity) and rabbit anti-ARG1 (1:100, Affinity). This was followed by incubation with a mixture of Alexa Fluor 594-conjugated AffiniPure Donkey Anti-Rabbit IgG (711-585-152, 1:500, Jackson ImmunoResearch, PA, US) and Alexa Fluor 488-conjugated AffiniPure Donkey Anti-Mouse IgG (715-547-003, 1:500, Jackson ImmunoResearch). After that, sections were washed, stained with DAPI, and sealed. Fluorescence microscopy (Nikon Eclipse E600, Japan was used to capture the images, and Image-Pro Plus 6.0 (MD, US) was used to calculate the intensity).
Real-Time PCR (RT-PCR)
Lumbar enlargements (n=3 for each group at each time point) were deeply anesthetized and were rapidly harvested on ice, homogenized and treated with TrizolTM Reagent (DP424, Tiangen Biotech, Beijing, CHN) to extract the total RNA. The cDNA for each sample was synthesized using a reverse transcription kit (KR118, Tiangen Biotech, Beijing, CHN). RT-PCR was performed with SYBR®Green Real-Time PCR Master Mixes (E096, Novo, Shanghai, CHN) using using CFX96 TouchTM Deep Well Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, US). All the primers are listed in Table 1. The cycling conditions were set as 95°C for 1 min, followed by 40 cycles of 95°C for 20s→50-60°C (depending on the primers) for 20s→72°C for 30s. GAPDH was used to standardize each gene expression. Data of Cycle Threshold (CT) in each sample then were processed using the 2−ΔΔCt method.
 |
Table 1 Primers for Each Gene for PCR Analysis |
Flow Cytometry
Lumbar enlargements (n=3 for each group at each time point) were harvested, washed and immediately filtered through a 40 μm nylon cell strainer (BD Biosciences, NY, US). Then, cells in the suspension were separated from myelin and debris through centrifugation using Percoll density gradient medium (70% and 30%, GE Healthcare, CT, US) and then carefully collected from the interface of the gradient. Then, cells were stained using the following antibodies or the respective isotype controls for 30 min on ice: Alexa Fluor 647 anti-rat CD86 (200314, 1:100, Biolegend, CA, US), PE anti-rat CD172a (SIRPα) (204706, 1:100, Biolegend) and PerCP/Cyanine5.5 anti-rat CD11b/c (201820, 1:100, Biolegend). After rinsing, the cells were tested using flow cytometry (Cytek, CA, USA) and the data were analyzed with FlowJo vX0.7 software.
Statistical Analysis
Continuous data were presented as mean ± standard deviation. Two analyses were performed to investigate BMP4ʹs effect on pain behavior and microglial polarization. Firstly, behavior tests were analyzed with two-way ANOVA, followed by a Bonferroni test. Secondly, protein expressions, gene levels, fluorescence intensity and cytometric data were assessed with one-way ANOVA, followed by Sidak’s multiple comparisons test at each time point.
All data were analyzed and charted with SPSS (version 19.0, IBM, NY, US) and Prism software (version 8.0.1, GraphPad Inc., CA, USA). Results were considered to be significant when P<0.05.
Results
Exogenous BMP4 Induced Allodynia, Microglial Activation and M1/M2 Polarization
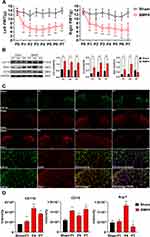
We have previously reported13 that the intrathecal administration of exogenous BMP4 can induce allodynia in normal rats. Similarly, in the present study, we also observed a prominent decrease in bilateral PWT in rats of the BMP4 group, starting from P1 (left: 7.18±1.62 g; right: 7.59±1.87 g, P<0.01) and lasting for the whole week (P7: left: 6.63±1.21 g; right: 4.88±3.06 g, P<0.01). In contrast, rats in the Sham group basically maintained at the untreated level (left and right: about 15 g) during the 1st week (Figure 1A).
Next, we investigated whether exogenous BMP4 could regulate microglial activity and polarization in the normal adult spinal cord. Firstly, the Western blotting results showed a marked upregulation of CD11b expressions in the BMP4 group compared with the Sham group, which lasted from P1 (2.07-fold, P<0.01) to P7 (2.08-fold, P<0.01). Similarly, the level of CD16, a characterized M1 marker, also elevated at P1 (2.77-fold, P<0.01) and lasted until P7 (2.26-fold, P<0.01) in the BMP4 group; Meanwhile, the expressions of ARG-1 (a recognized M2 marker) in the BMP4 group increased at P1 (2.85-fold, P<0.01) and P4 (4.57-fold, P<0.01), but then fell to the Sham level at P7 (0.69-fold, P<0.01) (Figure 1B). Secondly, the immunofluorescence further identified that CD11b positive cells were markedly increased in the BMP4 group, which were abundantly located at the bilateral dorsal horn of the spinal cord. Moreover, CD16 and ARG-1 showed the same expression patterns with the Western blotting results, and were both mainly accumulated in the CD11b positive cells (Figure 1C and D).
Exogenous BMP4 Induced Different Time Courses of M1 and M2 Polarized Genes
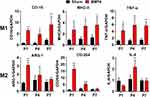
We further determined the precise pattern of microglial polarization by analyzing the expression patterns of series of M1/M2 genes using RT-PCR. As shown in Figure 2, all chosen M1 gene markers, including CD16, TNF-α and MHC-II mRNA, showed robust elevation at P1 (CD16: 8.14-fold, P<0.01; TNF-α: 3.52-fold, P=0.010) or P4 (MHC-II: 3.02-fold, P=0.015), and remained at a higher level at P7 (CD16: 4.10-fold, P<0.01; TNF-α: 3.84-fold, P<0.01; and MHC-II: 2.62-fold, P=0.017) in the BMP4 group compared with the Sham group. In contrast, M2 gene markers, such as ARG-1, CD-204 and IL-4, also increased at P1 (ARG-1:3.14-fold, P<0.01; CD-204: 14.99-fold, P<0.01) or P4 (IL-4: 1.98-fold, P<0.01), but then decreased rapidly and fell to the Sham levels at P7 in the BMP4 group (P>0.05).
Exogenous BMP4 Drove the Imbalance of the M1/M2 Ratio
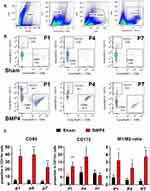
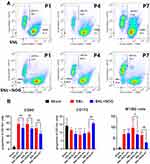
We then performed flow cytometry to verify whether exogenous BMP4 administration would give rise to an M1/M2 polarization ratio imbalance. Figure 3A shows the gating strategies of CD11b+ microglia. Then, as shown in Figure 3B and C, compared with the Sham group, the proportion of M1 microglia (characterized by CD11b+CD86+ cells) showed a marked increase at P1 (37.52±8.98% versus 4.75±1.17%, P<0.01), P4 (39.62±7.08% versus 4.59±1.10%, P<0.01) and P7 (22.47±5.29% versus 3.40±1.37%, P<0.01) in the BMP4 group. In contrast, the proportion of M2 microglia (recognized as CD11b+CD172+ cells) displayed elevated levels at P1 (12.05±2.73% versus 5.30±1.22%, P<0.01) and P4 (18.64±4.91% versus 8.13±3.39%, P=0.016), then fell to the Sham level at P7 (6.17±1.56% versus 7.54±2.05%, P>0.05). Moreover, the M1/M2 ratio in the BMP4 group dramatically increased at P1 (3.23±0.97 versus 0.96±0.36, P=0.013), P4 (2.24±0.67 versus 0.63±0.24, P=0.011) and P7 (3.73±0.85 versus 0.49±0.27, P<0.01) compared with the Sham group.
Together, all the above findings supported that BMP4 might have a role in the process of allodynia, microglial activation and polarization. After BMP4 treatment, the M1 polarization would be persistently activated through the whole 1st week, while M2 polarization peaked at the early stage, then fell to the untreated level thereafter, leading to a persistent imbalance of M1/M2 ratio.
Noggin Relieved Allodynia, Attenuated Microglial Activation and Changed the Pattern of Microglial Polarization in NP
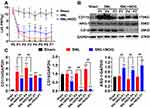
Rats’ NP model was achieved by L5 spinal nerve ligation. As shown in Figure 4A, rats in the SNL group developed a persistent and prominent decrease in the left PWT throughout the first week compared with the Sham group (P<0.01 at each time point), which was similar with previous report;15 Furthermore, in the SNL+NOG group, Noggin intrathecal administration significantly relieved SNL-induced PWT decrease at P3 (4.43±1.89 g versus 1.33±0.75 g, P<0.01), P4 (4.45±1.44 g versus 1.17±0.67 g, P<0.01), P5 (3.24±1.77 g versus 1.19±0.60 g, P<0.01) and P7 (4.24±1.60 g versus 1.79±0.60 g, P=0.012).
Next, using Western blotting method, we detected that rats in the SNL group showed a marked elevation in the expressions of CD11b from P1 to P7 (P<0.01 at each time point), CD16 at P7 (P<0.01) compared with the Sham group, while the ARG-1 levels remained comparable between the two groups at each time point (P>0.05); Moreover, compared with the SNL group, the CD11b and CD16 expressions in the SNL+NOG group significantly decreased from P1 (0.67-fold, P<0.01) to P4 (0.59-fold, P<0.01) and from P1 (0.56-fold, P<0.01) to P7 (0.08-fold, P<0.01), respectively; While the ARG-1 levels in the SNL+NOG group showed a prominent elevation from P1 (1.56-fold, P<0.01) to P7 (1.80-fold, P<0.01) in comparison with the SNL group (shown in Figure 4B and C).
Noggin Attenuated SNL Induced M1 Polarization and Promoted M2 Polarization at a Later Stage
We then performed RT-PCR to further determine Noggin’s effect on microglial polarization following SNL. As shown in Figure 5, the values for relative concentrations of each indicator in rats from the Sham group were defined as 1. Compared with the Sham group, rats in the SNL group showed a marked increase in M1-genes, including CD16 from P1 (8.22-fold, P<0.01) to P7 (17.10-fold, P<0.01) and TNF-α from P4 (4.07-fold, P<0.01) to P7 (11.81-fold, P<0.01), while MHC-II mRNA remained unchanged (P>0.05 during the 1st week). In the meantime, M2-genes in the SNL group, including ARG-1 and CD204, also showed a notable elevation from P4 (1.89-fold, P<0.01) to P7 (1.67-fold, P=0.026) and from P1 (10.56-fold, P<0.01) to P7 (12.50-fold, P<0.01), respectively, while IL-4 mRNA levels stayed unchanged. Moreover, compared with the SNL group, a significant decrease in M1-gene levels, including CD16 from P1 (0.33-fold, P<0.01) to P7 (0.31-fold, P<0.01) and TNF-α from P4 (0.35-fold, P=0.015) to P7 (0.58-fold, P<0.01), was observed in the SNL+NOG group, while MHC-II mRNA remained unchanged. Secondly, the levels of M2 gene markers, including ARG-1, CD204 and IL-4 in the SNL+NOG group, remained comparable with the SNL group at P1 and P4 (except for CD204 at P4, 1.87-fold, P=0.014), and then showed a marked increase at P7 (ARG-1: 1.79-fold, P=0.013; CD204: 1.88-fold, P<0.01; IL-4:1.94-fold, P=0.027).
Noggin Reversed the Imbalance of the M1/M2 Ratio in the Process of NP
Finally, we performed flow cytometry to verify whether Noggin treatment could reverse the imbalance of the M1/M2 ratio. Firstly, the proportions of the CD11b+CD86+ M1 subtype and CD11b+CD172+ M2 subtype in rats of the Sham group were 6.34±2.67% and 20.48±8.35%, respectively (data not shown). Secondly, as shown in Figure 6A and B, compared with the Sham group, the proportion of CD11b+CD86+ cells in rats of the SNL group significantly increased to 76.68±6.48% at P1 (P<0.01), 76.58±5.67% at P4 (P<0.01) and 63.52±4.47% at P7 (P<0.01), while the proportion of CD11b+CD172+ cells decreased at P4 (P4: 8.56±2.64%, P<0.01). Thirdly, compared with the SNL group, the proportion of CD11b+CD86+ cells in the SNL+NOG group showed a significant decrease at P1 (64.44±6.45%, P<0.01), P4 (62.44±1.88%, P<0.01), and P7 (46.36±4.70, P<0.01). Meanwhile, the proportion of CD11b+CD172+ cells in the SNL+NOG group remained comparable with the SNL group at P1 and P4 (P>0.05), and then increased to 15.71±0.95% at P7 (P=0.046). Finally, the M1/M2 ratio was statistically lower in the SNL+NOG group than the SNL group at P4 (6.68±0.88 versus 9.72±3.22, P=0.030) and P7 (2.97±0.44 versus 6.43±0.93, P<0.01).
In ensemble, all finds above corroborated that Noggin treatment could relieve SNL-induced allodynia with a time-dependent manner, probably through attenuating microglial activation; Besides, Noggin could persistently down-regulated the M1 polarization, while enhanced the M2 polarization at a later stage, thus reversing the imbalance of the M1/M2 ratio in the process of NP.
Discussion
In the present study, we firstly proved that intrathecal administration of exogenous BMP4 could induce allodynia through directing microglia towards continuously increasing M1 polarization together with inadequate M2 polarization, leading to the imbalance of M1/M2 ratio. Furthermore, antagonizing BMP4 signaling in the process of NP facilitated by SNL relieved the pain behavior through mitigating microglial activation and reversing the imbalance of the M1/M2 ratio. All the results above suggested that BMP4 might play a critical role in microglial polarization in the process of NP.
Compared with the scant available data regarding BMP4ʹs effect on microglia in the CNS, a growing body of evidence has illustrated its role in regulating macrophage activity and inflammation in peripheral organs. Intriguingly, the BMP4ʹs effect on macrophage remains controversial. On the one hand, substantial evidence17–21 has revealed that local elevation of BMP4 impedes endothelial barrier function, promotes leukocytes diapedesis and initiates macrophage activation via BMP4/reactive oxygen species (ROS)/cyclooxygenase-II (COX-2) cascade in the process of atherosclerosis; Besides, BMP4 has also proven to trigger macrophage M1 polarization through JNK pathways, therefore aggravating the inflammatory severity in the course of myocardial infarction, hypertrophy and apoptosis;22–27 Moreover, one of our recent studies28 further demonstrated that after accepting thoracic surgery, patients’ circulating BMP4 levels would sharply elevate and show a strong positive correlation with pro-inflammatory cytokines (including interleukin-1β (IL-1β) and TNF-α), and application of flurbiprofen (a COX-II inhibitor) could alleviate the elevation of both BMP4 and inflammatory cytokines. All the results indicate that BMP4 plays a pro-inflammatory role. On the other hand, other studies argued that BMP4 secreted by pulmonary arteries,29,30 tumors31,32 and adipose tissue33 can favor macrophage M2 polarization, thus exerting an anti-inflammatory or immuno-suppressive effect. In ensemble, the conflicting evidences above prompt that BMP4 may act as a molecular switch to induce both pro- and anti- inflammatory properties through directing either M1 or M2 polarization depending on the organ and specific phase of the disease.
Considering that resident microglia share similar morphology and immunoreactivity with circulating macrophages,34,35 we further assume that BMP4 might also play a role in regulating microglial activity in the CNS. So far, only a few studies have pointed out the possible relationship between BMP4 and microglial biology. For instance, Sato et al12 found that BMP4ʹs special receptors are expressed on microglia in normal adult spinal cord, suggesting that BMP4 might be a potential microglial regulator. Notably, during the course of experimental autoimmune encephalomyelitis36 and multiple sclerosis,37 the expressions of BMP4 are upregulated prominently on activated microglia at the sites of inflammation; Antagonizing BMP4 signaling decreases the microglial activity, therefore improving the neurological improvement after amyotrophic lateral sclerosis.38 All evidences above corroborate that BMP4 is strongly connected with microglial activation during the CNS inflammatory disease. In the present study, we demonstrated that the intrathecal administration of exogenous BMP4 on normal adult rats could induce persistent allodynia, together with microglial activation and polarization. Compared with the Sham group, a series of M1 and M2 polarized markers were elevated after BMP4 application. Thus, it can be inferred that BMP4 also has the ability to regulate the polarization of microglia at the resting state.
Another notable phenomenon was that the BMP4 induced both M1 and M2 polarization at the beginning of the application, suggesting that BMP4 might achieve the induction of both pro- and anti-inflammatory properties to the spinal cord at the early stage. One possible explanation is likely to be dependent on the diverse actions of BMP receptors (BMPRs). Generally, there are three main receptors of BMP4: BMPRIA, BMPRIB and BMPRII.39 Previous studies have already demonstrated that BMPRIA and BMPRIB exert distinct effects on gliosis after spinal cord injury.40 Besides, in the process of inflammation and oxidative injury following atherosclerosis,41,42 colitis43 and asthma,30 the expressions of BMPRII will be down-regulated, while BMPRI stays unchanged44 or even elevated.18 Moreover, using BMPRII siRNA and BMPRII± mice, Kim et al42 further proved that knockdown of BMPRII instead of other BMPR induces endothelial inflammation with a ligand independent manner, suggesting BMPRII might be a critical downstream receptor to dominate BMP4ʹs effect of either pro-inflammation or anti-inflammation. In the present study, we detected that intrathecal administration of BMP4 induced a long-lasting M1 polarization; Otherwise, M2 polarization was initiated at first, but then fell rapidly to the Sham levels at a later stage. Whether or not this M1/M2 ratio imbalance induced by BMP4 is accompanied with the dynamic changes of BMPRII expressions in microglia, and the actual role of each kind of BMPR in microglial polarization needs further elucidation.
NP is also known as an inflammatory disease, which is well characterized with microglial activation and polarization. Abundant of studies4–8 have identified that after the nerve injury, microglia will be polarized mainly towards M1 subtype, which contributes to the overproduction of series of pro-inflammatory cytokines and finally leads to the allodynia; Besides, treatments targeting on alleviating M1 polarization while restoring M2 polarization have proven to be effective for the pain relief.45–48 Importantly, Xu et al6 reported that following chronic sciatic nerve damage, both M1 and M2 subtype microglial genes increased immediately at day one. However, only M1-related genes remained elevation at day seven and thereafter, leaving M2 genes falling to the Sham level. Similarly, in the present study, we detected that after SNL, most of the M1 biomarkers, including gene and/or protein levels of CD16, TNF-α and CD86 (but not MHC-II), increased throughout the first week. On the contrary, among the M2 markers, the IL-4 gene and CD172 protein levels remained at or fell below the Sham level, which was in agreement with Xu’s study. Nevertheless, the ARG-1 and CD204 levels remained elevated for the whole week, representing an endogenous protective process in order to resolve the local inflammation. The discrepancy of M2 expression patterns between the two studies might be that different animal models and different polarized markers were chosen, and imply the fact introduced by our previous study49 that a special inflammatory microenvironment may favor some particular biomarkers but not others with a strict time pattern. Furthermore, using the cyto-flow method, we proved that the M1/M2 ratio showed a persistent and sharp increase after NP takes place, suggesting that switching the microglial M1/M2 polarization pattern within appropriate time window should be recommended.
We have previously reported that NP achieved by SNL is accompanied by the upregulation of endogenous BMP4.13 In light of the above findings that exogenous BMP4 induces allodynia and microglial polarization, we further hypothesized that antagonizing BMP4 should block its pro-nociceptive effect in the process of NP. As a recognized BMP4 antagonist, Noggin can directly bind with and de-activate BMP4 signaling with a high affinity.50 Previous evidence has shown that Noggin can abolish BMP4-induced Nicotinamide Adenine Dinucleotide Phosphate Oxidase 1 (NOX1), ROS and COX2 activation, and promote inflammation dissipation in the process of atherosclerosis18,21,22 and cardiac hypertrophy.26 Besides, in our previous study,28 we found that compared with the elevation of patients’ circulating BMP4 levels, which was highly correlated with the inflammation, endogenous Noggin levels stayed almost unchanged during surgery. Although the mechanism remained unclear, it may prompt that Noggin could be a new therapeutic target to treat inflammation in the clinical scenario. In the CNS, animal researches using models of multiple sclerosis and spinal cord injury51–53 have demonstrated that Noggin could attenuate demyelination and neuron loss induced by BMP4, which is released from activated microglia and astrocyte. Moreover, Noggin infusion has also been identified to have the ability to guide a shift of microglial status from the iron-storing (pro-inflammatory, M1) to the iron-releasing (anti-inflammatory, M2) subtype, thus providing beneficial effects for the survival of oligodendrocytes and improving remyelination after stroke takes place.54,55 All evidences above strongly supported that Noggin could exert anti-inflammatory properties in the repair-associated process in the CNS. Here, using a well-established NP model, we firstly demonstrated that Noggin treatment relieved SNL-induced allodynia and microglial activation. Besides, Noggin changed the microglial polarization dynamics in the process of NP by persistently decreasing M1 markers expressions while increasing M2 markers at a late stage, thereby reversing the imbalance of the M1/M2 ratio. Therefore, the above evidence indicates that Noggin might be a promising therapeutic approach to treat NP via regulating microglial polarization.
There were several limitations in our study. Firstly, we only detect the expression pattern of microglial polarization in 7 days following surgical interventions based mainly on our preliminary research, during which the changes of the endogenous BMP4 and M1/M2 markers’ expressions were prominent. Future research shall extend the observation period to investigate the BMP4ʹs effect on microglial activity in a longer range. Secondly, considering the potential diversity of each BMPR on the regulation of inflammation, the expression dynamics and the actual role of each BMPR (especially BMPRII) should be addressed in a future study.
Overall, the present study showed that BMP4 has the ability to induce allodynia by driving microglia towards both M1 and M2 polarization with different time patterns. Antagonizing BMP4 signaling could relieve pain behavior by mitigating microglial activation and reversing the imbalance of the M1/M2 ratio in the process of NP.
Data Sharing Statement
All individual deidentified participant data and statistical analysis code that underlie the results reported in this article will be available after publication. Data can be requested for academic use by contacting the corresponding author (Lin Yang: [email protected]).
Authorship Contribution
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work is funded by the National Key Research and Development Project of China (2018YFC2001902 to Xu JM) and the Foundation of Provincial Health Committee (HNA202101002 to Yang L).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol Rev. 2021;101(1):259–301. doi:10.1152/physrev.00045.2019
2. Tiwari V, Guan Y, Raja SN. Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev. 2014;45:19–27. doi:10.1016/j.neubiorev.2014.05.002
3. Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5–19. doi:10.1016/j.it.2016.10.001
4. Calvo M, Bennett DL. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol. 2012;234(2):271–282. doi:10.1016/j.expneurol.2011.08.018
5. Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi:10.2147/JPR.S53660
6. Xu F, Huang J, He Z, et al. Microglial polarization dynamics in dorsal spinal cord in the early stages following chronic sciatic nerve damage. Neurosci Lett. 2016;617:6–13. doi:10.1016/j.neulet.2016.01.038
7. Gong X, Chen Y, Fu B, Jiang J, Zhang M. Infant nerve injury induces delayed microglial polarization to the M1 phenotype, and exercise reduces delayed neuropathic pain by modulating microglial activity. Neuroscience. 2017;349:76–86. doi:10.1016/j.neuroscience.2017.02.051
8. Xu N, Tang XH, Pan W, et al. Spared nerve injury increases the expression of microglia M1 markers in the prefrontal cortex of rats and provokes depression-like behaviors. Front Neurosci. 2017;11:209. doi:10.3389/fnins.2017.00209
9. Lowery JW, Rosen V. Bone morphogenetic protein-based therapeutic approaches. Cold Spring Harb Perspect Biol. 2018;10(4):a022327. doi:10.1101/cshperspect.a022327
10. Wang Y, Cheng X, He Q, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31(16):6053–6058. doi:10.1523/JNEUROSCI.5524-09.2011
11. Cole AE, Murray SS, Xiao J. Bone morphogenetic protein 4 signalling in neural stem and progenitor cells during development and after injury. Stem Cells Int. 2016;2016:9260592. doi:10.1155/2016/9260592
12. Miyagi M, Mikawa S, Sato T, et al. BMP2, BMP4, noggin, BMPRIA, BMPRIB, and BMPRII are differentially expressed in the adult rat spinal cord. Neuroscience. 2012;203:12–26. doi:10.1016/j.neuroscience.2011.12.022
13. Yang L, Liu S, Wang Y. Role of bone morphogenetic protein-2/4 in astrocyte activation in neuropathic pain. Mol Pain. 2019;15:1744806919892100. doi:10.1177/17448069.19892100
14. Hashimoto M, Koda M, Furuya T, Murata A, Yamazaki M, Takahashi K. Intrathecal Noggin administration in rats temporally ameliorates mechanical allodynia induced by a chronic constriction injury. eNeurologicalSci. 2016;4:4–9. doi:10.1016/j.ensci.2016.03.001
15. Westin BD, Walker SM, Deumens R, Grafe M, Yaksh TL. Validation of a preclinical spinal safety model: effects of intrathecal morphine in the neonatal rat. Anesthesiology. 2010;113(1):183–199. doi:10.1097/ALN.0b013e3181dcd6ec
16. Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med. 2004;99:35–45. doi:10.1385/1-59259-770-X:035
17. Helbing T, Arnold L, Wiltgen G, et al. Endothelial BMP4 regulates leukocyte diapedesis and promotes inflammation. Inflammation. 2017;40(6):1862–1874. doi:10.1007/s10753-017-0627-0
18. Sorescu GP, Song H, Tressel SL, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95(8):773–779. doi:10.1161/01.RES.0000145728.22878.45
19. Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4- induced inflammation and atherosclerosis. Antioxid Redox Signal. 2006;8(9–10):1609–1619. doi:10.1089/ars.2006.8.1609
20. Wong WT, Tian XY, Chen Y, et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res. 2010;107(8):984–991. doi:10.1161/CIRCRESAHA.110.222794
21. Helbing T, Wiltgen G, Hornstein A, et al. Bone morphogenetic protein-modulator BMPER regulates endothelial barrier function. Inflammation. 2017;40(2):442–453. doi:10.1007/s10753-016-0490-4
22. Youn JY, Zhou J, Cai H. Bone morphogenic protein 4 mediates NOX1-dependent eNOS uncoupling, endothelial dysfunction, and COX2 induction in type 2 diabetes mellitus. Mol Endocrinol. 2015;29(8):1123–1133. doi:10.1210/ME.2014-1313
23. Hanna A, Frangogiannis NG. The role of the TGF-β superfamily in myocardial infarction. Front Cardiovasc Med. 2019;6:140. doi:10.3389/fcvm.2019.00140
24. Pallotta I, Sun B, Wrona EA, Freytes DO. BMP protein-mediated crosstalk between inflammatory cells and human pluripotent stem cell-derived cardiomyocytes. J Tissue Eng Regen Med. 2017;11(5):1466–1478. doi:10.1002/term.2045
25. Wu T, Ling QY, Zhong C, et al. Expression of BMP4 in myocardium and vascular tissue of obese mice. J Inflamm. 2015;12:8. doi:10.1186/s12950-015-0047-6
26. Sun B, Huo R, Sheng Y, et al. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension. 2013;61(2):352–360. doi:10.1161/HYPERTENSIONAHA.111.00562
27. Kercheva M, Gusakova AM, Ryabova TR, Suslova TE, Kzhyshkowska J, Ryabov VV. Serum levels of bone morphogenetic proteins 2 and 4 in patients with acute myocardial infarction. Cells. 2020;9(10):2179. doi:10.3390/cells9102179
28. Zhao X, Zhang J, Zhang W, et al. the relationship between circulating bone morphogenetic protein-4 and inflammation cytokines in patients undergoing thoracic surgery: a prospective randomized study. J Inflamm Res. 2021;14:4069–4077. doi:10.2147/JIR.S324775
29. Csiszar A, Labinskyy N, Jo H, Ballabh P, Ungvari Z. Differential proinflammatory and prooxidant effects of bone morphogenetic protein-4 in coronary and pulmonary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2008;295(2):H569–77. doi:10.1152/ajpheart.00180.2008
30. Kariyawasam HH, Xanthou G, Barkans J, Aizen M, Kay AB, Robinson DS. Basal expression of bone morphogenetic protein receptor is reduced in mild asthma. Am J Respir Crit Care Med. 2008;177(10):1074–1081. doi:10.1164/rccm.200709-1376OC
31. Martínez VG, Rubio C, Martínez-Fernández M, et al. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder cancer. Clin Cancer Res. 2017;23(23):7388–7399. doi:10.1158/1078-043.2.CCR-17-1004
32. Valencia J, Fernández-Sevilla L, Fraile-Ramos A, et al. Acute lymphoblastic leukaemia cells impair dendritic cell and macrophage differentiation: role of BMP4. Cells. 2019;8(7):722. doi:10.3390/cells8070722
33. Qian SW, Wu MY, Wang YN, et al. BMP4 facilitates beige fat biogenesis via regulating adipose tissue macrophages. J Mol Cell Biol. 2019;11(1):14–25. doi:10.1093/jmcb/mjy011
34. Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18(4):225–242. doi:10.1038/nri.2017.125
35. Haage V, Semtner M, Vidal RO, et al. Comprehensive gene expression meta-analysis identifies signature genes that distinguish microglia from peripheral monocytes/macrophages in health and glioma. Acta Neuropathol Commun. 2019;7(1):20. doi:10.1186/s40478-019-0665-y
36. Ara J, See J, Mamontov P, et al. Bone morphogenetic proteins 4, 6, and 7 are up-regulated in mouse spinal cord during experimental autoimmune encephalomyelitis. J Neurosci Res. 2008;86(1):125–135. doi:10.1002/jnr.21462
37. Costa C, Eixarch H, Martínez-Sáez E, et al. Expression of bone morphogenetic proteins in multiple sclerosis lesions. Am J Pathol. 2019;189(3):665–676. doi:10.1016/j.ajpath.2018.11.007
38. Shijo T, Warita H, Suzuki N, et al. Antagonizing bone morphogenetic protein 4 attenuates disease progression in a rat model of amyotrophic lateral sclerosis. Exp Neurol. 2018;307:164–179. doi:10.1016/j.expneurol.2018.06.009
39. Gomez-Puerto MC, Iyengar PV, García de Vinuesa A, Ten Dijke P, Sanchez-Duffhues G. Bone morphogenetic protein receptor signal transduction in human disease. J Pathol. 2019;247(1):9–20. doi:10.1002/path.5170
40. Sahni V, Mukhopadhyay A, Tysseling V, et al. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30(5):1839–1855. doi:10.1523/JNEUROSCI.4459-09.2010
41. Wang D, Prakash J, Nguyen P, et al. Bone morphogenetic protein signaling in vascular disease: anti-inflammatory action through myocardin-related transcription factor A. J Biol Chem. 2012;287(33):28067–28077. doi:10.1074/jbc.M112.379487
42. Kim CW, Song H, Kumar S, et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1350–1359. doi:10.1161/ATVBAHA.112.300287
43. Maric I, Kucic N, Turk Wensveen T, et al. BMP signaling in rats with TNBS-induced colitis following BMP7 therapy. Am J Physiol Gastrointest Liver Physiol. 2012;302(10):G1151–62. doi:10.1152/ajpgi.00244.2011
44. Rosendahl A, Pardali E, Speletas M, Ten Dijke P, Heldin CH, Sideras P. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002;27(2):160–169. doi:10.1165/ajrcmb.27.2.4779
45. Jin GL, Hong LM, Liu HP, et al. Koumine modulates spinal microglial M1 polarization and the inflammatory response through the Notch-RBP-Jκ signaling pathway, ameliorating diabetic neuropathic pain in rats. Phytomedicine. 2021;90:153640. doi:10.1016/j.phymed.2021.153640
46. Guo A, Li J, Luo L, et al. Valproic acid mitigates spinal nerve ligation-induced neuropathic pain in rats by modulating microglial function and inhibiting neuroinflammatory response. Int Immunopharmacol. 2021;92:107332. doi:10.1016/j.intimp.2020.107332
47. Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia - evidence from in vivo and in vitro studies. Neuropharmacology. 2016;108:207–219. doi:10.1016/j.neuropharm.2016.04.024
48. Popiolek-Barczyk K, Kolosowska N, Piotrowska A, et al. Parthenolide relieves pain and promotes M2 microglia/macrophage polarization in rat model of neuropathy. Neural Plast. 2015;2015:676473. doi:10.1155/2015/676473
49. Li H, Wang P, Tang L, et al. Distinct polarization dynamics of microglia and infiltrating macrophages: a novel mechanism of spinal cord ischemia/reperfusion injury. J Inflamm Res. 2021;14:5227–5239. doi:10.2147/JIR.S335382
50. Phan-Everson T, Etoc F, Li S, et al. Differential compartmentalization of BMP4/NOGGIN requires NOGGIN trans-epithelial transport. Dev Cell. 2021;56(13):1930–1944.e5. doi:10.1016/j.devcel.2021.05.003
51. Harnisch K, Teuber-Hanselmann S, Macha N, et al. Myelination in multiple sclerosis lesions is associated with regulation of bone morphogenetic protein 4 and its antagonist Noggin. Int J Mol Sci. 2019;20(1):154. doi:10.3390/ijms20010154
52. Matsuura I, Taniguchi J, Hata K, Saeki N, Yamashita T. BMP inhibition enhances axonal growth and functional recovery after spinal cord injury. J Neurochem. 2008;105(4):1471–1479. doi:10.1111/j.1471-4159.2008.05251.x
53. Lei ZN, Liu F, Zhang LM, Huang YL, Sun FY. Bcl-2 increases stroke-induced striatal neurogenesis in adult brains by inhibiting BMP-4 function via activation of β-catenin signaling. Neurochem Int. 2012;61(1):34–42. doi:10.1016/j.neuint.2012.04.004
54. Shin JA, Kim YA, Kim HW, et al. Iron released from reactive microglia by noggin improves myelin repair in the ischemic brain. Neuropharmacology. 2018;133:202–215. doi:10.1016/j.neuropharm.2018.01.038
55. Shin JA, Lim SM, Jeong SI, Kang JL, Park EM. Noggin improves ischemic brain tissue repair and promotes alternative activation of microglia in mice. Brain Behav Immun. 2014;40:143–154. doi:10.1016/j.bbi.2014.03.013
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.